The interaction of the alternative oxidase (AOX) pathway with nutrient metabolism is important for understanding how respiration modulates ATP synthesis and carbon economy in plants under nutrient deficiency. Although AOX activity reduces the energy yield of respiration, this enzymatic activity is upregulated under stress conditions to maintain the functioning of primary metabolism. The in vivo metabolic regulation of AOX activity by phosphorus (P) and nitrogen (N) and during plant symbioses with Arbuscular mycorrhizal fungi (AMF) and Rhizobium bacteria is still not fully understood. We highlight several findings and open questions concerning the in vivo regulation of AOX activity and its impact on plant metabolism during P deficiency and symbiosis with AMF. We also highlight the need for the identification of which metabolic regulatory factors of AOX activity are related to N availability and nitrogen‐fixing legume‐rhizobia symbiosis in order to improve our understanding of N assimilation and biological nitrogen fixation.
- alternative oxidase
- arbuscular mycorrhizal fungi
- nitrogen and phosphorus nutrition
- rhizobium
- plant primary metabolism
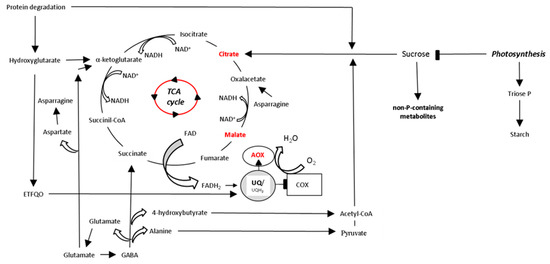
Regulation of AOX Activity by P Availability
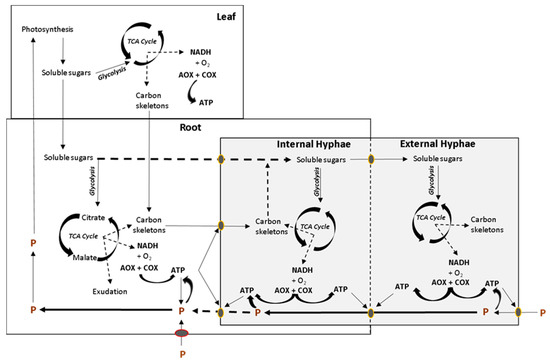
Regulation of AOX Activity by Arbuscular Mycorrhizal Symbiosis
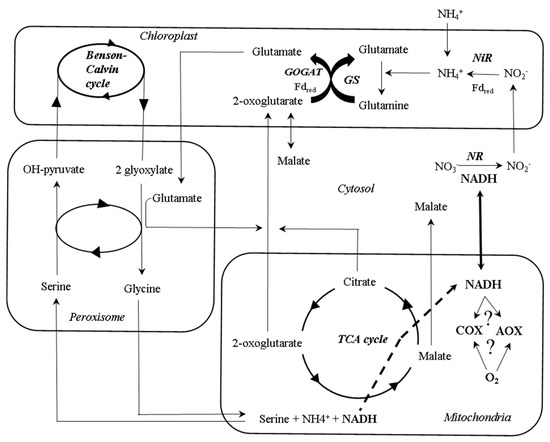
Regulation of AOX Activity by N Availability
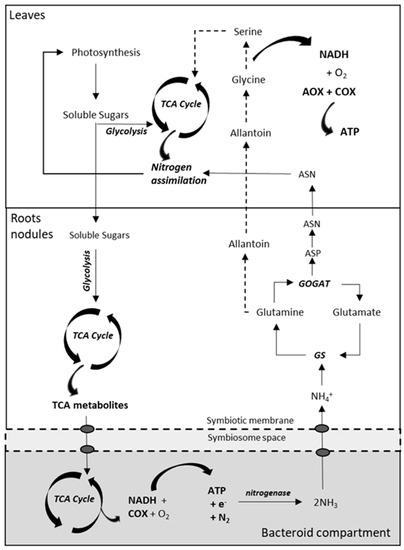
Regulation of AOX Activity in the Rhizobium-Legume Symbiosis
- George, T.S.; Hinsinger, P.; Turner, B.L. Phosphorus in soils and plants–facing phosphorus scarcity. Plant Soil. 2016, 401, 1–6. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhan, W.; Zhang, F. Phosphorus dynamics: From soil to Plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of macronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marchner, P., Ed.; Academic Press: Salt Lake City, UT, USA, 2012; pp. 135–189. [Google Scholar]
- Araújo, W.L.; Nunes-Nesi, A.; Nikoloski, Z.; Sweetlove, L.J.; Fernie, A.R. Metabolic control and regulation of the tricarboxylic acid cycle in photosynthetic and heterotrophic plant tissues. Plant Cell Environ. 2012, 35, 1–21. [Google Scholar] [CrossRef]
- Shane, M.W.; Lambers, H. Cluster roots: A curiosity in context. In Root Physiology: From Gene to Function, 1st ed.; Lambers, H., Colmer, T.D., Eds.; Springer: Dordrecht, The Netherlands , 2005; pp. 101–125. [Google Scholar]
- Lambers, H.; Shane, M.W.; Cramer, M.D.; Pearse, S.J.; Veneklaas, E.J. Root structure and functioning for efficient acquisition of phosphorus: Matching morphological and physiological traits. Ann. Bot. 2006, 98, 693–713. [Google Scholar] [CrossRef]
- Lambers, H.; Brundrett, M.; Raven, J.; Hopper, S. Plant mineral nutrition in ancient landscapes: High plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant Soil 2010, 334, 11–31. [Google Scholar] [CrossRef]
- Zemunik, G.; Lambers, H.; Turner, B.L.; Laliberté, E.; Oliveira, R.S. High abundance of non-mycorrhizal plant species in severely phosphorus-impoverished Brazilian campos rupestres. Plant Soil. 2018, 424, 255–271. [Google Scholar] [CrossRef]
- Florez-Sarasa, I.; Lambers, H.; Wang, X.; Finnegan, P.M.; Ribas-Carbo, M. The alternative respiratory pathway mediates carboxylate synthesis in white lupin cluster roots under phosphorus deprivation. Plant Cell Environ. 2014, 37, 922–928. [Google Scholar] [CrossRef]
- Shane, M.W.; Cramer, M.D.; Funayama-Noguchi, S.; Cawthray, G.R.; Millar, A.H.; Day, D.A.; Lambers, H. Developmental physiology of cluster-root carboxylate synthesis and exudation in harsh hakea. Expression of phosphoenolpyruvate carboxylase and the alternative oxidase. Plant Physiol. 2004, 135, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Vanlerberghe, G.C.; Mclntosh, L. Signals regulating the expression of the nuclear gene encoding alternative oxidase of plant mitochondria. Plant Physiol. 1996, 111, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Plaxton, W.C. Phosphorus: Back to the Roots. In Annual Plant Reviews Phosphorus Metabolism in Plants; Plaxton, W.C., Lambers, H., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; Volume 48, pp. 1–22. [Google Scholar]
- López-Bucio, J.; Nieto-Jacobo, M.F.; Ramırez-Rodrıguez, V.; Herrera-Estrella, L. Organic acid metabolism in plants: From adaptive physiology to transgenic varieties for cultivation in extreme soils. Plant Sci. 2000, 160, 1–13. [Google Scholar] [CrossRef]
- Alexova, R.; Nelson, C.J.; Millar, A.H. Temporal development of the barley leaf metabolic response to Pi limitation. Plant Cell Environ. 2017, 40, 645–657. [Google Scholar] [CrossRef]
- Theodorou, M.E.; Elrifi, I.R.; Turpin, D.H.; Plaxton, W.C. Effects of phosphorus limitation on respiratory metabolism in the green alga Selenastrum minutum. Plant Physiol. 1991, 95, 1089–1095. [Google Scholar] [CrossRef]
- Rychter, A.M.; Mikulska, M. The relationship between phosphate status and cyanide-resistant respiration in bean roots. Physiol. Plant 1991, 79, 663–667. [Google Scholar] [CrossRef]
- Hoefnagel, M.H.; Van Iren, F.; Libbenga, K.R.; Van der Plas, L.H. Possible role of adenylates in the engagement of the cyanide-resistant pathway in nutrient-starved Catharanthus roseus Cells. Physiol. Plant 1994, 90, 269–278. [Google Scholar] [CrossRef]
- Parsons, H.L.; Yip, J.Y.; Vanlerberghe, G.C. Increased respiratory restriction during phosphate-limited growth in transgenic tobacco cells lacking alternative oxidase. Plant Physiol. 1999, 121, 1309–1320. [Google Scholar] [CrossRef]
- Juszczuk, I.; Malusà, E.; Rychter, A.M. Oxidative stress during phosphate deficiency in roots of bean plants (Phaseolus vulgaris L.). J. Plant Physiol. 2001, 158, 1299–1305. [Google Scholar] [CrossRef]
- Sieger, S.M.; Kristensen, B.K.; Robson, C.A.; Amirsadeghi, S.; Eng, E.W.; Abdel-Mesih, A.; Møller, I.M.; Vanlerberghe, G.C. The role of alternative oxidase in modulating carbon use efficiency and growth during macronutrient stress in tobacco cells. J. Exp. Bot. 2005, 56, 1499–1515. [Google Scholar] [CrossRef] [PubMed]
- Funayama-Noguchi, S.; Noguchi, K.O.; Terashima, I. Comparison of the response to phosphorus deficiency in two lupin species, Lupinus albus and L. angustifolius, with contrasting root morphology. Plant Cell Environ. 2015, 38, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Del-Saz, N.F.; Romero-Munar, A.; Cawthray, G.R.; Palma, F.; Aroca, R.; Baraza, E.; Florez-Sarasa, I.; Lambers, H.; Ribas-Carbó, M. Phosphorus concentration coordinates a respiratory bypass, synthesis and exudation of citrate, and the expression of high-affinity phosphorus transporters in Solanum lycopersicum. Plant Cell Environ. 2018, 41, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Gonzàlez-Meler, M.A.; Giles, L.; Thomas, R.B.; Siedow, J.N. Metabolic regulation of leaf respiration and alternative pathway activity in response to phosphate supply. Plant Cell Environ. 2001, 24, 205–215. [Google Scholar] [CrossRef]
- Campbell, C.D.; Sage, R.F. Interactions between the effects of atmospheric CO2 content and P nutrition on photosynthesis in white lupin (Lupinus albus L.). Plant Cell Environ. 2006, 29, 844–853. [Google Scholar] [CrossRef]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Roessner, U.; Eickmeier, I.; Genc, Y.; Callahan, D.L.; Shirley, N.; Langridge, P.; Bacic, A. Metabolite profiling reveals distinct changes in carbon and nitrogen metabolism in phosphate-deficient barley plants (Hordeum vulgare L.). Plant Cell Physiol. 2008, 49, 691–703. [Google Scholar] [CrossRef]
- Warren, C.R. How does P affect photosynthesis and metabolite profiles of Eucalyptus globulus? Tree Physiol. 2011, 31, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Araújo, W.L.; Tohge, T.; Ishizaki, K.; Leaver, C.J.; Fernie, A.R. Protein degradation–an alternative respiratory substrate for stressed plants. Trends Plant Sci. 2011, 16, 489–498. [Google Scholar] [CrossRef]
- Hildebrandt, T.M.; Nesi, A.N.; Araújo, W.L.; Braun, H.P. Amino acid catabolism in plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Szal, B.; Podgorska, A. The role of mitochondria in leaf nitrogen metabolism. Plant Cell Environ. 2012, 35, 1756–1768. [Google Scholar] [CrossRef] [PubMed]
- Brundrett, M.C.; Tedersoo, L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Strack, D.; Fester, T.; Hause, B.; Schliemann, W.; Walter, M.H. Arbuscular mycorrhiza: Biological, chemical, and molecular aspects. J. Chem. Ecol. 2003, 29, 1955–1979. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3th ed.; Academic Press: London, UK, 2008; pp. 1–769. [Google Scholar]
- Nagy, R.; Drissner, D.; Amrhein, N.; Jakobsen, I.; Bucher, M. Mycorrhizal phosphate uptake pathway in tomato is phosphorus-repressible and transcriptionally regulated. New Phytol. 2009, 181, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.K.; Hodge, A.; Fitter, A.H.; Atkin, O.K. Mycorrhizal respiration: Implications for global scaling relationships. Trends Plant Sci. 2008, 13, 583–588. [Google Scholar] [CrossRef]
- Bago, B.; Pfeffer, P.E.; Shachar-Hill, Y. Carbon metabolism and transport in arbuscular mycorrhizas. Plant Physiol. 2000, 124, 949–958. [Google Scholar] [CrossRef]
- Baas, R.; Kuiper, D. Effects of vesicular-arbuscular mycorrhizal infection and phosphate on Plantago major ssp. pleiosperma in relation to internal cytokinin concentrations. Physiol. Plant 1989, 76, 211–215. [Google Scholar] [CrossRef]
- Hodge, A. Impact of elevated CO2 on mycorrhizal associations and implications for plant growth. Biol. Fertil. Soils. 1996, 23, 388–398. [Google Scholar] [CrossRef]
- Johnson, D.; Leake, J.R.; Read, D.J. Transfer of recent photosynthate into mycorrhizal mycelium of an upland grassland: Short-term respiratory losses and accumulation of 14C. Soil Biol. Biochem. 2002, 34, 1521–1524. [Google Scholar] [CrossRef]
- Atkin, O.K.; Sherlock, D.; Fitter, A.H.; Jarvis, S.; Hughes, J.K.; Campbell, C.; Hurry, V.; Hodge, A. Temperature dependence of respiration in roots colonized by arbuscular mycorrhizal fungi. New Phytol. 2009, 182, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Hohnjec, N.; Vieweg, M.F.; Pühler, A.; Becker, A.; Küster, H. Overlaps in the transcriptional profiles of Medicago truncatula roots inoculated with two different Glomus fungi provide insights into the genetic program activated during arbuscular mycorrhiza. Plant Physiol. 2005, 137, 1283–1301. [Google Scholar] [CrossRef] [PubMed]
- Schliemann, W.; Ammer, C.; Strack, D. Metabolite profiling of mycorrhizal roots of Medicago truncatula. Phytochemistry 2008, 69, 112–146. [Google Scholar] [CrossRef] [PubMed]
- Laparre, J.; Malbreil, M.; Letisse, F.; Portais, J.C.; Roux, C.; Bécard, G.; Puech-Pagès, V. Combining metabolomics and gene expression analysis reveals that propionyl-and butyryl-carnitines are involved in late stages of arbuscular mycorrhizal symbiosis. Mol. Plant 2014, 7, 554–566. [Google Scholar] [CrossRef]
- Ryan, M.H.; Tibbett, M.; Edmonds, T.; Suriyagoda, L.D.; Lambers, H.; Cawthray, G.R.; Pang, J. Carbon trading for phosphorus gain: The balance between rhizosphere carboxylates and arbuscular mycorrhizal symbiosis in plant phosphorus acquisition. Plant Cell Environ. 2012, 35, 2170–2180. [Google Scholar] [CrossRef] [PubMed]
- Nazeri, N.K.; Lambers, H.; Tibbett, M.; Ryan, M.H. Moderating mycorrhizas: Arbuscular mycorrhizas modify rhizosphere chemistry and maintain plant phosphorus status within narrow boundaries. Plant Cell Environ. 2014, 37, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Romero-Munar, A.; Del-Saz, N.F.; Ribas-Carbó, M.; Flexas, J.; Baraza, E.; Florez-Sarasa, I.; Fernie, A.R.; Gulías, J. Arbuscular mycorrhizal symbiosis with Arundo donax decreases root respiration increases both photosynthesis and plant biomass accumulation. Plant Cell Environ. 2017, 40, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Pearse, S.J.; Veneklaas, E.J.; Cawthray, G.; Bolland, M.D.; Lambers, H. Carboxylate composition of root exudates does not relate consistently to a crop species’ ability to use phosphorus from aluminium, iron or calcium phosphate sources. New Phytol. 2007, 173, 181–190. [Google Scholar] [CrossRef]
- Pang, J.; Ryan, M.H.; Tibbett, M.; Cawthray, G.R.; Siddique, K.H.M.; Bolland, M.D.A.; Denton, M.D.; Lambers, H. Variation in morphological and physiological parameters in herbaceous perennial legumes in response to phosphorus supply. Plant Soil. 2010, 331, 241–255. [Google Scholar] [CrossRef]
- Raven, J.A.; Lambers, H.; Smith, S.E.; Westoby, M. Costs of acquiring phosphorus by vascular land plants: Patterns and implications for plant coexistence. New Phytol. 2018, 217, 1420–1427. [Google Scholar] [CrossRef]
- Stribley, D.P.; Tinker, P.B.; Rayner, J.H. Relation of internal phosphorus concentration and plant weight in plants infected by vesicular-arbuscular mycorrhizas. New Phytol. 1980, 86, 261–266. [Google Scholar] [CrossRef]
- Del-Saz, N.F.; Romero-Munar, A.; Alonso, D.; Aroca, R.; Baraza, E.; Flexas, J.; Ribas-Carbo, M. Respiratory ATP cost and benefit of arbuscular mycorrhizal symbiosis with Nicotiana tabacum at different growth stages and under salinity. J. Plant Physiol. 2017, 218, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Smith, F.A.; Jakobsen, I. Functional diversity in arbuscular mycorrhizal (AM) symbioses: The contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake. New Phytol. 2004, 162, 511–524. [Google Scholar] [CrossRef]
- Bucher, M.; Hause, B.; Krajinski, F.; Küster, H. Through the doors of perception to function in arbuscular mycorrhizal symbioses. New Phytol. 2014, 204, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Watts-Williams, S.J.; Jakobsen, I.; Cavagnaro, T.R.; Grønlund, M. Local and distal effects of arbuscular mycorrhizal colonization on direct pathway Pi uptake and root growth in Medicago truncatula. J. Exp. Bot. 2015, 66, 4061–4073. [Google Scholar] [CrossRef]
- Bucher, M. Functional Biol.ogy of plant phosphate uptake at root and mycorrhiza interfaces. New Phytol. 2007, 173, 11–26. [Google Scholar] [CrossRef]
- Karandashov, V.; Bucher, M. Symbiotic phosphate transport in arbuscular mycorrhizas. Trends Plant Sci. 2005, 10, 22–29. [Google Scholar] [CrossRef]
- Sawers, R.J.; Svane, S.F.; Quan, C.; Grønlund, M.; Wozniak, B.; Gebreselassie, M.N.; Gonzalez-Muñoz, E.; Chavez, R.A.; Baxter, I.; Goudet, J.; et al. Phosphorus acquisition efficiency in arbuscular mycorrhizal maize is correlated with the abundance of root-external hyphae and the accumulation of transcripts encoding PHT1 phosphate transporters. New Phytol. 2017, 214, 632–643. [Google Scholar] [CrossRef]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smith, F.A. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef]
- Facelli, E.; Duan, T.; Smith, S.E.; Christophersen, H.M.; Facelli, J.M.; Smith, F.A. Opening the black box: Outcomes of interactions between arbuscular mycorrhizal (AM) and non-host genotypes of Medicago depend on fungal identity, interplay between P uptake pathways and external P supply. Plant Cell Environ. 2014, 37, 1382–1392. [Google Scholar] [CrossRef]
- Campos, C.; Cardoso, H.; Nogales, A.; Svensson, J.; Lopez-Ráez, J.A.; Pozo, M.J.; Nobre, T.; Schneider, C.; Arnholdt-Schmitt, B. Intra and inter-spore variability in Rhizophagus irregularis AOX gene. PLoS ONE 2015, 10, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Fellbaum, C.R.; Mensah, J.A.; Cloos, A.J.; Strahan, G.E.; Pfeffer, P.E.; Kiers, E.T.; Bücking, H. Fungal nutrient allocation in common mycorrhizal networks is regulated by the carbon source strength of individual host plants. New Phytol. 2014, 203, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Manoharan, L.; Rosenstock, N.P.; Olsson, P.A.; Hedlund, K. Long-term agricultural fertilization alters arbuscular mycorrhizal fungal community composition and barley (Hordeum vulgare) mycorrhizal carbon and phosphorus exchange. New Phytol. 2017, 213, 874–885. [Google Scholar] [CrossRef]
- Miller, A.J.; Cramer, M.D. Root nitrogen acquisition and assimilation. Plant Soil. 2005, 274, 1–36. [Google Scholar] [CrossRef]
- Lea, P.J.; Azevedo, R.A. Nitrogen use efficiency. 2. Amino acid metabolism. Ann. Appl. Biol. 2007, 151, 269–275. [Google Scholar] [CrossRef]
- Barber, S. Soil Nutrient Bioavailability: A Mechanistic Approach, 2nd ed.; Wiley: New York, NY, USA, 1995; p. 384. [Google Scholar]
- Rennenberg, H.; Wildhagen, H.; Ehlting, B. Nitrogen nutrition of poplar trees. Plant Biol. 2010, 12, 275–291. [Google Scholar] [CrossRef]
- Gan, H.; Jiao, Y.; Jia, J.; Wang, X.; Li, H.; Shi, W.; Peng, C.; Polle, A.; Zhi-Bin, L. Phosphorus and nitrogen physiology of two contrasting poplar genotypes when exposed to phosphorus and/or nitrogen starvation. Tree Physiol. 2016, 36, 22–38. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, T. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef]
- Terashima, I.; Evans, J.R. Effects of light and nitrogen nutrition on the organization of the photosynthetic apparatus in spinach. Plant Cell Physiol. 1988, 29, 143–155. [Google Scholar]
- Makino, A.; Osmond, B. Effects of nitrogen nutrition on nitrogen partitioning between chloroplasts and mitochondria in pea and wheat. Plant Physiol. 1991, 96, 355–362. [Google Scholar] [CrossRef]
- Byrd, G.T.; Sage, R.F.; Brown, R.H.A. comparison of dark respiration between C3 and C4 plants. Plant Physiol. 1992, 100, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Lusk, C.; Reich, P. Relationships of leaf dark respiration with light environment and tissue nitrogen content in juveniles of 11 cold-temperate tree species. Oecologia 2000, 123, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Richard-Molard, C.; Krapp, A.; Brun, F.; Ney, B.; Daniel-Vedele, F.; Chaillou, S. Plant response to nitrate starvation is determined by N storage capacity matched by nitrate uptake capacity in two Arabidopsis genotypes. J. Exp. Bot. 2008, 59, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Scheible, W.R.; Gonzalez-Fontes, A.; Lauerer, M.; Müller-Röber, B.; Caboche, M.; Stitt, M. Nitrate acts as a signal to induce organic acid metabolism and repress starch metabolism in tobacco. Plant Cell. 1997, 9, 783–798. [Google Scholar] [CrossRef] [PubMed]
- Lancien, M.; Ferrario-Mery, S.; Roux, Y.; Bismuth, E.; Masclaux, C.; Hirel, B.; Gadal, P.; Hodges, M. Simultaneous expression of NAD-dependent isocitrate dehydrogenase and other Krebs cycle genes after nitrate re-supply to short-term starved Nicotiana tabacum. Plant Physiol. 1999, 120, 717–726. [Google Scholar] [CrossRef]
- Noguchi, K.; Terashima, I. Responses of spinach leaf mitochondria to low N availablity. Plant Cell Environ. 2006, 29, 710–719. [Google Scholar] [CrossRef]
- Millenaar, F.F.; Gonzàlez-Meler, M.A.; Fiorani, F.; Welschen, R.; Ribas-Carbo, M.; Siedow, J.N.; Wagner, A.M.; Lambers, H. Regulation of alternative oxidase activity in six wild monocotyledonous species. An in vivo study at the whole root level. Plant Physiol. 2001, 126, 376–387. [Google Scholar] [CrossRef]
- MacFarlane, C.; Hansen, L.D.; Florez-Sarasa, I.; Ribas-Carbo, M. Plant mitochondria electron partitioning is independent of short-term temperature changes. Plant Cell Environ. 2009, 32, 585–591. [Google Scholar] [CrossRef]
- Rasmusson, A.G.; Fernie, A.R.; Van Dongen, J.T. Alternative oxidase: A defence against metabolic fluctuations? Physiol. Plant 2009, 137, 371–382. [Google Scholar] [CrossRef]
- Stitt, M. Nitrate regulation of metabolism and growth. Curr. Opin. Plant Biol. 1999, 2, 178–186. [Google Scholar] [CrossRef]
- Hachiya, T.; Terashima, I.; Noguchi, K. Increase in respiratory cost at high growth temperature is attributed to high protein turnover cost in Petunia x hybrida petals. Plant Cell Environ. 2007, 30, 1269–1283. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Foyer, C.H. A re-evaluation of the ATP: NADPH budget during C3 photosynthesis: A contribution from nitrate assimilation and its associated respiratory activity? J. Exp. Bot. 1998, 49, 1895–1908. [Google Scholar] [CrossRef]
- Scheurwater, I.; Clarkson, D.T.; Purves, J.V.; Van Rijt, G.; Saker, L.R.; Welschen, R.; Lambers, H. Relatively large nitrate efflux can account for the high specific respiratory costs for nitrate transport in slow-growing grass species. Plant Soil. 1999, 215, 123–134. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef]
- Escobar, M.A.; Geisler, D.A.; Rasmusson, A.G. Reorganization of the alternative pathways of the Arabidopsis respiratory chain by nitrogen supply: Opposing effects of ammonium and nitrate. Plant J. 2006, 45, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Hachiya, T.; Watanabe, C.K.; Boom, C.; Tholen, D.; Takahara, K.; Kawai-Yamada, M.; Uchimiya, H.; Uesono, Y.; Terashima, I.; Noguchi, K. Ammonium-dependent respiratory increase is dependent on the cytochrome pathway in Arabidopsis thaliana shoots. Plant Cell Environ. 2010, 33, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Hachiya, T.; Noguchi, K. Integrative response of plant mitochondrial electron transport chain to nitrogen source. Plant Cell Rep. 2011, 30, 195–204. [Google Scholar] [CrossRef]
- Podgórska, A.; Mazur, R.; Ostaszewska-Bugajska, M.; Kryzheuskaya, K.; Dziewit, K.; Borysiuk, K.; Wdowiak, A.; Burian, M.; Rasmusson, A.G.; Szal, B. Efficient photosynthetic functioning of Arabidopsis thaliana through electron dissipation in chloroplasts and electron export to mitochondria under ammonium nutrition. Front. Plant Sci. 2020, 103, 1–18. [Google Scholar] [CrossRef]
- Esteban, R.; Ariz, I.; Cruz, C.; Moran, J.F. Mechanisms of ammonium toxicity and the quest for tolerance. Plant Sci. 2016, 248, 92–101. [Google Scholar] [CrossRef]
- Frechilla, S.; Lasa, B.; Aleu, M.; Juanarena, N.; Lamsfus, C.; Aparicio-Tejo, P.M. Short-term ammonium supply stimulates glutamate dehydrogenase activity and alternative pathway respiration in roots of pea plants. J. Plant Physiol. 2002, 159, 811–818. [Google Scholar] [CrossRef]
- Lasa, B.; Frechilla, S.; Aparicio-Tejo, P.M.; Lamsfus, C. Alternative pathway respiration is associated with ammonium ion sensitivity in spinach and pea plants. Plant Growth Regul. 2002, 37, 49–55. [Google Scholar] [CrossRef]
- Patterson, K.; Cakmak, T.; Cooper, A.; Lager, I.; Rasmusson, A.G.; Escobar, M.A. Distinct signalling athways and transcriptome response signatures differentiate ammonium- and nitrate-supplied plants. Plant Cell Environ. 2010, 33, 1486–1501. [Google Scholar] [PubMed]
- Gupta, K.J.; Kumari, A.; Florez-Sarasa, I.; Fernie, A.R.; Igamberdiev, A.U. Interaction of nitric oxide with the components of the plant mitochondrial electron transport chain. J. Exp. Bot. 2018, 69, 3413–3424. [Google Scholar] [CrossRef] [PubMed]
- Kearns, A.; Whelan, J.; Young, S.; Elthon, T.E.; Day, D.A. Tissue specific expression of the alternative oxidase in soybean and siratro. Plant Physiol. 1992, 99, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, P.M.; Whelan, J.; Millar, A.H.; Zhang, Q.S.; Smith, M.K.; Wiskich, J.T.; Day, D.A. Differential expression of the multigene family encoding the soybean mitochondrial alternative oxidase. Plant Physiol. 1997, 114, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Sweetman, C.; Soole, K.L.; Jenkins, C.L.D.; Day, D.A. Genomic structure and expression of alternative oxidase genes in legumes. Plant Cell Environ. 2019, 42, 71–84. [Google Scholar] [CrossRef]
- Gonzàlez-Meler, M.A.; Siedow, J.N. Inhibition of respiratory enzymes by elevated CO2: Does it matter at the intact tissue and whole plant levels? Tree Physiol. 1999, 19, 253–259. [Google Scholar] [CrossRef]
- Martí, M.C.; Florez-Sarasa, I.; Camejo, D.; Ribas-Carbó, M.; Lázaro, J.J.; Sevilla, F.; Jiménez, A. Response of mitochondrial thioredoxin PsTrxo1, antioxidant enzymes, and respiration to salinity in pea (Pisum sativum L.) leaves. J. Exp. Bot. 2011, 62, 3863–3874. [Google Scholar] [CrossRef]
- Coba de la Peña, T.; Pueyo, J.J. Legumes in the reclamation of marginal soils, from cultivar and inoculant selection to transgenic approaches. Agron. Sustain. Dev. 2012, 32, 65–91. [Google Scholar] [CrossRef]
- Okazaki, S.; Kaneko, T.; Sato, S.; Saeki, K. Hijacking of leguminous nodulation signaling by the rhizobial type III secretion system. Proc. Natl. Acad. Sci. USA 2013, 110, 17131–17136. [Google Scholar] [CrossRef]
- Gourion, B.; Berrabah, F.; Ratet, P.; Stacey, G. Rhizobium-legume symbioses: The crucial role of plant immunity. Trends Plant Sci. 2015, 20, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Masson-Boivin, C.; Sachs, J.L. Symbiotic nitrogen fixation by rhizobia—The roots of a success story. Curr. Opin. Plant Biol. 2018, 44, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Coba de la Peña, T.; Fedorova, E.; Pueyo, J.J.; Lucas, M.M. The Symbiosome: Legume and Rhizobia Co-evolution toward a Nitrogen-Fixing Organelle? Front. Plant Sci. 2018, 8, 2229. [Google Scholar] [CrossRef]
- Day, D.A. Carbon metabolism and compartmentation in nitrogen fixing legume nodules. Plant Physiol. Biochem. 1991, 29, 185–201. [Google Scholar]
- Liu, J.; Contador, C.A.; Fan, K.; Hon-Ming, L. Interaction and regulation of carbon, nitrogen, and phosphorus metabolisms in root nodules of legumes. Front Plant Sci. 2018, 9, 1860. [Google Scholar] [CrossRef] [PubMed]
- Lodwig, E.M.; Hosie, A.H.; Bourdes, A.; Findlay, K.; Allaway, D.; Karunakaran, R.; Dowie, J.A.; Poole, P.S. Amino-acid cycling drives nitrogen fixation in the legume–Rhizobium symbiosis. Nature 2003, 422, 722–726. [Google Scholar] [CrossRef]
- Rao, T.P.; Ito, O. Differences in root system morphology and root respiration in relation to nitrogen uptake among six crop species. Jpn. Agric. Res. Q. 1998, 32, 97–104. [Google Scholar]
- Schulze, J.; Beschow, H.; Adgo, E.; Merbach, W. Efficiency of N2 fixation in Vicia faba L. in combination with different Rhizobium leguminosarum strains. J. Plant Nutr. Soil Sci. 2000, 163, 367–373. [Google Scholar]
- Mortimer, P.E.; Pérez-Fernández, M.A.; Valentine, A.J. The role of arbuscular mycorrhizal colonization in the carbon and nutrient economy of the tripartite symbiosis with nodulated Phaseolus vulgaris. S. Biol. Biochem. 2008, 40, 1019–1027. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Hsu, P.K.; Tsay, Y.F. Uptake, allocation and signaling of nitrate. Trends Plant Sci. 2012, 17, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Udvardi, M.; Poole, P.S. Transport and metabolism in legume-rhizobia symbioses. Annu. Rev. Plant Biol. 2013, 64, 781–805. [Google Scholar] [CrossRef] [PubMed]
- Downie, J.A. Legume nodulation. Curr. Biol. 2014, 24, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Winkler, R.D.; Blevins, D.G.; Polacco, J.C.; Randall, D.D. Ureide catabolism in soybeans. II. Pathway of catabolism in intact leaf tissue. Plant Physiol. 1987, 83, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Sprent, J.I. Legume Nodulation: A Global Perspective; Wiley-Blackwell: Oxford, UK, 2009; pp. 1–183. [Google Scholar]
- Marchal, K.; Vanderleyden, J. The “oxygen paradox” of dinitrogen-fixing bacteria. Biol. Fert. Soils 2000, 30, 363–373. [Google Scholar] [CrossRef]
- Dakora, F.D.; Atkins, C.A. Adaptation of nodulated soybean (Glycine max L. Merr.) to growth in rhizospheres containing non-ambient pO2. Plant Physiol. 1991, 96, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.M.; Walsh, K.B. Anatomy of the legume nodule cortex with respect to nodule permeability. Aust. J. Plant Physiol. 1994, 21, 49–68. [Google Scholar]
- Gordon, A.J.; Minchin, F.R.; Skot, L.; James, C.L. Stress-induced declines in soybean N2 fixation are related to nodule sucrose synthase activity. Plant Physiol. 1997, 114, 937–946. [Google Scholar] [CrossRef]
- Becana, M.; Navascués, J.; Pérez-Rontomé, C.; Walker, F.A.; Desbois, A.; Abian, J. Leghemoglobins with nitrated hemes in legume root nodules. In Biological Nitrogen Fixation; Bruijn, F.J., Ed.; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2015; pp. 705–713. [Google Scholar]
- Matamoros, M.A.; Fernández-García, N.; Wienkoop, S.; Loscos, J.; Saiz, A.; Becana, M. Mitochondria are an early target of oxidative modifications in senescing legume nodules. New Phytol. 2013, 197, 873–885. [Google Scholar] [CrossRef]
- Millar, A.H.; Finnegan, P.M.; Whelan, J.; Drevon, J.J.; Day, D.A. Expression and kinetics of the mitochondrial alternative oxidase in nitrogen-fixing nodules of soybean roots. Plant Cell Environ. 1997, 20, 1273–1282. [Google Scholar] [CrossRef]
- Bryce, J.H.; Day, D.A. Tricarboxylic acid cycle activity in mitochondria from soybean nodules and cotyledons. J. Exp. Bot. 1990, 41, 961–967. [Google Scholar] [CrossRef]
- Day, D.A.; Price, G.D.; Gresshoff, P.M. Isolation and oxidative properties of mitochondria and bacteroids from soybean root nodules. Protoplasma 1986, 134, 121–129. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Show, P.L.; Chang, J.S.; Ling, T.C.; Juan, J.C. Biosequestration of atmospheric CO2 and flue gas-containing CO2 by microalgae. Bioresour. Technol. 2015, 184, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Bergersen, F.J.; Turner, G.L. Leghaemoglobin and the supply of O2 to nitrogen-fixing root nodule bacteroids: Presence of two oxidase systems and ATP production at low free O2 concentration. Microbiology 1975, 91, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Bergersen, F.J.; Turner, G.L. Properties of terminal oxidase systems of bacteroids from root nodules of soybean and cowpea and of N2-fixing bacteria grown in continuous culture. Microbiology 1980, 118, 235–252. [Google Scholar] [CrossRef]
- Millar, A.H.; Day, D.A.; Bergersen, F.J. Microaerobic respiration and oxidative phosphorylation by soybean nodule mitochondria: Implications for nitrogen fixation. Plant Cell Environ. 1995, 18, 715–726. [Google Scholar] [CrossRef]
- de Visser, R.; Lambers, H. Growth and the efficiency of root respiration of Pisum sativum as dependent on the source of nitrogen. Physiol. Plant 1983, 58, 533–543. [Google Scholar] [CrossRef]
- Preisig, O.; Zufferey, R.; Thöny-Meyer, L.; Appleby, C.A.; Hennecke, H. A high-affinity cbb3-type cytochrome oxidase terminates the symbiosis-specific respiratory chain of Bradyrhizobium japonicum. J. Bacteriol. 1996, 178, 1532–1538. [Google Scholar] [CrossRef]
- López, M.; Herrera-Cervera, J.A.; Iribarne, C.; Tejera, N.A.; Lluch, C. Growth and nitrogen fixation in Lotus japonicus and Medicago truncatula under NaCl stress: Nodule carbon metabolism. J. Plant Physiol. 2008, 165, 641–650. [Google Scholar] [CrossRef]
- Wang, X.; Shen, J.; Liao, H. Acquisition or utilization, which is more critical for enhancing phosphorus efficiency in modern crops? Plant Sci. 2010, 179, 302–306. [Google Scholar] [CrossRef]
- Qin, L.; Zhao, J.; Tian, J.; Chen, L.; Sun, Z.; Guo, Y.; Lu, X.; Gu, M.; Xu, G.; Liao, H. The High affinity phosphate transporter GmPT5 regulates phosphate transport to nodules and nodulation in soybean. Plant Physiol. 2012, 159, 1634–1643. [Google Scholar] [CrossRef]
- Hernandez, G.; Valdes-Lopez, O.; Ramirez, M.; Goffard, N.; Weiller, G.; Aparicio-Fabre, R.; Fuentes, S.I.; Erban, A.; Kopka, J.; Udvardi, M.; et al. Global changes in the transcript and metabolic profiles during symbiotic nitrogen fixation in phosphorus-stressed common bean plants. Plant Physiol. 2009, 151, 1221–1238. [Google Scholar] [CrossRef] [PubMed]
This entry is adapted from the peer-reviewed paper 10.3390/ijms21124201
