Heart Failure (HF) is a clinical syndrome characterized by symptoms such as dyspnoea or fatigue on exertion or at rest, and clinical signs (i.e. lower extremity oedema, elevated jugular venous pressure, pulmonary crackles, etc.) caused by a structural and/or functional cardiac abnormality, ultimately leading to reduced cardiac output. Despite advances in the knowledge on HF, decisions on diagnosis and treatment of HF remain challenging. In everyday clinical practice, biomarkers such as plasma natriuretic peptides such as brain natriuretic peptide (BNP) and N-terminal fragment BNP (NT-proBNP), play an important role in HF diagnosis, therapy monitoring and risk stratification, while many other serum biomarkers have also been studied without definite evidence on how to use them in clinical practice. Biomarkers that could be measured in other biological fluids other than blood, easily and non-invasively, and outside the hospital setting, have attracted research interest. During the last years, saliva has emerged as a body fluid for this purpose.
This review assessed the potential role of salivary biomarkers in diagnosis and progression monitoring of patients with HF. 18 salivary biomarkers were analyzed and the levels of all biomarkers studied were found to be higher in HF patients compared to controls, except for amylase, sodium, and chloride that had smaller saliva concentrations in HF patients. Natriuretic peptides are the most commonly used plasma biomarkers in the management of HF. Their saliva levels show promising results, although the correlation of saliva to plasma values is weakened in higher plasma values. In most of the publications, differences in biomarker levels between HF patients and controls were found to be statistically significant. Due to small number of patients included, larger studies need to be conducted in order to facilitate the use of saliva biomarkers in clinical practice.
- heart failure
- biomarkers
- saliva
- diagnosis
1. Introduction
Heart Failure (HF) is a clinical syndrome characterized by symptoms such as dyspnea or fatigue on exertion or at rest, and clinical signs (i.e., lower extremity oedema, elevated jugular venous pressure, pulmonary crackles, etc.) caused by a structural and/or functional cardiac abnormality, ultimately leading to reduced cardiac output [1]. HF is one of the leading causes of morbidity and mortality worldwide, with a prevalence ranging from 6% to 13% [2]. An analysis in 2012 estimated that the global cost of HF was $108 billion per year, of which $65 billon was attributed to direct and $43 billion to indirect costs [3].
The etiology of HF is quite variable, with most common causes being coronary artery disease, hypertension, arrhythmias, valvular and structural abnormalities, toxic damage from recreational substance use and chemotherapy, immune, inflammatory and metabolic causes, infiltrative diseases, genetic abnormalities, etc. [1]. Many patients may have more than one causative factor for HF. The classification of HF syndromes currently used in clinical practice for the management of patients is based on the left ventricular ejection fraction (LVEF), rather than the etiology. Accordingly, patients are characterized as HF with preserved, mid-range and reduced LVEF (HFpEF, HFmrEF and HFrEF when LVEF is ≥50%, 40–49%, and <40%, respectively) [1].
Prognosis varies depending on the cause but is generally poor [4]. In patients with HFrEF, therapy is based on well-established algorithms supported by large, multi-center, randomized clinical trials that have shown increased survival and improved quality of life [1]. On the contrary, for patients with HFpEF (usually older patients with many co-morbidities) there is no clear evidence that specific medications or other therapeutic approaches may increase survival. The European Society of Cardiology has published guidelines and several consensus and position papers on HF management [1][5].
However, due to the variations in causative factors, presenting symptoms and therapeutic options, decisions on diagnosis and treatment of HF remain challenging. In everyday clinical practice, biomarkers play an important and expanding role in HF diagnosis, therapy monitoring and risk stratification. Plasma natriuretic peptides such as brain natriuretic peptide (BNP) and N-terminal fragment BNP (NT-proBNP) have already been established in the diagnostic algorithm of HF, while other serum biomarkers such as soluble interleukin 1 receptor-like 1 (ST2), galectin-3 (Gal-3), copeptin, adrenomedullin, high sensitivity troponin (hsTn), growth differentiation factor 15 (GDF-15), adiponectin, C-reactive protein (CRP) and neprilysin have been tested without definite evidence yet to recommend them for clinical practice [1][6][7][8][9][10][11][12][13][14][15][16][17].
As in many other chronic diseases, an important goal in HF research is to identify biomarkers that could improve accuracy in diagnosis and monitoring. Another important issue is to detect biomarkers that could be measured in other biological fluids (instead of blood), because this procedure would be less invasive and easier to accomplish in all clinical settings. Towards that direction, point-of-care devices that can be used for HF monitoring outside the hospital setting, in primary health care or at home have been developed [8][18][19].
During the last years, saliva has emerged as a body fluid containing several proteins that could be used as potential biomarkers, with an important benefit that saliva can be easily collected with non-invasive procedures [20]. In addition, salivary diagnostics is currently catching onto the emerging field of Lab-on-Chip (LoC) and Point-of-Care (PoC) devices [20]. Saliva values of myoglobin, cardiac troponin I, creatine phosphokinase MB, myeloperoxidase, the natriuretic peptides (BNP and NT-proBNP), CRP, etc., have been used for the diagnosis of cardiovascular diseases in general and in most cases they have been found to correlate well with plasma concentrations [21][22][23][24][25]. On the other hand, very few have been tested specifically for HF diagnosis, monitoring and prognosis [26][27][28][29][30][31][32][33][34][35][36][37][38][39][40].
2. Review Question
Which salivary biomarkers have been used for the diagnosis and therapy monitoring of patients with HF, and which of them provided a reliable means of disease detection?
3. Eligibility Criteria
Inclusion criteria for the search were case-control, cross-sectional studies that compared the values of one or more salivary biomarkers among healthy subjects and patients with HF. No randomized control trials were found in our search. Articles published before 1950, articles written in a language other than English, reviews, letters to the editors, and articles correlating salivary biomarkers with other diseases such as liver failure, cancer or periodontal diseases, were excluded.
4. Search Strategy
An advanced literature search was conducted using the following Mesh terms: (“Saliva” (Mesh) and “Biomarkers” (Mesh) or salivary biomarkers) and (“Heart Failure” (Mesh)). Study titles, abstracts, and full texts were extracted from PubMed until December 2020. After duplicates (22 in total) were removed, the search yielded a total of 107 titles. Titles were evaluated for relevance. From the relevant ones, the abstracts were reviewed and re-evaluated for relevance to our study question. Subsequently, the full text of accepted abstracts was reviewed. Of the full texts reviewed, those that did not contribute to our study question were excluded. Finally, the number of studies included in this review was 15 (Figure 1).
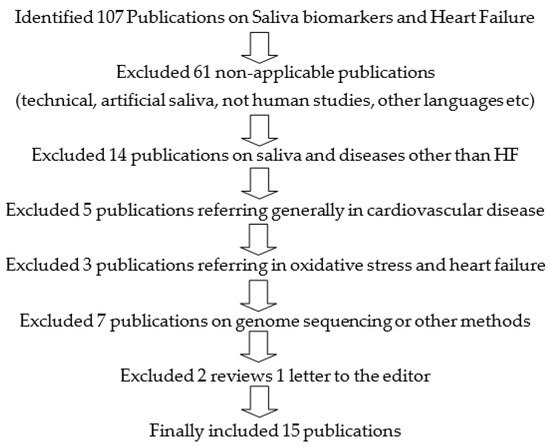
Figure 1. Search strategy on “Saliva Biomarkers and Heart Failure.
5. Results
Fifteen studies fulfilled the inclusion criteria [26][27][28][29][30][31][32][33][34][35][36][37][38][39][40]. Among these studies, eighteen separate salivary biomarkers were analyzed; salivary amylase, mainly its major form salivary alpha amylase (sAA), uric acid (UA), 8-isoprostaglandin F2α (8-isoPGF2α), lactate, galectin-3 (Gal-3), BNP, interleukin 6 (IL-6) and interleukin 10 (IL-10), CRP, protein S100-A7 (S10A7), cortisol, NT-proBNP, 8-epiprostaglandin F2α (8-epiPGF2α), endothelin, sodium, chloride, and potassium.
Only three studies measured salivary natriuretic peptides [29][30][35]. The first compared salivary NT-proBNP between controls (n = 40) and HF patients (n = 45). The salivary NT-proBNP concentrations from the healthy participants were below the limit of detection (LOD 16 pg/mL), while in HF patients salivary NT-proBNP ranged from 18.3 to 748.7 pg/mL with a median value of 76.8 pg/mL. The salivary NT-proBNP immunoassay showed sensitivity of 82.2% and specificity of 100%, positive predictive value of 100% and negative predictive value of 83.3%, with overall diagnostic accuracy at 90.6%. On the other hand, there was no correlation between salivary and plasma NT-proBNP concentrations in HF patients (R2 = 0.006, p = 0.66) [35]. The second study measured serum and salivary BNP from 75 hospitalized patients with HF, and also found no significant correlations between serum and salivary BNP (r = −0.064, p = 0.628), with a large positive bias of 480 pg/mL, indicating that serum concentrations of BNP were much higher than the salivary concentrations, and as serum BNP levels increased, the difference became larger [29]. In the third study, mean salivary BNP levels were higher in both hospitalised HF (p < 0.001) and outpatient HF patients (p = 0.02) compared to the control subjects (6.50 ng/L vs. 5.87 ng/L vs. 5.64 ng/L, respectively). A moderate correlation between salivary BNP and plasma NT-proBNP concentrations (p < 0.001, r = 0.459) was found [30]. Despite the rather small number of participants, all studies found higher natriuretic peptide levels in the saliva in HF patients compared to controls, but larger studies are needed to validate the clinical importance of the saliva values of natriuretic peptides as well as their correlation to plasma levels.
In chronic HF, inflammatory and neurohormonal activation takes place as a response to the failing heart. Activation of the renin-angiotensin system is responsible for the over-expression of the stress hormones, i.e., angiotensin-converting enzyme and angiotensin II. Cortisol has been implicated in the progress of chronic HF, since it binds to the mineralocorticoid receptor with an affinity equal to that of aldosterone and its pathophysiological role may be influenced by oxidative stress [41][42][43]. Analysis of saliva instead of serum is advantageous because salivary cortisol represents the unbound (i.e., free) hormone which is considered biologically active, while the vast majority of serum cortisol is bound to cortisol-binding globulin and albumin [44]. The number of patients included in these three studies is small (229 [32], 81 [34], and 27 [38], respectively). The LOD was 0.05 ng/mL, 0.07 ng/mL and 0.15 ng/mL respectively with values of HF patients ranging from 0.40–0.92 ng/mL, 0.19–0.55 ng/mL, and 6.88–8.33 ng/mL, respectively, in each study. Only in the third study were cortisol levels measured in controls, with values ranging from 5.43 to 6.88 ng/mL. Salivary cortisol was commonly increased in HF patients, while higher levels were associated with a reduced event-free interval and high evening levels were associated with increased mortality risk [32][34][38].
Galectin-3 (Gal-3) is a β-galactoside–binding lectin that plays a role in inflammatory and immune-mediated disorders. It is mainly expressed in activated macrophages and pathologically damaged cardiomyocytes and is considered as an active contributor to cardiac remodeling (including myocardial fibrogenesis) and development of HF. Gal-3 induces fibroblast proliferation and heterogeneous deposition of collagen types, eventually leading to loss of cardiac function [45][46]. Two studies have measured salivary Gal-3. In the first study, 105 HF patients (hospitalised or at routine outpatient visits) had a significantly higher cumulative risk of cardiovascular death or hospitalization when their salivary Gal-3 levels were higher than 172.58 ng/mL [28]. In the second study, samples from 63 HF patients were compared to healthy controls and were significantly elevated (both in saliva and serum), with a moderate correlation (r = 0.4, p < 0.01) between serum and salivary Gal-3 levels [33].
Almost all of the serum amylase activity in healthy adults is found at the pancreas and salivary glands [47]. Plasma amylase levels were found to be elevated in severe HF, possibly due to the mesenteric venous congestion and impaired peripheral tissue perfusion [48]. Two studies, with a small number of patients each, measured salivary amylase in HF patients. The first (which included 33 NYHA II and 17 NYHA III patients) found decreased salivary levels of amylase in the HF population; this was attributed to the impaired secretory function of salivary glands in HF patients that led to lower content and activity of salivary amylase compared to healthy controls [26]. The second study (which included 24 NYHA I-III patients and 24 controls) found no statistically significant difference between the control and HF groups in sAA levels, although there was a strong tendency for the morning values to be higher in HF patients, especially if measured within 30 min after awakening. It should be noted that there was a strong inter- and intra-subject variation and a small number of participants, while all HF patients were on b-blocker therapy that reduces sAA levels [36].
Apart from the activation of compensatory neurohormonal mechanisms, HF is also associated with hyper-lactatemia, oxidative stress, and hyperuricemia [27]. In one study, salivary lactate and 8-isoPGF2α from 44 patients with acute HF strongly correlated with serum NT-proBNP, while salivary uric acid did not. The LOD was 10 pg/mL and 6 µg/mL for 8-iso-PGF2α and lactate, respectively. Lactate levels positively correlated with NYHA class, while 8-isoprostaglandin F2α levels did not correlate to NYHA class [27]. In another study, salivary 8-epiPGF2α levels were significantly higher in patients with ischemic and dilated cardiomyopathy compared to controls and patients with coronary heart disease (p = 0.001). 8-epiPGF2α levels negatively correlated with LVEF and positively correlated with NYHA class [37]. Salivary UA was also examined by Klimiuk et al. [27] and was significantly higher in HF compared to healthy subjects in stimulated saliva. Moreover, in non-stimulated saliva the UA levels were higher in worse NYHA class patients.
C-reactive protein (CRP) is a phylogenetically highly conserved plasma protein that participates in the systemic response to inflammation. CRP and other inflammatory cytokines were examined as markers of HF severity and prognosis in the study by Dekker et al. [29]. Although serum and salivary levels of CRP were found to have a moderate correlation (r = 0.594, p < 0.001), salivary CRP levels were not associated with NYHA class [29]. In the same study in 75 hospitalized HF patients, a weak correlation for serum–salivary IL-6 (r = 0.288, p = 0.037), and no correlations for serum–salivary IL-10 (r = 0.068, p = 0.629) or serum–salivary BNP (r = −0.064, p = 0.628) were reported [29]. Interestingly, no biomarkers in this study were associated with NYHA class and only visible oral inflammation was found to be a significant predictor of HF severity. Two major classes of cytokines have also been implicated in HF, vasoconstrictor cytokines such as endothelin and vasodepressor proinflammatory cytokines such as Tumor Necrosis Factor-α (TNF-α), IL-6, and IL-1 [48][49]. These inflammatory mediators are expressed by all nucleated cell types residing in the myocardium, including the cardiac myocyte. In patients with HF, circulating as well as intra-cardiac levels of these cytokines have been reported to be elevated [50]. Salivary endothelin concentrations were raised two to six-fold in chronic HF patients vs. controls (p = 0.005), with a positive correlation to respective plasma concentrations (p = 0.032) and were able to detect chronic HF with 63% sensitivity 63% and 92% specificity. Endothelin salivary levels have also been shown to reflect symptom severity by NYHA class [39]. TNF-α has been studied extensively in periodontal inflammations and oral cancers and has been identified in detectable concentrations in human saliva with values correlating well to plasma values [51][52], indicating that salivary TNF-α could potentially serve as a HF biomarker, providing that supporting data emerged [53][54][55][56][57].
Mass spectrometry was used to analyze the saliva proteome in HF patients (n = 75) and healthy controls (n = 36), as reported by Zhang et al. Of the 728 proteins detected, only the protein S100-A7 (S10A7) was significantly different between saliva from NYHA III/IV HF patients compared to healthy controls. However, the underlying mechanisms relating S10A7 to the pathogenesis of HF are not clear and further investigations are needed [31].
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics11050824
References
- Ponikowsky, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; Gonzales-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2016, 37, 2129–2200.
- Van Riet, E.E.S.; Hoes, A.W.; Wagenaar, K.P.; Limburg, A.; Landman, M.A.; Rutten, F.H. Epidemiology of heart failure: The prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur. J. Heart Fail. 2016, 18, 242–252.
- Urbich, M.; Globe, G.; Pantiri, K.; Heisen, M.; Bennison, C.; Wirtz, H.; Di Tanna, G.L. A Systematic Review of Medical Costs Associated with Heart Failure in the USA (2014–2020). Pharm. Econ. 2020, 38, 1219–1236.
- Laukkanen, K.S.; Salonen, R.; Rauramaa, R.; Salonen, J.T. The predictive value of cardiorespiratory fitness for cardiovascular events in men with various risk profiles: A prospective population-based cohort study. Eur. Heart J. 2004, 25, 1428–1437.
- Pieske, B.; Tschöpe, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2020, 22, 391–412.
- George, M.; Jena, A.; Srivatsan, V.; Muthukumar, R.; Dhandapani, V.E. GDF 15-A Novel Biomarker in the Offing for Heart Failure. Curr. Cardiol. Rev. 2016, 12, 37–46.
- Aimo, A.; Januzzi, J.L.; Vergaro, G.; Ripoli, A.; Latini, R.; Masson, S.; Magnoli, M.; Anand, I.S.; Cohn, J.N.; Tavazzi, L.; et al. Prognostic Value of High-Sensitivity Troponin T in Chronic Heart Failure: An Individual Patient Data Meta-Analysis. Circulation 2018, 137, 286–297.
- Taylor, K.S.; Verbakel, J.Y.; Feakins, B.G.; Price, C.P.; Perera, R.; Bankhead, C.; Pluddemann, A. Diagnostic Accuracy of Point-Of-Care Natriuretic Peptide Testing for Chronic Heart Failure in Ambulatory Care: Systematic Review and Metac. BMJ 2018, 361, k1450.
- McAloon, C.J.; Ali, D.; Hamborg, T.; Banerjee, P.; O’Hare, P.; Randeva, H.; Osman, F. Extracellular Cardiac Matrix Biomarkers in Patients with Reduced Ejection Fraction Heart Failure as Predictors of Response to Cardiac Resynchronisation Therapy: A Systematic Review. Open Heart 2017, 4, e000639.
- Bai., W.; Huang, J.; Zhu, M.; Liu, X.; Tao, X. Association between Elevated Adiponectin Level and Adverse Outcomes in Patients with Heart Failure: A Systematic Review and Meta-Analysis. Braz. J. Med. Biol. Res. 2019, 52, e8416.
- Srivatsan, V.; George, M.; Shanmugam, E. Utility of galectin-3 as a Prognostic Biomarker in Heart Failure: Where Do We Stand? Eur. J. Prev. Cardiol. 2015, 22, 1096–1110.
- Lakhani, I.; Wong, M.V.; Kai, F.H.J.; Gong, M.; Bin, W.K.; Xia, Y.; Lee, S.; Roever, L.; Liu, T.; Tse, G.; et al. Diagnostic and Prognostic Value of Serum C-reactive Protein in Heart Failure with Preserved Ejection Fraction: A Systematic Review and Meta-Analysis. Heart Fail. Rev. 2020, 6.
- Ramanathan, K.; Padmanabhan, G. Soluble Neprilysin: A Versatile Biomarker for Heart Failure, Cardiovascular Diseases and Diabetic complications: A Systematic Review. Indian Heart J. 2020, 72, 14–19.
- Zhang, X.; Schulz, B.L.; Punyadeera, C. The Current Status of Heart Failure Diagnostic Biomarkers Expert. Rev. Mol. Diagn. 2016, 16, 487–500.
- Gamiño-Arroyo, A.E.; Prado-Galbarro, F.J.; García-Pérez, S.; Sánchez-Piedra, C. Effectiveness of Natriuretic Peptide-Guided Treatment of Chronic Heart Failure. A Meta-Analysis. Arch. Cardiol. Mex 2018, 88, 171–177.
- Pearson, M.J.; King, N.; Smart, N.A. Effect of Exercise Therapy on Established and Emerging Circulating Biomarkers in Patients with Heart Failure: A Systematic Review and Meta-Analysis. Open Heart 2018, 5, e000819.
- Chow, S.L.; Maisel, A.S.; Anand, I.; Bozkurt, B.; de Boer, R.A.; Felker, G.M.; Fonarow, G.C.; Greenberg, B.; Januzzi, J.L., Jr.; Kiernan, M.S.; et al. Role of Biomarkers for the Prevention, Assessment, and Management of Heart Failure: A Scientific Statement from the American Heart Association. Circulation 2017, 135, e1054–e1091.
- Alawieh, H.; Chemaly, T.E.; Alam, S.; Khraiche, M. Towards Point-of-Care Heart Failure Diagnostic Platforms: BNP and NT-proBNP Biosensors. Sensors 2019, 19, 5003.
- Tripoliti, E.E.; Ioannidou, P.; Toumpaniaris, P.; Rammos, A.; Pacitto, A.; Lourme, J.C.; Goletsis, Y.; Naka, K.K.; Errachid, A.; Fotiadis, D.I.I. Point-of-care testing devices for heart failure analyzing blood and saliva samples. IEEE Rev. Βiomed. Εng. 2019, 13, 17–31.
- Bellagambi, F.G.; Lomonaco, T.; Salvo, P.; Vivaldi, F.; Hangouët, M.; Ghimenti, S.; Biagini, D.; Di Francesco, F.; Fuoco, R.; Errachid, A. Saliva sampling: Methods and devices. An overview. TrAC 2020, 124, 115781.
- Rehman, S.A.; Khurshid, Z.; Niazi, F.H.; Naseem, M.; Al Waddani, H.; Sahibzada, H.A.; Khan, R.S. Role of Salivary Biomarkers in Detection of Cardiovascular Diseases (CVD). Proteomes 2017, 5, 21.
- Floriano, P.N.; Christodoulides, N.; Miller, C.S.; Ebersole, J.L.; Spertus, J.; Rose, B.G.; Kinane, D.F.; Novak, J.; Steinhubl, S.; Acosta, S.; et al. Use of saliva-based nano-biochip tests for acute myocardial infarction at the point of care: A feasibility study. Clin. Chem. 2009, 55, 1530–1538.
- Foley, J.D., III; Sneed, J.D.; Steinhubl, S.R.; Kolasa, J.; Ebersole, J.L.; Lin, Y.; Kryscio, R.J.; McDevitt, J.T.; Campbell, C.L.; Miller, C.S. Oral fluids that detect cardiovascular disease. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 207–214.
- Gohel, V.; Jones, J.A.; Wehler, C.J. Salivary biomarkers and cardiovascular disease: A systematic review. Clin. Chem. Lab. Med. 2018, 56, 1432–1442.
- Miller, C.S.; Foley, J.D., 3rd; Floriano, P.N.; Christodoulides, N.; Ebersole, J.L.; Campbell, C.L.; Bailey, A.L.; Rose, B.G.; Kinane, D.F.; Novak, M.J.; et al. Utility of salivary biomarkers for demonstrating acute myocardial infarction. J. Dent. Res. 2014, 93, 72S–79S.
- Klimiuk, A.; Zalewska, A.; Sawicki, R.; Knapp, M.; Maciejczyk, M. Salivary Oxidative Stress Increases with the Progression of Chronic Heart Failure. J. Clin. Med. 2020, 9, 769.
- Ghimenti, S.; Lomonaco, T.; Bellagambi, F.G.; Biagini, D.; Salvo, P.; Trivella, M.G.; Scali, M.S.; Barletta, V.; Marzilli, M.; Di Francesco, F.; et al. Salivary Lactate and 8-isoprostaglandin F 2α as Potential Non-Invasive Biomarkers for Monitoring Heart Failure: A Pilot Study. Sci. Rep. 2020, 10, 7441.
- Zhang, X.; Karunathilaka, N.; Senanayake, S.; Subramaniam, V.N.; Chan, W.; Kostner, K.; Fraser, J.; Atherton, J.J.; Punyadeera, C. The Potential Prognostic Utility of Salivary galectin-3 Concentrations. Heart Fail. Clin. Res. Cardiol. 2020, 109, 685–692.
- Dekker, R.L.; Lennie, T.A.; Moser, D.K.; Miller, C.S.; Ebersole, J.L.; Chung, M.L.; Campbell, C.L.; Bailey, A.; Tovar, E.G. Salivary Biomarkers, Oral Inflammation, and Functional Status in Patients with Heart Failure. Biol. Res. Nurs. 2017, 19, 153–161.
- Joharimoghadam, A.; Tajdini, M.M.; Bozorgi, A. Salivary B-type Natriuretic Peptide: A New Method for Heart Failure Diagnosis and Follow-Up. Kardiol. Pol. 2017, 75, 71–77.
- Zhang, X.; Walsh, T.; Atherton, J.J.; Kostner, K.; Schulz, B.; Punyadeera, C. Identification and Validation of a Salivary Protein Panel to Detect Heart Failure Early. Theranostics 2017, 7, 4350–4358.
- Hammer, F.; Deutschbein, T.; Marx, A.; Güder, G.; Michalski, R.; Ertl, G.; Allolio, B.; Angermann, C.E.; Stork, S.; Fassnacht, M. High Evening Salivary Cortisol Is an Independent Predictor of Increased Mortality Risk in Patients with Systolic Heart Failure. Int. J. Cardiol. 2016, 203, 69–73.
- Zhang, X.; Wan, Y.; Chata, R.; Brazzale, A.; Atherton, J.J.; Kostner, K.; Dimeski, G.; Punyadeera, C. A Pilot Study to Demonstrate Diagnostic Potential of galectin-3 Levels in Saliva. J. Clin. Pathol. 2016, 69, 1100–1104.
- Alhurani, A.S.; Dekker, R.; Tovar, E.; Bailey, A.; Lennie, T.A.; Randall, D.C.; Moser, D.K. Examination of the Potential Association of Stress with Morbidity and Mortality Outcomes in Patient with Heart Failure. SAGE Open Med. 2014.
- Foo, J.Y.Y.; Wan, Y.; Kostner, K.; Arivalagan, A.; Atherton, J.; Cooper-White, J.; Dimeski, G.; Punyadeera, C. NT-ProBNP Levels in Saliva and Its Clinical Relevance to Heart Failure. PLoS ONE 2012, 7, e48452.
- Suska, A.; Alehagen, U.; Lundstrom, I.; Dahlstrom, U. Salivary Alpha-Amylase Activity, a New Biomarker in Heart Failure? J. Clin. Exp. Cardiolog. 2012.
- Wolfram, R.; Oguogho, A.; Palumbo, B.; Sinzinger, H. Enhanced Oxidative Stress in Coronary Heart Disease and Chronic Heart Failure as Indicated by an Increased 8-epi-PGF (2alpha). Eur. J. Heart Fail. 2005, 7, 167–172.
- Jekell, A.; Hossain, A.; Alehagen, U.; Dahlström, U.; Rosén, A. Elevated Circulating Levels of Thioredoxin and Stress in Chronic Heart Failure. Eur. J. Heart Fail. 2004, 6, 883–890.
- Denver, R.; Tzanidis, A.; Martin, P.; Krum, H. Salivary Endothelin Concentrations in the Assessment of Chronic Heart Failure. Lancet 2000, 355, 468–469.
- White, A.G.; Gordon, H.; Leiter, L. Studies in Edema. II. The Effect of Congestive Heart Failure on Saliva Electrolyte Concentrations. J. Clin. Investig. 1950, 11, 1445–1447.
- Tzanis, G.; Dimopoulos, S.; Agapitou, V.; Nanas, S. Exercise intolerance in chronic heart failure: The role of cortisol and the catabolic state. Curr. Heart Fail. Rep. 2014, 11, 70–79.
- Yamaji, M.; Tsutamoto, T.; Kawahara, C.; Nishiyama, K.; Yamamoto, T.; Fuji, M.; Horie, M. Serum cortisol as a useful predictor of cardiac events in patients with chronic heart failure: The impact of oxidative stress. Circ. Heart Fail. 2009, 2, 608–615.
- Güder, G.; Bauersachs, J.; Frantz, S.; Weismann, D.; Allolio, B.; Ertl, G.; Angermann, C.; Stork, S. Complementary and incremental mortality risk prediction by cortisol and aldosterone in chronic heart failure. Circulation 2007, 115, 1754–1761.
- Lewis, J.G.; Bagley, C.J.; Elder, P.A.; Bachmann, A.W.; Torpy, D.J. Plasma free cortisol fraction reflects levels of functioning corticosteroid-binding globulin. Clin. Chim. Acta 2005, 359, 189–194.
- Van der Velde, A.R.; Gullestad, L.; Ueland, T.; Aukrust, P.; Guo, Y.; Adourian, A.; Muntendam, P.; van Veldhuisen, D.J.; de Boer, R.A. Prognostic value of changes in galectin-3 levels over time in patients with heart failure: Data from CORONA and COACH. Circ. Heart Fail. 2013, 6, 219–226.
- Zhong, X.; Qian, X.; Chen, G.; Song, X. The role of galectin-3 in heart failure and cardiovascular disease. Clin. Exp. Pharmacol. Physiol. 2019, 46, 197–203.
- Pieper-Bigelow, C.; Strocchi, A.; Levitt, M.D. Where does serum amylase come from and where does it go? Gastroenterol. Clin. N. Am. 1990, 19, 793–810.
- Parissis, J.T.; Adamopoulos, S.N.; Venetsanou, K.F.; Karas, S.M.; Kremastinos, D.T. Elevated plasma amylase levels in advanced chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy: Correlation with circulating interleukin-6 activity. J. Interf. Cytokine Res. 2003, 23, 329–333.
- Vozgirdaite, D.; Ben Halima, H.; Bellagambi, F.G.; Alcacer, A.; Palacio, F.; Zine, N.; Bausells, J.; Errachid, A. Development of an ImmunoFET for the detection of TNF-α in saliva: Application to heart failure monitoring. Chemosensors 2021, 9, 26.
- Bozkurt, B.; Mann, D.L.; Deswal, A. Biomarkers of inflammation in heart failure. Heart Fail. Rev. 2010, 15, 331–341.
- Afakan, B.; Ozturk, V.O.; Pasali, C.; Bozkurt, E.; Kose, T.; Emingil, G. Gingival crevicular fluid and salivary HIF-1α, VEGF, and TNF-α levels in periodontal health and disease. Periodontology 2019, 90, 788–797.
- Nandan, S.R.K.; Kulkarni, P.G. Salivary Tumour Necrosis Factor-α as a Biomarker in Oral Leukoplakia and Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2019, 20, 2087–2093.
- Levine, B.; Kalman, J.; Mayer, L.; Fillit, H.M.; Packer, M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N. Engl. J. Med. 1990, 323, 236–241.
- Tousoulis, D. Novel biomarkers in heart failure. What they add in daily clinical practice? Hell. J. Cardiol. 2018, 59, 193–195.
- Loppnow, H.; Werdan, K.; Werner, C. The enhanced plasma levels of soluble tumor necrosis factor receptors (sTNF-R1; sTNF-R2) and interleukin-10 (IL-10) in patients suffering from chronic heart failure are reversed in patients treated with beta-adrenoceptor antagonist. Auton. Autacoid Pharmacol. 2002, 22, 83–92.
- Mann, D.L. Innate immunity and the failing heart: The cytokine hypothesis revisited. Circ. Res. 2015, 116, 1254–1268.
- Ohtsuka, T.; Hamada, M.; Hiasa, G.; Sasaki, O.; Suzuki, M.; Hara, Y.; Shigematsu, Y.; Hiwada, K. Effect of beta-blockers on circulating levels of inflammatory and anti-inflammatory cytokines in patients with dilated cardiomyopathy. JACC 2001, 37, 412–417.
