More than one year into the novel coronavirus disease 2019 (COVID-19) pandemic, healthcare systems across the world continue to be overwhelmed with soaring daily cases. The treatment spectrum primarily includes ventilation support augmented with repurposed drugs and/or convalescent plasma transfusion (CPT) from recovered COVID-19 patients. CPT is a promising COVID-19 therapeutic option that merits internationally coordinated RCTs to achieve a scientific risk-benefit consensus.
- COVID-19
- convalescent plasma transfusion
- therapeutics
- clinical trials
1. Introduction
Multiple cases of acute respiratory syndrome with unclear precursors were recorded during December 2019 in Wuhan, the capital of Hubei province in central China. Subsequent analysis of samples from patients’ lower respiratory tract indicated the presence of an unprecedented strain of human coronavirus, referred to as the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) [1][2][3], and its corresponding disease was dubbed coronavirus disease 2019 (COVID-19). Since the World Health Organization (WHO) declared the COVID-19 outbreak as a global pandemic on 11 March 2020, the cumulative number of global confirmed cases has surpassed 142 million with over 3 million deaths as of 21 April 2021 [4]. In response to growing pressures from the pandemic, more than 2000 clinical trials have been actively implemented during 2020, yet a unified treatment approach has not been recognized by the global healthcare and scientific communities to date [5]. Adding to the complexity of the current situation, government bodies and regulatory agencies overseeing national healthcare response plans have been subject to an unprecedented rate of trial reports published by multiple sources with often conflicting findings.
Global- and national-scale recovery timelines remain unclear amidst the uncertainty in treatment approaches and the emergence of new COVID-19 mutant strains [6]. Standard supportive care guidelines released by the WHO remain the status quo and mainly include symptom management through assisted ventilation and fluid management [7]. Remdesevir has been the only repurposed drug authorized by the United States (US) Food and Drug Administration (FDA) solely for emergency use [8]. Other drugs such as Hydroxychloroquinine [9], Ivermectin [10], and Oseltamivir [11] have been reportedly found to be comparably effective to standard supportive care for COVID-19 patients. Nevertheless, the generalized effectiveness of repurposed drugs remains subject to debate in the absence of large-scale data. As such, international efforts have been focused on vaccine development, which is naturally the product of several years of research and clinical trials before being authorized for general use.
The multi-phase vaccine development process encompasses pre-clinical testing (non-human trials), safety and dosage assessments (Phase 1), expanded safety trials (Phase 2), scalable efficacy trials (Phase 3), limited use authorization, and ultimately, full-scale approval. Given the urgency of the COVID-19 pandemic, a total of 89 vaccines are currently in human clinical trials—many of which are combining Phase 1 and 2 trials—while 27 have reached the final stages of testing [12]. Despite the timely progress in vaccine developments, two dynamic challenges hinder their post-efficacy stage for the global population. The primary set of challenges, especially for developing countries, lies in the supply chain and logistics in terms of scaled-up manufacturing and quality control, regional and local coordination of supply, and equality in distribution and governmental subsidies [13][14]. These factors exclusively stem from the post-efficacy stage, presumably after addressing any lurking uncertainties, such as the optimal timing and dosage of booster vaccine shots. Another transient challenge lies in the ongoing emergence of new SARS-CoV-2 variants [6][15]. The coronavirus genome is highly susceptible to mutations [16], which increases the likelihood of variant forms to evolve with time—a process termed genetic drift [17]. Hence, there is a sizable risk that a newly mutated virus might not be recognized by vaccine-acquired immune recognition from pre-mutation genomes.
Based on the above uncertainties in the COVID-19 response, numerous healthcare practitioners across the world have resorted to the conventional approach of convalescent plasma transfusion (CPT) therapy [18]. CPT therapy was first proposed in the late 1800s to treat a variety of previously unknown infectious diseases that lacked conclusive treatment options [19][20]. The general concept of CPT is based on the notion of relaying passive immunity by screening blood samples taken from a recovered individual (donor) for specific neutralizing antibodies, which are then administered to a patient suffering from the same disease to ameliorate symptoms and reduce mortality [21]. CPT has been particularly effective when used for diseases targeting the respiratory system, including the severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and influenza (H5N1 and H1N1) outbreaks [22].
Investigating the efficacy of CPT for treating COVID-19 patients has been recommended by the FDA since March 2020 [23]. Consequently, local-scale randomized clinical trials (RCTs), matched-control studies (MCSs), and case reports (CRs) conducted during 2020 offered preliminary evidence on the safety and efficacy of CPT therapy for COVID-19 patients [24]. However, the findings are subject to debate within the healthcare and scientific communities due to the lack of concrete evidence from statistically representative large-scale RCTs [25]. For instance, some studies were terminated early while reporting inconclusive results and a non-significant correlation between CPT and clinical improvement [26].
COVID-19 infections are generally mild and/or asymptomatic in the majority of the pediatric population with less than 2% of COVID-19 cases reported for infant and child age groups [27][28][29][30]. Additionally, current recommendations for the treatment of severe COVID-19 in children is limited to monitoring and supportive care [31]. Consequently, very few studies on convalescent plasma transfusion (CPT) therapy for COVID-19 pediatric patient groups are available to date. Rodriguez et al. [32] and Shankar et al. [33] provide case reports on individual high-risk patients with pre-existing conditions, namely, congenital heart disease (6 weeks old; female) and lymphoblastic leukemia (4 years old; female), respectively. In both cases, patients show a considerable reduction in symptoms after CPT therapy, which suggests a positive clinical correlation in infants and children with underlying conditions. Moreover, Figlerowicz et al. [34] reports the first attempt of CPT therapy on a 6-year-old child diagnosed with COVID-19-induced aplastic anemia without pre-existing conditions. Following the administration of antiviral drugs without signs of recovery during the first 5 weeks, a single dose of convalescent plasma (200 mL; 1:700 titer) resulted in recovery from COVID-19 within 3 weeks, while hematologic deficiencies persisted. The most representative attempt involving COVID-19 pediatric patients to date is the recent work of Małecki et al. [35]. Their study included a group of 13 patients (median age of 12 years), of which six CPT-recipient patients recovered within 3 days of transfusion, while the remaining seven control patients recovered after 12 days. Based on the limited data and a gap period of 10 days between infection and transfusion, the study suggests CPT may be a promising treatment for COVID-19 in children. Given the limited data on pediatric patients, underlying conditions, and/or notable lag times in administering CPT, the aforementioned studies were excluded from the review to avoid introducing significant biases within the pediatric age group.
2. Included Studies
The literature search identified a total of 181 articles, of which 63 articles remained after removing duplicates. Following the title and abstract screening against the inclusion/exclusion criteria, 23 articles were excluded. During the full-text screening, one RCT article was excluded, since the majority of the trial’s CPT patients received plasma transfusions with non-detectable levels of SARS-CoV-2-neutralizing antibodies based on post-trial measurements (antibody titers < 1:80) [36]. The remaining 39 articles included in this review were geographically distributed across the following countries: China (13), USA (11), Mexico (2), South Korea (2), Turkey (2), Argentina (1), France (1), Hungary (1), Italy (1), Iran (1), Iraq (1), Netherlands (1), Qatar (1), and Spain (1).
3. Characteristics of Included Studies
The 39 studies included four RCTs [26][37][38][39], 14 MCSs [40][41][42][43][44][45][46][47][48][49][50][51][52][53], and 21 CRs [54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74]. The majority of studies (28) were published in peer-reviewed journals, while 11 studies were available as pre-prints. Despite still being under peer-review, the raw data reported in these pre-prints remain highly relevant to our analysis, regardless of the methodology and recommendations provided.
An important uniformity across the studies was that all patients were included on the basis of demonstrating severe or life-threatening complications associated with COVID-19. Hence, all patients received one or more form of concomitant supportive care including mechanical ventilation and/or repurposed drugs as per WHO and local healthcare guidance. Almost half the studies (20) applied an antibody inclusion threshold to donor samples before transfusion, while the remaining (19) did not apply any screening to samples. The volume of transfused plasma ranged from 200 to 1200 mL, while the post-CPT therapy follow-up occurred between 7 and 60 days. Patient cohort age groups ranged from 23 to 96 years with a higher male proportion in each study. The total number of patients per study ranged from 49 to 103 for RCTs, from 20 to 3166 for MCSs, and from 1 to 31 for CRs. Table 1 lists the main characteristics relevant to our analysis and the results presented in the following section.
Table 1. Studies showing effects of convalescent plasma therapy in COVID-19 cases.
| Study, Country | CPT (and Control) Patient Count |
CPT and (Control) Mortality (%) |
CPT and (Control) Cohort Age Group | Antibody Threshold a | Plasma Volume (mL) |
Follow-Up Days |
|---|---|---|---|---|---|---|
| Randomized Clinical Trials (RCTs) | ||||||
| [37], Spain | 38; (43) | 0; (9) | 61; (60) | - | 250–300 | 29 |
| [39], Netherlands | 43; (43) | 14; (26) | 63; (61) | NA(t) ≥ 1:80 | ≥300 | 30 |
| [26], China | 52; (51) | 16; (24) | 70; (69) | NA(t) ≥ 1:640 | 200 | 28 |
| [38], Iraq | 21; (28) | 5; (29) | 56; (48) | EIgG(i) > 1.25 | ≥200 | 30 |
| Matched-Control Studies (MCSs) | ||||||
| [50], Iran | 115; (74) | 15; (24) | 54; (57) | EIgG(i) > 1.1 | ≥500 | 30 |
| [52], Turkey | 888; (888) | 25; (28) | 60; (61) | - | 200–600 | 17 |
| [45], USA | 47; (1340) | 23; (42) | 59; (-) | NA(t) ≥ 1:500 | ≥200 | 30 |
| [40], China | 10; (10) | 0; (30) | 53; (53) | NA(t) ≥ 1:640 | 200 | - |
| [43], USA | 20; (20) | 10; (30) | 60; (-) | - | ≥200 | 14 |
| [53], USA | 35,322; (-) b | [8.3–26.7] c; (-) | 60; (-) | S/Co [4.62–18.45] | 200 | 7 |
| [48], USA | 39; (156) | 13; (24) | 55; (54) | EIgG(t) ≥ 1:320 | 500 | 14 |
| [42], Qatar | 40; (40) | 3; (13) | 48; (56) | - | 400 | 28 |
| [41], Italy | 46; (23) | 7; (30) | 63; (-) | NA(t) ≥ 1:80 | ≥250 | 7 |
| [51], USA | 64; (177) | 13; (16) | 61; (61) | - | ≥200 | 28 |
| [46], Argentina | 868; (2298) | 25; (44) | 56; (64) | EIgG(i) > 1350 | 200–250 | 28 |
| [47], USA | 321; (582) | 6; (12) | 53; (60) | EIgG(i) ≥ 1350 | ≥200 | 60 |
| [49], China | 138; (1430) | 2; (4) | 65; (63) | - | ≥200 | 14 |
| [44], China | 6; (15) | 83; (93) d | 62; (73) | - | ≥200 | - |
| Case Reports (CRs) | ||||||
| [60], South Korea | 2 | 0 | 69 | - | 250 | 15 |
| [65], USA | 1 | 0 | 35 | - | 400 | 10 |
| [66], China | 1 | 0 | 38 | - | 300 | 31 |
| [61], Hungary | 2 | 0 | - | - | 600 | 14 |
| [55], China | 16 | 0 | 65 | TA [10.9–115 AU/mL] | 200–1200 | 8 |
| [67], Turkey | 1 | 0 | 55 | EIgG(i) > 1.1 | 350 | 11 |
| [70], USA | 31 | 13 | - | - | ≥200 | 7 |
| [69], France | 17 | 6 | 58 | NA(t) ≥ 1:40 | 800 | 7 |
| [62], South Korea | 1 | 0 | 68 | - | 500 | 23 |
| [71], USA | 29 | 17 | 58 | - | 200 | 28 |
| [57], China | 6 | 0 | 61 | NA(t) ≥ 1:40 | 200 | 60 |
| [58], USA | 3 | 0 | 24 | - | 400 | 31 |
| [68], China | 1 | 0 | 100 | NA(t) ≥ 1:640 | 300 | 13 |
| [56], Mexico | 8 | 0 | 57 | EIgG(t) > 1:100 | 500 | 23 |
| [72], Mexico | 10 | 20 | 52 | - | ≥200 | 8 |
| [63], China | 1 | 0 | 66 | EIgG(t) > 1:160 | 400 | 26 |
| [54], China | 5 | 0 | 65 | NA(t) ≥ 1:40 | 400 | 47 |
| [73], USA | 24 | 42 | 69 | EIgG(t) ≥ 1:320 | 500 | 9 |
| [64], China | 1 | 0 | 65 | - | 800 | 11 |
| [74], China | 6 | 0 | 58 | - | ≥200 | 25 |
| [59], China | 4 | 0 | 57 | - | ≥200 | 38 |
a Different serologic tests to report antibody levels across studies, including the enzyme-linked immunosorbent assay for immunoglobulin G (EIgG) and neutralizing antibody (NA) indices (i) or titers (t), signal-to-cutoff ratio (S/Co), and total antibodies (TA) in arbitrary units per volume (AU/mL). b Joyner et al. [53] reports on an Expanded Access Program for the treatment of COVID-19 patients with CPT. The program did not include a control group and was, therefore, excluded from the boxplot and random-effects model. c Range of 7- and 30-day mortality rates corresponding to different times of transfusion (<3 or ≥4 days) after diagnosis and antibody S/Co levels. d The CPT and control group mortality rates (83 and 93%) reported by Zeng et al. [44] are not shown as outliers in Figure 1 for visualization purposes.
4. Statistical and Random-Effects Model Results
Figure 1 shows the boxplots of mortality rates as reported from the 39 studies and aggregated per RCTs, MCSs, and CRs with a total patient count of N = 319, 9655, and 170, respectively. The mortality rates for both the control and CPT recipient groups are shown, except for the CR studies which, by definition, are not assigned a control group. The median mortality rates of the control groups (RCT-Control and MCS-Control) are shown to be comparable at 24 and 28%, respectively. However, the MCS-Control group records a higher variance with mortality rates reaching around 45% compared to the 30% limit of the RCT-Control groups. Similarly, the median mortality rates of the corresponding CPT groups (RCT-CPT and MCS-CPT) are comparable at around 14%, while the MCS-CPT group shows a higher variance with mortality rates reaching 25%, compared to the 16% limit reported by the RCT-CPT group. The 28-fold difference in total patient numbers between the RCT (319) and MCS groups (9655) explains the higher variance in mortality rates from the latter group.
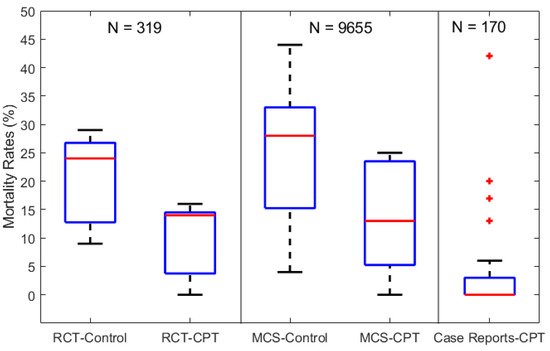
Figure 1. Boxplots of mortality rates in COVID-19 patients from control and CPT recipient groups of RCTs, MCSs, and CRs. The total number of patients included in each category of studies is labeled above as N = 319, 9655, and 170 for the RCTs, MCSs, and CRs, respectively.
The results from both the RCT and MCS groups in Figure 1 indicate an overall reduction of 46% in mortality rates (~26 to 14%) in patients receiving CPT therapy compared to the control groups receiving standard supportive care and/or repurposed drugs. On the other hand, the CR-CPT group shows significantly lower mortality rates (less than 5%) compared to the RCT and MCS groups, but with outliers reaching 43%. This reflects the potential of high bias when aggregating individual case reports. For instance, several CR studies consisted of COVID-19 patients with impaired immunities, which suppressed their response to standard supportive care [58][69][73]. However, their symptoms were significantly reduced and showed signs of recovery after 3–5 days of CPT with antibody titer levels higher than 1:80, compared to undetectable levels pre-CPT.
To address the risk of bias and generalize the boxplot results, a random-effects model was set up based on the parameters listed in Table 1. Mortality rates were derived based on the longest follow-up period in each study, and odds ratios (ORs) were aggregated for each of the RCT and MCS study categories (CRs do not have control groups). A meta-regression analysis based on the MATLAB LMM function [75] was used to evaluate the significance of the parameters listed in Table 1 (i.e., cohort age, follow-up duration, plasma volume/dose) with respect to mortality across the studies. Hypothesis testing using the two-tailed test at a 95% confidence interval (5% significance) was carried out.
The random-effects analysis showed median mortality rates of 11 vs. 23% for the CPT and control groups, respectively, across the four RCT studies with an OR of 0.41. This suggests that CPT was correlated with a 59% reduction in the likelihood of mortality among COVID-19 patients. This association was further observed based on the MCS random-effects model results, for which mortality rates of 21 vs. 32% for the CPT and control groups with an OR of 0.47 (i.e., 53% reduction in the likelihood of mortality). To further investigate these findings, an overall random-effects model was used to aggregate results from both RCT and MC studies. Similarly, mortality rates of 17 vs. 32% for the CPT and control groups were recorded, respectively, with an OR of 0.49—a 51% reduction in the overall likelihood of mortality across all trials. The meta-regression analyses indicated that cohort age and the follow-up duration did not affect the aggregate OR computed for all clinical trials.
5. Conclusions
Aggregated results show an overall reduction of 46% in mortality in patients receiving CPT therapy compared to control groups receiving standard supportive care and/or repurposed drugs. Despite the promising preliminary evidence from trials and case studies to date, several challenges remain in terms of the technical, safety and resource aspects of CPT trials as presented in Section 4. Early intervention is shown to be a critical factor for CPT efficacy in treating COVID-19 patients which is coupled to the scalability of testing and contact tracing. The recruitment of plasma donors, consistency of adequate ranges of SARS-CoV-2 antibody titer, risks of adverse effects to CPT donors/recipients, and robust management of healthcare resources are some of the major challenges to consider before implementing large-scale RCTs.
This entry is adapted from the peer-reviewed paper 10.3390/v13050849
References
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534.
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273.
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269.
- WHO. Coronavirus (COVID-19). Available online: (accessed on 26 January 2021).
- Siemieniuk, R.A.; Bartoszko, J.J.; Ge, L.; Zeraatkar, D.; Izcovich, A.; Kum, E.; Pardo-Hernandez, H.; Rochwerg, B.; Lamontagne, F.; Han, M.A. Drug treatments for covid-19: Living systematic review and network meta-analysis. BMJ 2020, 370, m2980.
- Van Oosterhout, C.; Hall, N.; Ly, H.; Tyler, K.M. COVID-19 Evolution during the Pandemic–Implications of New SARS-CoV-2 Variants on Disease Control and Public Health Policies; Taylor & Francis: Milton Park/Abingdon, UK, 2021.
- WHO. Clinical Management of Severe Acute Respiratory Infections (SARI) When COVID-19 Disease Is Suspected; Interim Guidance; WHO: Geneva, Switzerland, 2020.
- FDA. Coronavirus (COVID-19) Update: FDA Issues Emergency Use Authorization for Potential COVID-19 Treatment; Food and Drug Administration: Silver Spring, MD, USA, 2020.
- Gautret, P.; Lagier, J.-C.; Parola, P.; Meddeb, L.; Mailhe, M.; Doudier, B.; Courjon, J.; Giordanengo, V.; Vieira, V.E.; Dupont, H.T. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 2020, 56, 105949.
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020, 178, 104787.
- Cunningham, A.C.; Goh, H.P.; Koh, D. Treatment of COVID-19: Old Tricks for New Challenges; Springer: Berlin/Heidelberg, Germany, 2020.
- Corum, J.; Grady, D.; Wee, S.-L.; Zimmer, C. Coronavirus Vaccine Tracker; The New York Times: New York, NY, USA, 2020; p. 5.
- Kim, J.H.; Marks, F.; Clemens, J.D. Looking beyond COVID-19 vaccine phase 3 trials. Nat. Med. 2021, 27, 205–211.
- Newton, P.N.; Bond, K.C.; Adeyeye, M.; Antignac, M.; Ashenef, A.; Awab, G.R.; Bannenberg, W.J.; Bower, J.; Breman, J.; Brock, A. COVID-19 and risks to the supply and quality of tests, drugs, and vaccines. Lancet Glob. Health 2020, 8, e754–e755.
- Koyama, T.; Weeraratne, D.; Snowdon, J.L.; Parida, L. Emergence of drift variants that may affect COVID-19 vaccine development and antibody treatment. Pathogens 2020, 9, 324.
- Yao, H.-P.; Lu, X.; Chen, Q.; Xu, K.; Chen, Y.; Cheng, L.; Liu, F.; Wu, Z.; Wu, H.; Jin, C. Patient-Derived Mutations Impact Pathogenicity of SARS-CoV-2. CELL-D-20-01124. 2020. Available online: (accessed on 6 May 2021).
- Lynch, M.; Ackerman, M.S.; Gout, J.-F.; Long, H.; Sung, W.; Thomas, W.K.; Foster, P.L. Genetic drift, selection and the evolution of the mutation rate. Nat. Rev. Genet. 2016, 17, 704.
- Chen, L.; Xiong, J.; Bao, L.; Shi, Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect. Dis. 2020, 20, 398–400.
- Luke, T.C.; Kilbane, E.M.; Jackson, J.L.; Hoffman, S.L. Meta-analysis: Convalescent blood products for Spanish influenza pneumonia: A future H5N1 treatment? Ann. Intern. Med. 2006, 145, 599–609.
- Marano, G.; Vaglio, S.; Pupella, S.; Facco, G.; Catalano, L.; Liumbruno, G.M.; Grazzini, G. Convalescent plasma: New evidence for an old therapeutic tool? Blood Transfus. 2016, 14, 152.
- Bozzo, J.; Jorquera, J.I. Use of human immunoglobulins as an anti-infective treatment: The experience so far and their possible re-emerging role. Expert Rev. Anti-Infect. Ther. 2017, 15, 585–604.
- Mair-Jenkins, J.; Saavedra-Campos, M.; Baillie, J.K.; Cleary, P.; Khaw, F.-M.; Lim, W.S.; Makki, S.; Rooney, K.D.; Group, C.P.S.; Nguyen-Van-Tam, J.S. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta-analysis. J. Infect. Dis. 2015, 211, 80–90.
- FDA. Recommendations for Investigational COVID-19 Convalescent Plasma, Content Current as of: 04/13/2020; Food and Drug Administration: Silver Spring, MD, USA, 2020.
- Joyner, M.J.; Klassen, S.A.; Senefeld, J.; Johnson, P.W.; Carter, R.E.; Wiggins, C.C.; Shoham, S.; Grossman, B.J.; Henderson, J.P.; Musser, J.M. Evidence favouring the efficacy of convalescent plasma for COVID-19 therapy. medRxiv 2020.
- Valk, S.J.; Piechotta, V.; Chai, K.L.; Doree, C.; Monsef, I.; Wood, E.M.; Lamikanra, A.; Kimber, C.; McQuilten, Z.; So-Osman, C. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: A rapid review. Cochrane Database Syst. Rev. 2020.
- Li, L.; Zhang, W.; Hu, Y.; Tong, X.; Zheng, S.; Yang, J.; Kong, Y.; Ren, L.; Wei, Q.; Mei, H. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: A randomized clinical trial. JAMA 2020, 324, 460–470.
- Dong, Y.; Mo, X.; Hu, Y.; Qi, X.; Jiang, F.; Jiang, Z.; Tong, S. Epidemiology of COVID-19 among children in China. Pediatrics 2020, 145, e20200702.
- DeBiasi, R.L.; Song, X.; Delaney, M.; Bell, M.; Smith, K.; Pershad, J.; Ansusinha, E.; Hahn, A.; Hamdy, R.; Harik, N. Severe coronavirus disease-2019 in children and young adults in the Washington, DC, metropolitan region. J. Pediatr. 2020, 223, 199–203.e1.
- Zimmermann, P.; Curtis, N. Coronavirus infections in children including COVID-19: An overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr. Infect. Dis. J. 2020, 39, 355.
- Ludvigsson, J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020, 109, 1088–1095.
- Ye, Z.; Rochwerg, B.; Wang, Y.; Adhikari, N.K.; Murthy, S.; Lamontagne, F.; Fowler, R.A.; Qiu, H.; Wei, L.; Sang, L. Treatment of patients with nonsevere and severe coronavirus disease 2019: An evidence-based guideline. CMAJ 2020, 192, E536–E545.
- Rodriguez, Z.; Shane, A.L.; Verkerke, H.; Lough, C.; Zimmerman, M.G.; Suthar, M.; Wrammert, J.; MacDonald, H.; Wolf, M.; Clarke, S. COVID-19 convalescent plasma clears SARS-CoV-2 refractory to remdesivir in an infant with congenital heart disease. Blood Adv. 2020, 4, 4278.
- Shankar, R.; Radhakrishnan, N.; Dua, S.; Arora, S.; Rana, M.; Sahu, D.K.; Rai, S.; Gupta, D.K. Convalescent plasma to aid in recovery of COVID-19 pneumonia in a child with acute lymphoblastic leukemia. Transfus. Apher. Sci. 2021, 60, 102956.
- Figlerowicz, M.; Mania, A.; Lubarski, K.; Lewandowska, Z.; Służewski, W.; Derwich, K.; Wachowiak, J.; Mazur-Melewska, K. First case of convalescent plasma transfusion in a child with COVID-19-associated severe aplastic anemia. Transfus. Apher. Sci. 2020, 59, 102866.
- Małecki, P.; Faltin, K.; Mania, A.; Mazur-Melewska, K.; Cwalińska, A.; Zawadzka, A.; Bukowska, A.; Lisowska, K.; Graniczna, K.; Figlerowicz, M. Effects and Safety of Convalescent Plasma Administration in a Group of Polish Pediatric Patients with COVID-19: A Case Series. Life 2021, 11, 247.
- Agarwal, A.; Mukherjee, A.; Kumar, G.; Chatterjee, P.; Bhatnagar, T.; Malhotra, P. Convalescent plasma in the management of moderate covid-19 in adults in India: Open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ 2020, 371, m3939.
- Avendano-Sola, C.; Ramos-Martinez, A.; Munez-Rubio, E.; Ruiz-Antoran, B.; de Molina, R.M.; Torres, F.; Fernandez-Cruz, A.; Callejas-Diaz, A.; Calderon, J.; Payares-Herrera, C. Convalescent Plasma for COVID-19: A multicenter, randomized clinical trial. medRxiv 2020.
- Rasheed, A.M.; Fatak, D.F.; Hashim, H.A.; Maulood, M.F.; Kabah, K.K.; Abdulamir, A.S. The therapeutic potential of convalescent plasma therapy on treating critically-ill COVID-19 patients residing in respiratory care units in hospitals in Baghdad, Iraq. medRxiv 2020.
- Gharbharan, A.; Jordans, C.C.; GeurtsvanKessel, C.; den Hollander, J.G.; Karim, F.; Mollema, F.P.; Stalenhoef, J.E.; Dofferhoff, A.; Ludwig, I.; Koster, A. Convalescent Plasma for COVID-19. A randomized clinical trial. medRxiv 2020.
- Duan, K.; Liu, B.; Li, C.; Zhang, H.; Yu, T.; Qu, J.; Zhou, M.; Chen, L.; Meng, S.; Hu, Y. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. USA 2020, 117, 9490–9496.
- Perotti, C.; Baldanti, F.; Bruno, R.; Del Fante, C.; Seminari, E.; Casari, S.; Percivalle, E.; Glingani, C.; Musella, V.; Belliato, M. Mortality reduction in 46 patients with severe COVID-19 treated with hyperimmune plasma. A proof-of-concept, single-arm, multicenter trial. Haematologica 2020, 105, 2834–2840.
- Omrani, A.S.; Zaqout, A.; Baiou, A.; Daghfal, J.; Elkum, N.; Alattar, R.A.; Bakdach, D.; Abusriwil, H.; Mostafa, A.M.; Alhariri, B. Convalescent plasma for the treatment of patients with severe coronavirus disease 2019: A preliminary report. J. Med. Virol. 2021, 93, 1678–1686.
- Hegerova, L.; Gooley, T.A.; Sweerus, K.A.; Maree, C.; Bailey, N.; Bailey, M.; Dunleavy, V.; Patel, K.; Alcorn, K.; Haley, R. Use of convalescent plasma in hospitalized patients with COVID-19: Case series. Blood J. Am. Soc. Hematol. 2020, 136, 759–762.
- Zeng, Q.-L.; Yu, Z.-J.; Gou, J.-J.; Li, G.-M.; Ma, S.-H.; Zhang, G.-F.; Xu, J.-H.; Lin, W.-B.; Cui, G.-L.; Zhang, M.-M. Effect of convalescent plasma therapy on viral shedding and survival in patients with coronavirus disease 2019. J. Infect. Dis. 2020, 222, 38–43.
- Donato, M.; Park, S.; Baker, M.; Korngold, R.; Morawski, A.; Geng, X.; Tan, M.T.; Rowley, S.; Chow, K.; Brown, E. Clinical and laboratory evaluation of patients with SARS-CoV-2 pneumonia treated with high-titer convalescent plasma: A prospective study. medRxiv 2020.
- Salazar, M.R.; Gonzalez, S.E.; Regairaz, L.; Ferrando, N.S.; Gonzalez, V.; Carrera, P.M.; Muñoz, L.; Pesci, S.A.; Vidal, J.M.; Kreplak, N. Effect of convalescent plasma on mortality in patients with COVID-19 pneumonia. medRxiv 2020.
- Salazar, E.; Christensen, P.A.; Graviss, E.A.; Ngyuen, D.T.; Castillo, B.; Chen, J.; Lopez, B.V.; Eager, T.; Yi, X.; Zhao, P. Early transfusion of a large cohort of COVID-19 patients with high titer anti-SARS-CoV-2 spike protein IgG convalescent plasma confirms a signal of significantly decreased mortality. medRxiv 2020.
- Liu, S.T.; Lin, H.-M.; Baine, I.; Wajnberg, A.; Gumprecht, J.P.; Rahman, F.; Rodriguez, D.; Tandon, P.; Bassily-Marcus, A.; Bander, J. Convalescent plasma treatment of severe COVID-19: A propensity score–matched control study. Nat. Med. 2020, 26, 1708–1713.
- Xia, X.; Li, K.; Wu, L.; Wang, Z.; Zhu, M.; Huang, B.; Li, J.; Wang, Z.; Wu, W.; Wu, M. Improved clinical symptoms and mortality on severe/critical COVID-19 patients utilizing convalescent plasma transfusion. Blood 2020, 136, 755–759.
- Abolghasemi, H.; Eshghi, P.; Cheraghali, A.M.; Fooladi, A.A.I.; Moghaddam, F.B.; Imanizadeh, S.; Maleki, M.M.; Ranjkesh, M.; Rezapour, M.; Bahramifar, A. Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: Results of a multicenter clinical study. Transfus. Apher. Sci. 2020, 59, 102875.
- Rogers, R.; Shehadeh, F.; Mylona, E.; Rich, J.; Neill, M.; Touzard-Romo, F.; Geffert, S.; Larkin, J.; Bailey, J.A.; Lu, S. Convalescent plasma for patients with severe COVID-19: A matched cohort study. medRxiv 2020.
- Altuntas, F.; Ata, N.; Yigenoglu, T.N.; Bascı, S.; Dal, M.S.; Korkmaz, S.; Namdaroglu, S.; Basturk, A.; Hacıbekiroglu, T.; Dogu, M.H. Convalescent plasma therapy in patients with COVID-19. Transfus. Apher. Sci. 2020, 60, 102955.
- Joyner, M.J.; Senefeld, J.W.; Klassen, S.A.; Mills, J.R.; Johnson, P.W.; Theel, E.S.; Wiggins, C.C.; Bruno, K.A.; Klompas, A.M.; Lesser, E.R. Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: Initial three-month experience. medRxiv 2020.
- Shen, C.; Wang, Z.; Zhao, F.; Yang, Y.; Li, J.; Yuan, J.; Wang, F.; Li, D.; Yang, M.; Xing, L. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA 2020, 323, 1582–1589.
- Chen, S.; Lu, C.; Li, P.; Wang, L.; Wang, H.; Yang, Q.; Chen, L.; Li, J.; Ma, H.; Sang, Q. Effectiveness of Convalescent Plasma for Treatment of COVID-19 Patients. medRxiv 2020.
- Martinez-Resendez, M.F.; Castilleja-Leal, F.; Torres-Quintanilla, A.; Rojas-Martinez, A.; Garcia-Rivas, G.; Ortiz-Lopez, R.; Trevino, V.; Lara-Medrano, R.; Villanueva-Lozano, H.; Ramirez-Elizondo, T. Initial experience in Mexico with convalescent plasma in COVID-19 patients with severe respiratory failure, a retrospective case series. medRxiv 2020.
- Jin, C.; Gu, J.; Yuan, Y.; Long, Q.; Zhang, Q.; Zhou, H.; Wu, W.; Zhang, W. Treatment of 6 COVID-19 patients with convalescent plasma. medRxiv 2020.
- Jin, H.; Reed, J.C.; Liu, S.T.; Ho, H.-E.; Lopes, J.P.; Ramsey, N.B.; Waqar, O.; Rahman, F.; Aberg, J.A.; Bouvier, N.M. Three patients with X-linked agammaglobulinemia hospitalized for COVID-19 improved with convalescent plasma. J. Allergy Clin. Immunol. Pract. 2020, 8, 3594–3596.e3.
- Zhang, B.; Liu, S.; Tan, T.; Huang, W.; Dong, Y.; Chen, L.; Chen, Q.; Zhang, L.; Zhong, Q.; Zhang, X. Treatment with convalescent plasma for critically ill patients with severe acute respiratory syndrome coronavirus 2 infection. Chest 2020, 158, e9–e13.
- Ahn, J.Y.; Sohn, Y.; Lee, S.H.; Cho, Y.; Hyun, J.H.; Baek, Y.J.; Jeong, S.J.; Kim, J.H.; Ku, N.S.; Yeom, J.-S. Use of convalescent plasma therapy in two COVID-19 patients with acute respiratory distress syndrome in Korea. J. Korean Med. Sci. 2020, 35, e149.
- Bobek, I.; Gopcsa, L.; Réti, M.; Bekő, G.; Hancz, L.; Lakatos, B.; Molnár, E.; Nagy, S.; Reményi, P.; Sebestyén, G. Successful administration of convalescent plasma in critically ill COVID-19 patients in Hungary: The first two cases. Orv. Hetil. 2020, 161, 1111–1121.
- Im, J.H.; Nahm, C.H.; Baek, J.H.; Kwon, H.Y.; Lee, J.-S. Convalescent plasma therapy in coronavirus disease 2019: A case report and suggestions to overcome obstacles. J. Korean Med. Sci. 2020, 35, e239.
- Peng, H.; Gong, T.; Huang, X.; Sun, X.; Luo, H.; Wang, W.; Luo, J.; Luo, B.; Chen, Y.; Wang, X. A synergistic role of convalescent plasma and mesenchymal stem cells in the treatment of severely ill COVID-19 patients: A clinical case report. Stem Cell Res. Ther. 2020, 11, 291.
- Xu, T.-M.; Lin, B.; Chen, C.; Liu, L.-G.; Xue, Y. Non-optimal effectiveness of convalescent plasma transfusion and hydroxychloroquine in treating COVID-19: A case report. Virol. J. 2020, 17, 80.
- Anderson, J.; Schauer, J.; Bryant, S.; Graves, C.R. The use of convalescent plasma therapy and remdesivir in the successful management of a critically ill obstetric patient with novel coronavirus 2019 infection: A case report. Case Rep. Women’s Health 2020, 27, e00221.
- Bao, Y.; Lin, S.Y.; Cheng, Z.H.; Xia, J.; Sun, Y.P.; Zhao, Q.; Liu, G.J. Clinical features of COVID-19 in a young man with massive cerebral hemorrhage—Case report. SN Compr. Clin. Med. 2020, 2, 703–709.
- Çınar, O.E.; Sayınalp, B.; Karakulak, E.A.; Karataş, A.A.; Velet, M.; İnkaya, A.Ç.; Ortaç, N.E.E.; Öcal, S.; Aksu, S.; Haznedaroğlu, İ.C. Convalescent (immune) plasma treatment in a myelodysplastic COVID-19 patient with disseminated tuberculosis. Transfus. Apher. Sci. 2020, 59, 102821.
- Kong, Y.; Cai, C.; Ling, L.; Zeng, L.; Wu, M.; Wu, Y.; Zhang, W.; Liu, Z. Successful treatment of a centenarian with coronavirus disease 2019 (COVID-19) using convalescent plasma. Transfus. Apher. Sci. 2020, 59, 102820.
- Hueso, T.; Pouderoux, C.; Péré, H.; Beaumont, A.-L.; Raillon, L.-A.; Ader, F.; Chatenoud, L.; Eshagh, D.; Szwebel, T.-A.; Martinot, M. Convalescent plasma therapy for B-cell–depleted patients with protracted COVID-19. Blood J. Am. Soc. Hematol. 2020, 136, 2290–2295.
- Hartman, W.R.; Hess, A.S.; Connor, J.P. Hospitalized COVID-19 patients treated with Convalescent Plasma in a mid-size city in the midwest. Transl. Med. Commun. 2020, 5, 17.
- Jamous, F.; Meyer, N.; Buus, D.; Ateeli, H.; Taggart, K.; Devasahayam, J.; Hanson, T.; Alzoubaidi, M.; Nazir, J. Critical Illness Due to Covid-19: A Description of the Surge in a Single Center in Sioux Falls. South Dak. Med. 2020, 73, 312–317.
- Olivares-Gazca, J.C.; Priesca-Marín, J.M.; Ojeda-Laguna, M.; Garces-Eisele, J.; Soto-Olvera, S.; Palacios-Alonso, A.; Izquierdo-Vega, J.; Chacon-Cano, R.; Arizpe-Bravo, D.; López-Trujillo, M.A. Infusion of convalescent plasma is associated with clinical improvement in critically ill patients with COVID-19: A pilot study. Rev. Investig. Clin. 2020, 72, 159–164.
- Tremblay, D.; Seah, C.; Schneider, T.; Bhalla, S.; Feld, J.; Naymagon, L.; Wang, B.; Patel, V.; Jun, T.; Jandl, T. Convalescent Plasma for the Treatment of Severe COVID-19 Infection in Cancer Patients. Cancer Med. 2020, 9, 8571–8578.
- Ye, M.; Fu, D.; Ren, Y.; Wang, F.; Wang, D.; Zhang, F.; Xia, X.; Lv, T. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J. Med. Virol. 2020, 92, 1890–1901.
- Worsley, K.J.; Taylor, J.; Carbonell, F.; Chung, M.; Duerden, E.; Bernhardt, B.; Lyttelton, O.; Boucher, M.; Evans, A. A Matlab toolbox for the statistical analysis of univariate and multivariate surface and volumetric data using linear mixed effects models and random field theory. NeuroImage 2009, 47, S102.
