Functional nanocomposites with biopolymers and zinc oxide (ZnO) nanoparticles is an emerging application of photocatalysis in antifouling coatings. The reduced chemical stability of ZnO photocatalyst in the acidic media in which chitosan is soluble affects the performance of chitosan nanocomposites in antifouling applications. In this study, a thin shell of amorphous tin oxide (SnOx) was grown on the surface of ZnO to form ZnO–SnOx core–shell nanoparticles that improved the chemical stability of the photocatalyst, as examined at pH 3 and 6. The photocatalytic activity of ZnO–SnOx in the degradation of methylene blue (MB) dye under visible light showed a higher efficiency than that of ZnO nanoparticles due to improved electron-hole separation. Meanwhile, the incorporation of photocatalysts into the chitosan matrix enhanced the thermal stability of the coatings. Chitosan-based antifouling coatings with varying percentages of ZnO or ZnO–SnOx nanoparticles, with or without the glutaraldehyde (GA) crosslinking of chitosan, were developed and studied for the prevention of biofouling.
- chitosan
- ZnO
- nanocomposite
- chemically resistant
- photocatalytic
- antifouling
1. Introduction
2. Characterization of ZnO–SnOx Core–Shell Nanoparticles
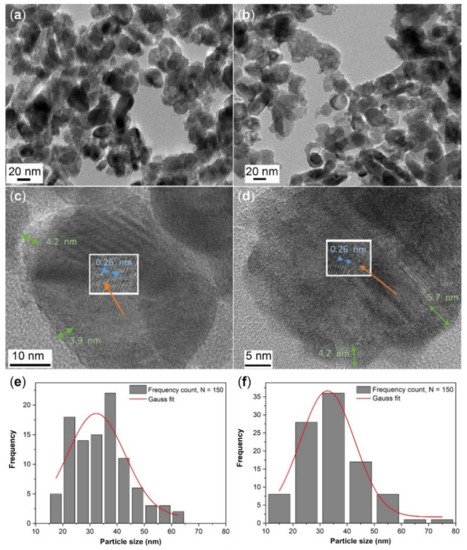
2.1. Microstructural Analysis
2.2. Colloidal Suspension and Stability
| Particles | Diameter (nm) | Zeta Potential (mV) |
|---|---|---|
| Bare ZnO | 68.4 ± 0.6 | 42.54 ± 0.27 |
| ZnO–SnOx (5 mM) | 230.6 ± 1.8 | 14.48 ± 0.34 |
| ZnO–SnOx (10 mM) | 250.3 ± 0.9 | 7.20 ± 0.14 |
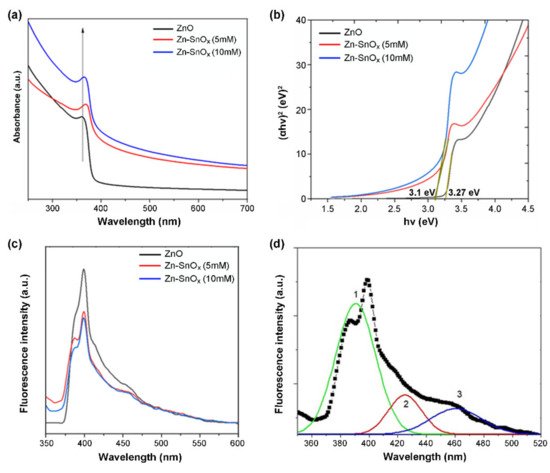
2.3. Spectroscopic Analysis
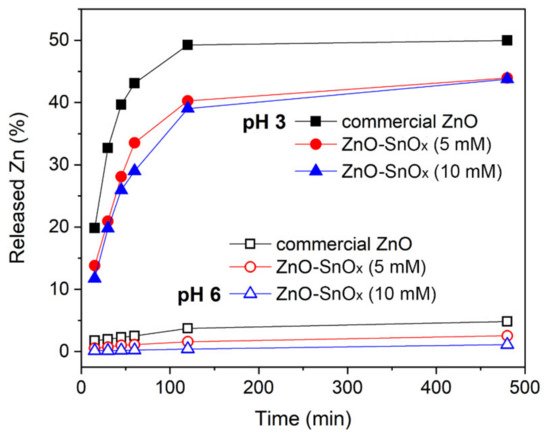
3. Enhancement in Chemical Stability of ZnO–SnOx Core–Shell Nanoparticles
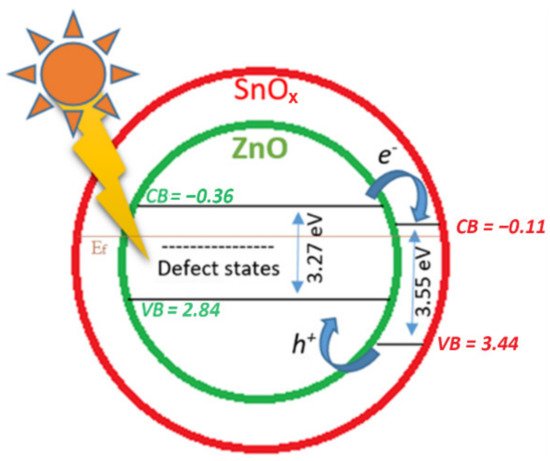
4. Discussion
This entry is adapted from the peer-reviewed paper 10.3390/ijms22094513
References
- Wahl, M. Marine epibiosis. I. Fouling and antifouling: Some basic aspects. Mar. Ecol. Prog. Ser. 1989, 58, 175–189.
- Schultz, M.P.; Bendick, J.A.; Holm, E.R.; Hertel, W.M. Economic impact of biofouling on a naval surface ship. Biofouling 2010, 27, 87–98.
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology—Past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004, 50, 75–104.
- Sonak, S.; Pangam, P.; Giriyan, A.; Hawaldar, K. Implications of the ban on organotins for protection of global coastal and marine ecology. J. Environ. Manag. 2009, 90, S96–S108.
- Dobretsov, S.; Thomason, J.C. The development of marine biofilms on two commercial non-biocidal coatings: A comparison between silicone and fluoropolymer technologies. Biofouling 2011, 27, 869–880.
- Sokolova, A.; Cilz, N.; Daniels, J.; Stafslien, S.J.; Brewer, L.H.; Wendt, D.E.; Bright, F.V.; Detty, M.R. A comparison of the antifouling/foul-release characteristics of non-biocidal xerogel and commercial coatings toward micro- and macrofouling organisms. Biofouling 2012, 28, 511–523.
- Kumar, S.; Ye, F.; Dobretsov, S.; Dutta, J. Chitosan nanocomposite coatings for food, paints, and water treatment applications. Appl. Sci. 2019, 9, 2409.
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63.
- Kumar, S.; Mudai, A.; Roy, B.; Basumatary, I.B.; Mukherjee, A.; Dutta, J. Biodegradable hybrid nanocomposite of chitosan/gelatin and green synthesized zinc oxide nanoparticles for food packaging. Foods 2020, 9, 1143.
- Kumar, S.; Shukla, A.; Baul, P.P.; Mitra, A.; Halder, D. Biodegradable hybrid nanocomposites of chitosan/gelatin and silver nanoparticles for active food packaging applications. Food Packag. Shelf Life 2018, 16, 178–184.
- Neto, C.G.T.; Giacometti, J.A.; Job, A.E.; Ferreira, F.C.; Fonseca, J.L.C.; Pereira, M.R. Thermal analysis of chitosan based networks. Carbohyd. Polym. 2005, 62, 97–103.
- Al-Naamani, L.; Dobretsov, S.; Dutta, J.; Burgess, J.G. Chitosan-zinc oxide nanocomposite coatings for the prevention of marine biofouling. Chemosphere 2017, 168, 408–417.
- Al-Fori, M.; Dobretsov, S.; Myint, M.T.Z.; Dutta, J. Antifouling properties of zinc oxide nanorod coatings. Biofouling 2014, 30, 871–882.
- Kumar, S.; Boro, J.C.; Ray, D.; Mukherjee, A.; Dutta, J. Bionanocomposite films of agar incorporated with ZnO nanoparticles as an active packaging material for shelf life extension of green grape. Heliyon 2019, 5, e01867.
- Arrieta, M.P.; Peponi, L.; López, D.; López, J.; Kenny, J.M. Chapter 12—An overview of nanoparticles role in the improvement of barrier properties of bioplastics for food packaging applications. In Food Packaging; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 391–424.
- Li, L.-H.; Deng, J.-C.; Deng, H.-R.; Liu, Z.-L.; Xin, L. Synthesis and characterization of chitosan/ZnO nanoparticle composite membranes. Carbohyd. Res. 2010, 345, 994–998.
- Al-Naamani, L.; Dobretsov, S.; Dutta, J. Chitosan-zinc oxide nanoparticle composite coating for active food packaging applications. Innov. Food Sci. Emerg. Technol. 2016, 38, 231–237.
- Jayasuriya, A.C.; Aryaei, A.; Jayatissa, A.H. ZnO nanoparticles induced effects on nanomechanical behavior and cell viability of chitosan films. Mater. Sci. Eng. C 2013, 33, 3688–3696.
- Girigoswami, K.; Viswanathan, M.; Murugesan, R.; Girigoswami, A. Studies on polymer-coated zinc oxide nanoparticles: UV-blocking efficacy and in vivo toxicity. Mater. Sci. Eng. C 2015, 56, 501–510.
- Karbowniczek, J.; Cordero-Arias, L.; Virtanen, S.; Misra, S.K.; Valsami-Jones, E.; Tuchscherr, L.; Rutkowski, B.; Górecki, K.; Bała, P.; Czyrska-Filemonowicz, A.; et al. Electrophoretic deposition of organic/inorganic composite coatings containing ZnO nanoparticles exhibiting antibacterial properties. Mater. Sci. Eng. C 2017, 77, 780–789.
- Naskar, A.; Khan, H.; Sarkar, R.; Kumar, S.; Halder, D.; Jana, S. Anti-biofilm activity and food packaging application of room temperature solution process based polyethylene glycol capped Ag-ZnO-graphene nanocomposite. Mater. Sci. Eng. C 2018, 91, 743–753.
- Bian, S.-W.; Mudunkotuwa, I.A.; Rupasinghe, T.; Grassian, V.H. Aggregation and dissolution of 4 nm ZnO nanoparticles in aqueous environments: Influence of pH, ionic strength, size, and adsorption of humic acid. Langmuir 2011, 27, 6059–6068.
- Wang, Q.Z.; Chen, X.G.; Liu, N.; Wang, S.X.; Liu, C.S.; Meng, X.H.; Liu, C.G. Protonation constants of chitosan with different molecular weightand degree of deacetylation. Carbohyd. Polym. 2006, 65, 194–201.
- Jóźwiak, T.; Filipkowska, U.; Szymczyk, P.; Rodziewicz, J.; Mielcarek, A. Effect of ionic and covalent crosslinking agents on properties of chitosan beads and sorption effectiveness of Reactive Black 5 dye. React. Funct. Polym. 2017, 114, 58–74.
- Azevedo, J.; Tilley, S.D.; Schreier, M.; Stefik, M.; Sousa, C.; Araujo, J.P.; Mendes, A.; Gratzel, M.; Mayer, M.T. Tin oxide as stable protective layer for composite cuprous oxide water-splitting photocathodes. Nano Energy 2016, 24, 10–16.
- Li, Z.; Wang, R.; Xue, J.; Xing, X.; Yu, C.; Huang, T.; Chu, J.; Wang, K.-L.; Dong, C.; Wei, Z.; et al. Core–shell 2 nanoparticles for efficient inorganic perovskite solar cells. J. Am. Chem. Soc. 2019, 141, 17610–17616.
- Fu, Q.-M.; Peng, J.-L.; Yao, Z.-C.; Zhao, H.-Y.; Ma, Z.-B.; Tao, H.; Tu, Y.-F.; Tian, Y.; Zhou, D.; Han, Y.-B. Highly sensitive ultraviolet photodetectors based on ZnO/SnO2 core-shell nanorod arrays. Appl. Surf. Sci. 2020, 527, 146923.
- Zhang, B.; Fu, W.; Li, H.; Fu, X.; Wang, Y.; Bala, H.; Sun, G.; Wang, X.; Wang, Y.; Cao, J.; et al. Actinomorphic ZnO/SnO2 core–shell nanorods: Two-step synthesis and enhanced ethanol sensing propertied. Mater. Lett. 2015, 160, 227–230.
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Variation of shell thickness in ZnO-SnO2 core-shell nanowires for optimizing sensing behaviors to CO, C6H6, and C7H8 gases. Sensor. Actuat. B Chem. 2020, 302, 127150.
- Zhu, H.-Y.; Xiao, L.; Jiang, R.; Zeng, G.-M.; Liu, L. Efficient decolorization of azo dye solution by visible light-induced photocatalytic process using SnO2/ZnO heterojunction immobilized in chitosan matrix. Chem. Eng. J. 2011, 172, 746–753.
- Ashraf, M.A.; Li, C.; Zhang, D.; Zhao, L.; Fakhri, A. Fabrication of silver phosphate-ilmenite nanocomposites supported on glycol chitosan for visible light-driven degradation, and antimicrobial activities. Int. J. Biol. Macromol. 2021, 169, 436–442.
- Wang, D.; Lin, J.; Huang, J.; Zhang, H.; Lin, S.; Chen, L.; Ni, Y.; Huang, L. A chitosan/dopamine-TiO2 composite nanofiltration membrane for antifouling in water purification. Cellulose 2021, 1–15.
- Kim, K.-M.; Choi, M.-H.; Lee, J.-K.; Jeong, J.; Kim, Y.-R.; Kim, M.-K.; Paek, S.-M.; Oh, J.-M. Physicochemical properties of surface charge-modified ZnO nanoparticles with different particle sizes. Int. J. Nanomed. 2014, 9 (Suppl. 2), 41–56.
- Kahouli, M.; Barhoumi, A.; Bouzid, A.; Al-Hajry, A.; Guermazi, S. Structural and optical properties of ZnO nanoparticles prepared by direct precipitation method. Superlattices Microstruct. 2015, 85, 7–23.
- Wang, L.; Li, J.; Wang, Y.; Yu, K.; Tang, X.; Zhang, Y.; Wang, S.; Wei, C. Construction of 1D SnO2-coated ZnO nanowire heterojunction for their improved n-butylamine sensing performances. Sci. Rep. 2016, 6, 35079.
- Geng, X.; Zhang, C.; Luo, Y.; Liao, H.; Debliquy, M. Light assisted room-temperature NO2 sensors with enhanced performance based on black SnO1-α@ZnO1-β@SnO2-γ nanocomposite coatings deposited by solution precursor plasma spray. Ceram. Int. 2017, 43, 5990–5998.
- Mahmoudi Chenari, H.; Zamiri, R.; Maria Tobaldi, D.; Shabani, M.; Rebelo, A.; Kumar, J.S.; Salehizadeh, S.A.; Graça, M.P.F.; Soares, M.J.; António Labrincha, J.; et al. Nanocrystalline ZnO–SnO2 mixed metal oxide powder: Microstructural study, optical properties, and photocatalytic activity. J. Sol-Gel Sci. Technol. 2017, 84, 274–282.
- Bora, T.; Sathe, P.; Laxman, K.; Dobretsov, S.; Dutta, J. Defect engineered visible light active ZnO nanorods for photocatalytic treatment of water. Catal. Today 2017, 284, 11–18.
- Thein, M.T.; Chim, J.E.; Pung, S.-Y.; Pung, Y.-F. Highly UV light driven WOx@ZnO nanocomposites synthesized by liquid impregnation method. J. Ind. Eng. Chem. 2017, 46, 119–129.
- Major, S.; Kumar, S.; Bhatnagar, M.; Chopra, K.L. Effect of hydrogen plasma treatment on transparent conducting oxides. Appl. Phys. Lett. 1986, 49, 394–396.
- Kim, W.; Choi, M.; Yong, K. Generation of oxygen vacancies in ZnO nanorods/films and their effects on gas sensing properties. Sensor Actuat. B-Chem. 2015, 209, 989–996.
- Dai, J.; Xu, C.; Guo, J.; Xu, X.; Zhu, G.; Lin, Y. Brush-like SnO2/ZnO hierarchical nanostructure: Synthesis, characterization and application in UV photoresponse. AIP Adv. 2013, 3, 062108.
- Haghighi, S.; Haghighatzadeh, A. Actinomorphic ZnO microneedles decorated with SnO2 nanospheres: Synthesis, characterization and optical studies. Appl. Phys. A 2020, 126, 107.
- Derikvandi, H.; Nezamzadeh-Ejhieh, A. A comprehensive study on electrochemical and photocatalytic activity of SnO2-ZnO/clinoptilolite nanoparticles. J. Mol. Catal. A Chem. 2017, 426, 158–169.
- Kumar, S.G.; Rao, K.S.R.K. Zinc oxide based photocatalysis: Tailoring surface-bulk structure and related interfacial charge carrier dynamics for better environmental applications. RSC Adv. 2015, 5, 3306–3351.
- Vo, D.-T.; Lee, C.-K. Cells capture and antimicrobial effect of hydrophobically modified chitosan coating on Escherichia coli. Carbohydr. Polym. 2017, 164, 109–117.
- Beppu, M.M.; Vieira, R.S.; Aimoli, C.G.; Santana, C.C. Crosslinking of chitosan membranes using glutaraldehyde: Effect on ion permeability and water absorption. J. Membrane Sci. 2007, 301, 126–130.
- Nowacki, K.; Galiński, M.; Stępniak, I. Synthesis and characterization of modified chitosan membranes for applications in electrochemical capacitor. Electrochim. Acta 2019, 320, 134632.
- Zhao, Z.; Zheng, J.; Wang, M.; Zhang, H.; Han, C.C. High performance ultrafiltration membrane based on modified chitosan coating and electrospun nanofibrous PVDF scaffolds. J. Membrane Sci. 2012, 394–395, 209–217.
- Amato, A.; Migneco, L.M.; Martinelli, A.; Pietrelli, L.; Piozzi, A.; Francolini, I. Antimicrobial activity of catechol functionalized-chitosan versus Staphylococcus epidermidis. Carbohyd. Polym. 2018, 179, 273–281.
