You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Biomedical
The loss of one or multiple fingers can lead to psychological problems as well as functional impairment. Various options exist for replacement and restoration after hand or finger loss. Prosthetic hand or finger prostheses improve esthetic outcomes and the quality of life for patients. Myoelectrically controlled hand prostheses have been used to attempt to produce different movements.
- finger loss
- hand loss
- finger prosthesis
- hand prostheses
- prostheses
- myoelectric control
- dental implant
1. Introduction
Hands and fingers have a vital role in daily life for various functions and esthetics. The loss of a hand or finger (one or more) may be a result of trauma, disease, or congenital abnormalities. The loss of hands and fingers can lead to psychological problems, functional impairment, and social dysfunction [1]. Various options exist for replacement and restoration after hand and finger loss. For finger loss, the amount of tissue loss, the extent of finger loss (carpel, metacarpal, phalanges), and the number of fingers lost must be considered in treatment planning [2][3]. Prosthetic hand or finger prostheses improve esthetic outcomes and quality of life for patients. Passive hand or finger prostheses are mostly fabricated from silicone retained from adhesive or as implants that are robust and difficult or impossible regarding movement. Such passive devices may be able to perform limited activities [4][5]; however, a bionic hand substantially improves the functional outcome [6].
There has been considerable development in the biomedical field due to the development of engineering and digital technologies [7][8][9][10]. Nowadays, commercial prosthetic hands are becoming advanced and offer a range of individual hand/finger movements. The use of myoelectrical control in hand prostheses to produce different movements is common in the literature [11][12][13]; however, the precise control of prosthetic hands or fingers is still a problem. Although different methods have been proposed to control a prosthetic hand or finger, the expected function is difficult to achieve. Various methods to command a prosthetic hand include a joystick, push buttons, keyboard, vision, speech, electromyography (EMG), electroencephalography (EEG), and electroneurography (ENG). Among all, electromyographical techniques are most commonly used in prosthetics. EMG is also sometimes called surface EMG (sEMG).
3. Signals and Finger Motor Function
Signals are important for the control of the myoelectrical (prosthetic) hands or fingers. Figure 1 shows a myoelectrically controlled hand prosthesis (designed in computer software), a design to collect EMG signals, and the generated signals [14].
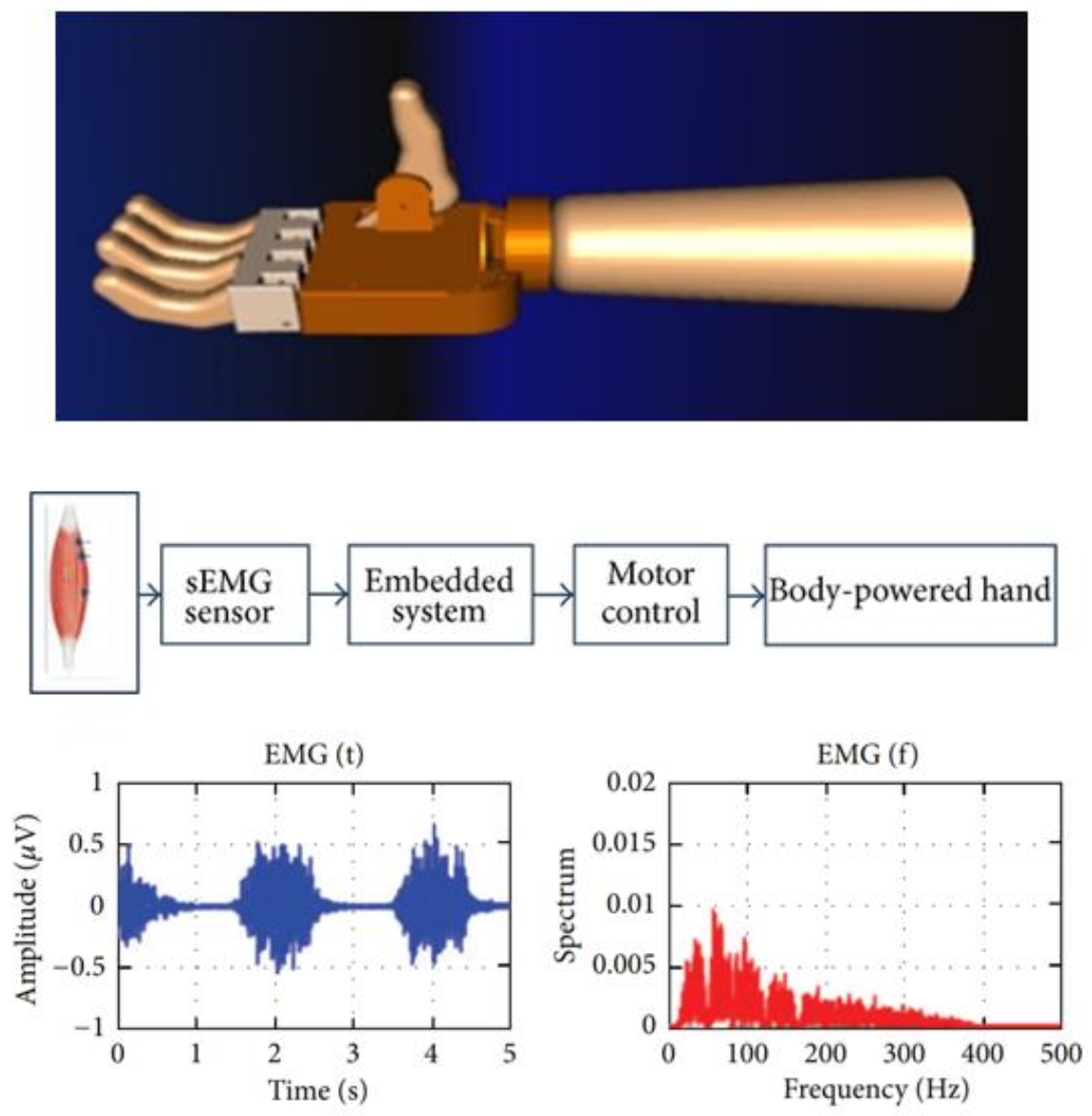 Figure 1. Myoelectrically controlled hand prosthesis designed in computer software, a design to collect the electromyography (EMG) signals, and sample generated signals [14].
Figure 1. Myoelectrically controlled hand prosthesis designed in computer software, a design to collect the electromyography (EMG) signals, and sample generated signals [14].The motor and sensory components of the hand provide an individual with high functionality and elegant behavior through a complex integrated system [15]. Human hands can perform numerous actions, varying from handling heavy objects to fine digital movements while directly connecting with the brain. It would ideal if similar movements can be performed by prosthetic hands and fingers. An intelligent interface that controls prosthetic hands and fingers can suggest movement by interpreting a muscle’s electrical activity via the surface EMG signal [16][17]. Figure 2 shows a myoelectrically controlled hand prosthesis, the generation of signals, and EMG channels [6]. The myoelectric activities of the residual limb for the phantom limb movements (PLM) may help for pattern recognition in the context of myoelectrical control [17].
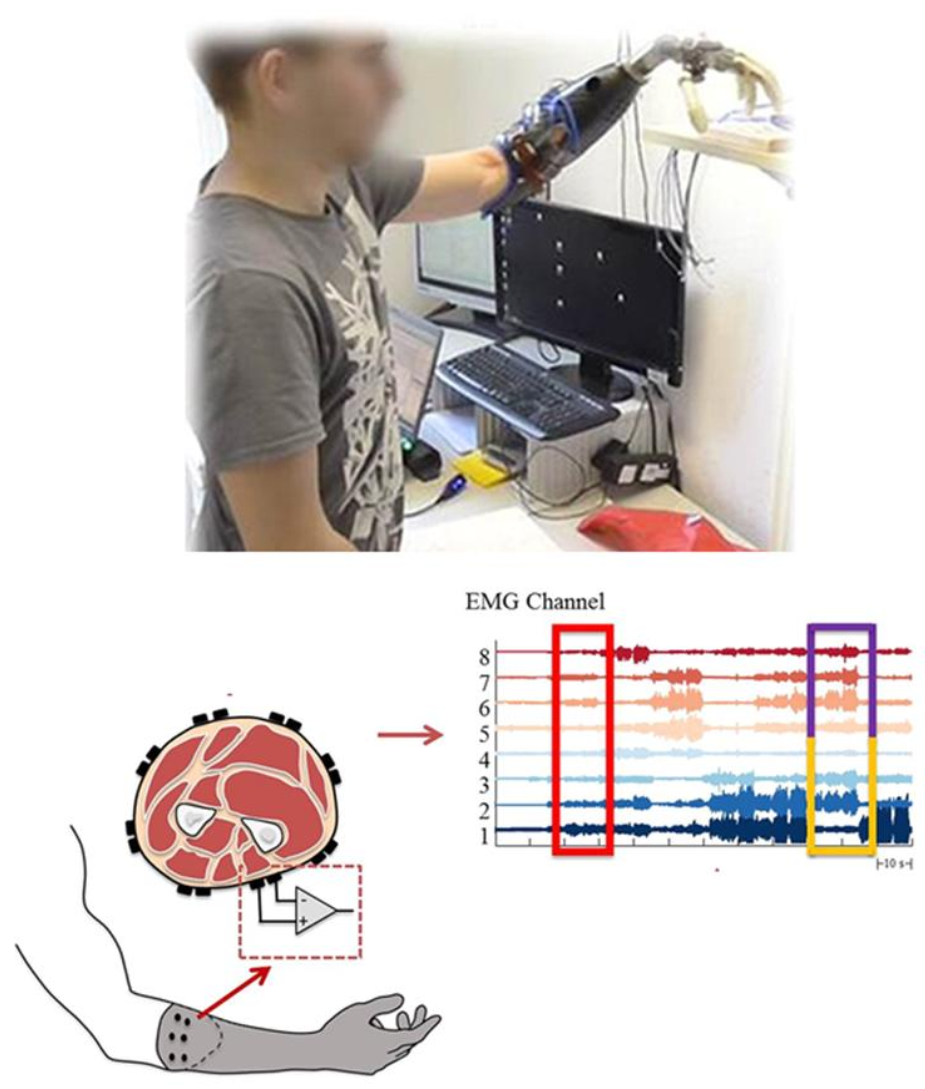 Figure 2. Active myoelectrically controlled hand prosthesis, generation of signals, and signals in the electromyography (EMG) [10].
Figure 2. Active myoelectrically controlled hand prosthesis, generation of signals, and signals in the electromyography (EMG) [10].Finger movements require specific stimulation of a group of muscles [18]. Such stimulation is principally controlled by the motor cortex through the corticospinal tract. The motor cortex’s output operates via corticospinal projection, which is important for the movements of the finger. Normally, an action potential propagates and activates a motor neuron which in turn activates the muscle fibers. When the depolarization of the postsynaptic membrane of muscle fiber takes place, the depolarization spreads along the fiber supplemented by the movement of ions and generates an electromagnetic field [19]. Myoelectric or EMG signals are read by microelectrodes and the signals are amplified, from which the muscle’s naturally generated electricity can be measured [16]. The signals are assigned to control the movement of the prosthesis after processing. Specific EMG pulse sequences are linked to a specific movement of the prosthesis. The predefined movements are controlled by specific signals [20].
The effectiveness of prosthetic hands can be assessed by user performance when performing functional tasks. Chadwell et al. [21] compared the upper limb activity between myoelectric prosthesis users and normal adults and found that temporal patterns in upper limb activity were present. It was also found that prosthesis users were greatly reliant on their intact limbs for daily activities. There was no significant correlation between the kinematic and the gaze-related performance between myoelectric prosthesis users and normal adults.
Access to the neural control information for an arm is lost during amputation. Hence, improving the function of hands or finger prosthesis poses a challenge. Various invasive methods have been proposed to generate proper signals for myoelectrical prostheses, such as peripheral nerve signal transmission [22], motor cortex [23], or targeted muscle reinnervation (TMR) [24] methods. In TMR, the muscle of the amputee is reinnervated with the residual nerves of the amputated limb. Following the reinnervation, the target muscles produce EMG signals, and these signals can control the prosthetic hand/arm and have promising results [25]. Engdahl et al. [26] studied four types of control for a prosthetic hand (TMR, myoelectric, cortical interfaces, and peripheral nerve interfaces) in upper-limb amputees and found that the participants showed a maximum positive response for the myoelectrical interface, while they showed the least interest for the cortical interface. More skillful individual and combined finger control has not yet been gained [27]. Khushaba et al. [27] investigated the accuracy of individual and combined finger movements using surface EMG signals using Bayesian data fusion in postprocessing for the proper classification of EMG data belonging to different movements. Similarly, Isaković et al. [28] purposed a feedback interface for a myoelectric prosthesis with multiple degrees of freedom that allows proprioceptive and sensory information (i.e., the grasping force). In this method, nine subjects performed a sequential algorithm in a time comparable to that reported when performed by experts and found that amplitudes can be significantly streamlined to a quarter of initial time without reducing the outcome when using prior knowledge in setting the baseline stimulation.
Atzori et al. [20] studied the natural control of robotic hands via sEMG using healthy subjects and amputees. A neural network was used to evaluate the effects of the pre-processing, layer architecture, data augmentation, and optimization. The results show that convolutional neural networks with simple architecture can produce precise results comparable to the average classical classification methods. They also show that several factors (pre-processing, net architecture, and parameters for optimization) are important for the analysis of sEMG data. More accuracy for computer vision and object recognition tasks is seen with larger networks.
Recently, multimodal data acquisition has been applied for the EMG-based detection of hand/finger movement [20][29][30][31]. Accelerometers can be combined with EMG electrodes to produce excellent capabilities to recognize hand movements [32]. Similarly, convolutional neural networks and deep learning have revolutionized machine learning, computer vision, and speech recognition. Deep learning is used in deep neural network applications and reinforcement learning to maximize the performance of a machine or software agent by itself making errors [33]. The neural networks have been applied to EMG hand movement recognition. The highest accuracy can be seen when combined convolutional neural networks with the retrained network. Table 1 shows the summary of developments in myoelectric finger prostheses and research outcomes.
Table 1. Summary on the developments in myoelectric finger prostheses.
| Year | Developments | Study |
|---|---|---|
| 1922 | Passive hooks and shoulder harness were designed by Weimar. | [34] |
| 1948 | The concept of the prosthesis ideally should replace not only mechanical function but also cutaneous and kinesthetic sensation. | [35] |
| 1948 | Myoelectric control was first implemented by Reinhold Reiter, a physics student at Munich University and patent application. | [36] |
| 1968 | The first commercial myoelectric hands were available from the middle of the 1960s. | [37] |
| 1968 | The concept of natural appearance during function “dynamic cosmesis” which contributes to the complexity of design both in terms of segmental trajectories and in terms of mechanical noise is highlighted. | [38] |
| 1970 onwards | Various advancements in the Myoelectric control prostheses through research and training systems. Availability of advanced commercial limb prostheses, such as the i-Limb from Touch Bionics, BeBionic from RSL Steeper, and Michelangelo by Ottobock. |
[11][14][19][20][21][24][39][40][41][42] |
Furthermore, EMG-driven prosthesis performance is influenced by various factors, such as electrode position shifting, varying muscle contraction levels, forearm orientation, and limb position [16]. In addition, Khushaba et al. [16] investigated the combined effect of forearm orientation and muscle contraction levels on the generalizability of EMG pattern recognition. They first verified whether the subjects could produce EMG activity in all different classes of movement at three muscular contraction levels (low, medium, and high) by analyzing the RMS of EMG activity across all channels, orientations, and movements. Figure 3 shows the representative radar plot of the EMG signals RMS values collected from one subject performing wrist flexion (C4) when the wrist was fully pronated. The RMS values from the different channels were then grouped in separate columns for each force level, thereby constructing an RMS matrix. They found a significant difference (p = 0.003) between the means of the different force levels. They also mentioned that multiple factors can influence the accuracy of EMG pattern recognition.
Figure 3. The results of root mean square (RMS) of the electromyogram (EMG) signals from six channels through flexion of the wrist at three different levels [16].
There are various myoelectric prostheses available in the market. Some commonly used models are iLimb, Vincent Hand, iLimb Pulse, SensorHand, BeBionic, BeBionic V2, and Michelangelo [43][44][45][46][47]. Table 2 shows the details of the myoelectric hand/finger prostheses.
Table 2. Various myoelectric hand/finger prostheses details
| Developed Year | Name | Producer | Size | Number of Joints | Degree of Freedom | Joint Coupling Method | Study |
|---|---|---|---|---|---|---|---|
| 2009 | i-Limb | Touch Bionics | 180–18-mm-long, 80–75-mm-wide, 35–41-mm-thick |
11 | 6 | Tendron linking MCP to PIP | [43] |
| 2010 | Vincent Hand | Vincent Systems | - | 11 | 6 | Linkage spanning MCP to PIP | [44] |
| 2010 | i-Limb Pulse | Touch Bionics | 180–182-mm-long, 80–75-mm-wide, 35–45-mm-thick |
11 | 6 | Tendron linking MCP to PIP | [43] |
| 2011 | SensorHand | Otto Bock | Glove sizes | 2 | 1 | DC Motor Worm Gear | [45] |
| 2011 | BeBionic | RSL Steeper | 198-mm-long, 90-mm-wide, 50-mm-thick |
11 | 6 | DC Motor Worm Gear | [46] |
| 2011 | BeBionic V2 | RSL Steeper | 190–200-mm-long, 84–92-mm-wide, 50-mm-thick |
11 | 6 | DC Motor Lead Screw | [46] |
| 2012 | Michelangelo | Otto Bock | - | 6 | 2 | - | [47] |
8. Conclusions
Motor cortex output through corticospinal projection is important in producing individuated finger movements. In the market, various myoelectrical hand prostheses are available but feature limited hand movement, control, and control speed. The use of a finger bone implant can aid the retention of myoelectrically controlled finger or hand prostheses regarding the production of different movements. Further research and development are necessary for the precise control of various prosthetic hand movements with sufficient speed and robust control.
This entry is adapted from the peer-reviewed paper 10.3390/app11104464
References
- Aydin, C.; Nemli, S.K.; Yilmaz, H. Esthetic, functional, and prosthetic outcomes with implant-retained finger prostheses. Prosthet. Orthot. Int. 2013, 37, 168–174.
- Aydin, C.; Karakoca, S.; Yilmaz, H. Implant-retained digital prostheses with custom-designed attachments: A clinical report. J. Prosthet. Dent. 2007, 97, 191–195.
- Manurangsee, P.; Isariyawut, C.; Chatuthong, V.; Mekraksawanit, S. Osseointegrated finger prosthesis: An alternative method for finger reconstruction. J. Hand Surg. 2000, 25, 86–92.
- Thongpulsawasdi, N.; Amornvit, P.; Rokaya, D.; Keawcharoen, K. Adhesive vs Implant Retained Fingers Prosthesis: A Comparative Study on Esthetic and Functional Outcome. World Appl. Sci. J. 2014, 29, 1015–1019.
- Rokaya, D.; Kitisubkanchana, J.; Wonglamsam, A.; Santiwong, P.; Srithavaj, T.; Humagain, M. Nepalese Esthetic Dental (NED) Proportion in Nepalese Population. Kathmandu Univ. Med. J. 2015, 13, 244–249.
- Aszmann, O.C.; Vujaklija, I.; Roche, A.D.; Salminger, S.; Herceg, M.; Sturma, A.; Hruby, L.A.; Pittermann, A.; Hofer, C.; Amsuess, S.; et al. Elective amputation and bionic substitution restore functional hand use after critical soft tissue injuries. Sci. Rep. 2016, 6, 34960.
- Humagain, M.; Rokaya, D. Integrating Digital Technologies in Dentistry to Enhance the Clinical Success. Kathmandu Univ. Med. J. 2019, 17, 256–257.
- Chang, Y.S. Advances in technology are changing the future of medicine. Asia Pac. Allergy 2016, 6, 137–138.
- Adam, N.R.; Wieder, R.; Ghosh, D. Data science, learning, and applications to biomedical and health sciences. Ann. N. Y. Acad. Sci. 2017, 1387, 5–11.
- Avetisyan, A.; Markaryan, M.; Rokaya, D.; Tovani-Palone, M.R.; Zafar, M.S.; Khurshid, Z.; Vardanyan, A.; Heboyan, A. Characteristics of Periodontal Tissues in Prosthetic Treatment with Fixed Dental Prostheses. Molecules 2021, 26, 1331.
- Weir, R.; Grahn, E.; Duff, S. A new externally powered, myoelectrically controlled prosthesis for persons with partial-hand amputations at the metacarpals. J. Prosthet. Orthot. 2001, 13, 26–31.
- Agnew, P.J. Functional effectiveness of a myo-electric prosthesis compared with a functional split-hook prosthesis: A single subject experiment. Prosthet. Orthot. Int. 1981, 5, 92–96.
- Pistohl, T.; Cipriani, C.; Jackson, A.; Nazarpour, K. Abstract and proportional myoelectric control for multi-fingered hand prostheses. Ann. Biomed. Eng. 2013, 41, 2687–2698.
- Salem, F.H.A.; Mohamed, K.S.; Mohamed, S.B.K.; Gehani, A.A.E. The development of body-powered prosthetic hand controlled by EMG signals using DSP processor with virtual prosthesis implementation. Conf. Pap. Eng. 2013, 598945.
- Montagnani, F.; Controzzi, M.; Cipriani, C. Independent long fingers are not essential for a grasping hand. Sci. Rep. 2016, 6, 35545.
- Khushaba, R.N.; Al-Timemy, A.; Kodagoda, S.; Nazarpour, K. Combined influence of forearm orientation and muscular contraction on EMG pattern recognition. Expert Syst. Appl. 2016, 61, 154–161.
- Touillet, A.; Peultier-Celli, L.; Nicol, C.; Jarrassé, N.; Loiret, I.; Martinet, N.; Paysant, J.; De Graaf, J.B. Characteristics of phantom upper limb mobility encourage phantom-mobility-based prosthesis control. Sci. Rep. 2018, 8, 15459.
- Schieber, M.H.; Lang, C.E.; Reilly, K.T.; McNulty, P.; Sirigu, A. Selective activation of human finger muscles after stroke or amputation. Adv. Exp. Med. Biol. 2009, 629, 559–575.
- Wang, N.; Lao, K.; Zhang, X. Design and myoelectric control of an anthropomorphic prosthetic hand. J. Bionic Eng. 2017, 14, 47–59.
- Atzori, M.; Cognolato, M.; Müller, H. Deep learning with convolutional neural networks applied to electromyography data: A resource for the classification of movements for prosthetic hands. Front. Neurorobot. 2016, 10, 9.
- Chadwell, A.; Kenney, L.; Granat, M.H.; Thies, S.; Head, J.; Galpin, A.; Baker, R.; Kulkarni, J. Upper limb activity in myoelectric prosthesis users is biased towards the intact limb and appears unrelated to goal-directed task performance. Sci. Rep. 2018, 8, 11084.
- Urbanchek, M.G.; Baghmanli, Z.; Moon, J.D.; Sugg, K.B.; Langhals, N.B.; Cederna, P.S. Quantification of regenerative peripheral interface signal transmission. Plast. Reconstr. Surg. 2012, 130, 55–56.
- Chestek, C.A.; Gilja, V.; Nuyujukian, P.; Foster, J.D.; Fan, J.M.; Kaufman, M.T.; Churchland, M.M.; Rivera-Alvidrez, Z.; Cunningham, J.P.; Ryu, S.I.; et al. Long-term stability of neural prosthetic control signals from silicon cortical arrays in rhesus macaque motor cortex. J. Neural Eng. 2011, 8, 045005.
- Kuiken, T.A.; Li, G.; Lock, B.A.; Lipschutz, R.D.; Miller, L.A.; Stubblefield, K.A.; Englehart, K.B. Targeted muscle reinnervation for real-time myoelectric control of multifunction artificial arms. JAMA 2009, 301, 619–628.
- Atzori, M.; Müller, H. Control capabilities of myoelectric robotic prostheses by hand amputees: A scientific research and market overview. Front. Neurosci. 2015, 9, 162.
- Engdahl, S.M.; Christie, B.P.; Kelly, B.; Davis, A.; Chestek, C.A.; Gates, D.H. Surveying the interest of individuals with upper limb loss in novel prosthetic control techniques. J. NeuroEng. Rehabil. 2015, 12, 53.
- Khushaba, R.N.; Kodagoda, S.; Takruri, M.; Dissanayake, G. Toward improved control of prosthetic fingers using surface electromyogram (EMG) signals. Exp. Syst. Appl. 2012, 39, 10731–10738.
- Isaković, M.; Malešević, J.; Keller, T.; Kostić, M.; Štrbac, M. Optimization of semiautomated calibration algorithm of multichannel electrotactile feedback for myoelectric hand prosthesis. Appl. Bionics Biomech. 2019, 9298758.
- Došen, S.; Cipriani, C.; Kostić, M.; Controzzi, M.; Carrozza, M.C.; Popović, D.B. Cognitive vision system for control of dexterous prosthetic hands: Experimental evaluation. J. NeuroEng. Rehabil. 2010, 7, 42.
- Light, C.M.; Chappell, P.H.; Hudgins, B.; Engelhart, K. Intelligent multifunction myoelectric control of hand prostheses. J. Med. Eng. Technol. 2002, 26, 139–146.
- Cotton, D.P.J.; Cranny, A.; Chappell, P.H.; White, N.M.; Beeby, S.P. Control strategies for a multiple degree of freedom prosthetic hand. Meas. Control. 2007, 40, 24–27.
- Gijsberts, A.; Atzori, M.; Castellini, C.; Müller, H.; Caputo, B. Movement error rate for evaluation of machine learning methods for sEMG-based hand movement classification. IEEE Trans. Neural. Syst. Rehabil. Eng. 2014, 22, 735–744.
- Mnih, V.; Kavukcuoglu, K.; Silver, D.; Rusu, A.A.; Veness, J.; Bellemare, M.G.; Graves, A.; Riedmiller, M.; Fidjeland, A.K.; Ostrovski, G.; et al. Human-level control through deep reinforcement learning. Nature 2015, 518, 529–533.
- Lange, F. Lehrbuch der Orthopädie; Gustav Fischer: New York, NY, USA, 1922.
- Weiner, N. Cybernetics, or Control and Communication in the Animal and the Machine; Wiley: New York, NY, USA, 1948.
- Reiter, R. Eine neue Elektrokunsthand. Grenzgeb Med. 1968, 4, 133–135.
- Sherman, E.D. A Russian bioelectric-controlled prosthesis: Report of a research team from the Rehabilitation Institute of Montreal. Can. Med. Assoc. J. 1964, 91, 1268–1270.
- Scott, R.N.; Tucker, F.R. Surgical implications of myoelectric control. Clin. Orthop. Relat. Res. 1968, 61, 248–260.
- Dunfield, V.A.; Scott, R.N. A surgically implanted myotelemetry unit. In Proceedings of the 3rd Canadian Medical and Biological Engineering Conference, Halifax, NS, Canada, 9–11 September 1970; p. 32.
- Herberts, P.; Petersen, I. Possibilities for control of powered devices by myoelectric signals. Scand. J. Rehabil. Med. 1970, 2, 164–170.
- Englehart, K.B.; Hudgins, B. A robust, real-time control scheme for multifunction myoelectric control. IEEE Trans. Biomed. Eng. 2003, 50, 848–854.
- Belter, J.T.; Segil, J.L.; Dollar, A.M.; Weir, R.F. Mechanical design and performance specifications of anthropomorphic prosthetic hands: A review. J. Rehabil. Res. Dev. 2013, 50, 599–618.
- Touch Bionics. Available online: (accessed on 25 March 2021).
- Vincent Hand. Available online: (accessed on 25 March 2021).
- SensorHand Speed. Available online: (accessed on 25 March 2021).
- RSL Steeper. Available online: (accessed on 25 March 2021).
- Michelangelo Prosthetic Hand. Available online: (accessed on 25 March 2021).
This entry is offline, you can click here to edit this entry!
