Allograft inflammatory factor-1 (AIF-1) is a calcium-binding scaffold/adaptor protein often associated with inflammatory diseases. Originally cloned from active macrophages in humans and rats, this gene has also been identified in other vertebrates and in several invertebrate species. Among metazoans, AIF-1 protein sequences remain relatively highly conserved. Generally, the highest expression levels of AIF-1 are observed in immunocytes, suggesting that it plays a key role in immunity. In mammals, the expression of AIF-1 has been reported in different cell types such as activated macrophages, microglial cells, and dendritic cells. Its main immunomodulatory role during the inflammatory response has been highlighted. Among invertebrates, AIF-1 is involved in innate immunity, being in many cases upregulated in response to biotic and physical challenges. AIF-1 transcripts result ubiquitously expressed in all examined tissues from invertebrates, suggesting its participation in a variety of biological processes, but its role remains largely unknown.
- AIF-1
- Iba1
- immunity
- macrophages
- invertebrates
- inflammation
- bacterial challenge
1. Vertebrata
2. Invertebrata
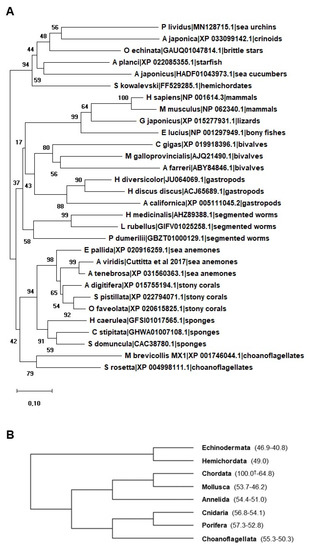

2.1. Porifera
2.2. Cnidaria
2.3. Mollusca
2.4. Annelida
2.5. Echinodermata
This entry is adapted from the peer-reviewed paper 10.3390/biology9110355
References
- Chen, Z.W.; Ahren, B.; Östenson, C.G.; Cintra, A.; Bergman, T.; Moller, C.; Fuxe, K.; Mutt, V.; Jörnvall, H.; Efendic, S. Identification, isolation, and characterization of daintain (allograft inflammatory factor 1), a macrophage polypeptide with effects on insulin secretion and abundantly present in the pancreas of prediabetic BB rats. Proc. Natl. Acad. Sci. USA 1997, 94, 13879–13884.
- Schluesener, H.J.; Seid, K.; Kretzschmar, J.; Meyermann, R. Allograft-inflammatory factor-1 in rat experimental autoimmune encephalomyelitis, neuritis, and uveitis: Expression by activated macrophages and microglial cells. Glia 1998, 24, 244–511.
- Autieri, M.V. cDNA cloning of human allograft inflammatory factor-1: Tissue distribution, cytokine induction, and mRNA expression in injured rat carotid arteries. Biochem. Biophys. Res. Commun. 1996, 228, 29–37.
- Kuschel, R.; Deininger, M.H.; Meyermann, R.; Bornemann, A.; Yablonka-Reuveni, Z.; Schluesener, H.J. Allograft inflammatory factor-1 is expressed by macrophages in injured skeletal muscle and abrogates proliferation and differentiation of satellite cells. J. Neuropathol. Exp. Neurol. 2000, 59, 323–332.
- Autieri, M.V.; Carbone, C.; Mu, A. Expression of allograft inflammatory factor-1 is a marker of activated human vascular smooth muscle cells and arterial injury. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1737–1744.
- Elizondo, D.M.; Andargie, T.E.; Yang, D.; Kacsinta, A.D.; Lipscomb, M.W. Inhibition of allograft inflammatory factor-1 in dendritic cells restrains CD4+ T cell effector responses and induces CD25+Foxp3+ T regulatory subsets. Front. Immunol. 2017, 8, 1502.
- Elizondo, D.M.; Andargie, T.E.; Haddock, N.L.; da Silva, R.L.L.; de Moura, T.R.; Lipscomb, M.W. IL-10 producing CD8+ CD122+ PD-1+ regulatory T cells are expanded by dendritic cells silenced for Allograft Inflammatory Factor-1. J. Leukoc. Biol. 2018, 105, 123–130.
- Miyata, M.; Iinuma, K.; Miyazaki, T. DNA cloning and characterization of an allograft inflammatory factor-1 homologue in red sea bream (Chrysophrys major). Aquaculture 2001, 194, 63–74.
- Elizondo, D.M.; Brandy, N.Z.D.; Louzada da Silva, R.; Haddock, N.; Haddock, N.; Kacsinta, A.; Moura, T.; Lipscomb, M. Allograft Inflammatory Factor-1 governs hematopoietic stem cell differentiation into cDC1 and monocyte-derived dendritic cells through IRF8 and RelB in vitro. Front. Immunol. 2019, 10, 173.
- Yang, Z.F.; Ho, D.W.; Lau, C.K.; Lam, C.T.; Lum, C.T.; Poon, R.T.; Fan, S.T. Allograft inflammatory factor-1 (AIF-1) is crucial for the survival and pro-inflammatory activity of macrophages. Int. Immunol. 2005, 17, 1391–1397.
- Tian, Y.; Kelemen, S.E.; Autieri, M.V. Inhibition of AIF-1 expression by constitutive siRNA expression reduces macrophage migration, proliferation, and signal transduction initiated by atherogenic stimuli. Am. J. Physiol. Cell Physiol. 2006, 290, C1083–C1091.
- Zhao, Y.Y.; Huang, X.Y.; Chen, Z.W. Daintain/AIF-1 (Allograft Inflammatory Factor-1) accelerates type 1 diabetes in NOD mice. Biochem. Biophys. Res. Commun. 2012, 427, 513–517.
- Sommerville, L.J.; Kelemen, S.E.; Ellison, S.P.; England, R.N.; Autieri, M.V. Increased atherosclerosis and vascular smooth muscle cell activation in AIF-1 transgenic mice fed a high-fat diet. Atherosclerosis 2012, 220, 45–52.
- Deininger, M.H.; Weinschenk, T.; Meyermann, R.; Schluesener, H.J. The allograft inflammatory factor-1 in Creutzfeldt-Jakob disease brains. Neuropathol. Appl. Neurobiol. 2003, 29, 389–399.
- Sikora, M.; Kopeć, B.; Piotrowska, K.; Pawlik, A. Role of allograft inflammatory factor-1 in pathogenesis of diseases. Immunol. Lett. 2020, 218, 1–4.
- Kimura, M.; Kawahito, Y.; Obayashi, H.; Ohta, M.; Hara, H.; Adachi, T.; Tokunaga, D.; Hojo, T.; Hamaguchi, M.; Omoto, A.; et al. A critical role for allograft inflammatory factor-1 in the pathogenesis of rheumatoid arthritis. J. Immunol. 2007, 178, 3316–3322.
- Harney, S.M.; Vilariño-Güell, C.; Adamopoulos, I.E.; Sims, A.M.; Lawrence, R.W.; Cardon, L.R.; Newton, J.L.; Meisel, C.; Pointon, J.J.; Darke, C.; et al. Fine mapping of the MHC Class III region demonstrates association of AIF1 and rheumatoid arthritis. Rheumatol. Oxf. 2008, 47, 1761–1767.
- Pawlik, A.; Kurzawski, M.; Szczepanik, T.; Dziedziejko, V.; Safranow, K.; Borowiec-Chłopek, Z.; Giedrys-Kalemba, S.; Drozdzik, M. Association of allograft inflammatory factor-1 gene polymorphism with rheumatoid arthritis. Tissue Antigens 2008, 72, 171–175.
- Berglund, L.M.; Kotova, O.; Osmark, P.; Grufman, H.; Xing, C.; Lydrup, M.-L.; Goncalves, I.; Autieri, M.V.; Gomez, M.F. NFAT regulates the expression of AIF-1 and IRT-1: Yin and yang splice variants of neointima formation and atherosclerosis. Cardiovasc. Res. 2012, 93, 414–423.
- Albiero, M.; Rattazzi, M.; Menegazzo, L.; Boscaro, E.; Cappellari, R.; Pagnin, E.; Bertacco, E.; Poncina, N.; Dyar, K.; Ciciliot, S.; et al. Myeloid calcifying cells promote atherosclerotic calcification via paracrine activity and allograft inflammatory factor-1 overexpression. Basic Res. Cardiol. 2013, 108, 368.
- Yu, Z.; Song, Y.B.; Cui, Y.; Fu, A.Q. Effects of AIF-1 inflammatory factors on the regulation of proliferation of breast cancer cells. J. Biol. Regul. Homeost. Agents 2019, 33, 1085–1095.
- Liu, S.; Tan, W.-Y.; Chen, Q.-R.; Chen, X.-P.; Fu, K.; Zhao, Y.-Y.; Chen, Z.-W. Daintain/AIF-1 promotes breast cancer proliferation via activation of the NF-kB/cyclin D1 pathway and facilitates tumor growth. Cancer Sci. 2008, 99, 952–957.
- Prinz, M.; Priller, J. Microglia and brain macrophages in the molecular age: From origin to neuropsychiatric disease. Nat. Rev. Neurosci. 2014, 15, 300–312.
- Zhao, Y.Y.; Yan, D.-J.; Chen, Z.-W. Role of AIF-1 in the regulation of inflammatory activation and diverse disease processes. Cell. Immunol. 2013, 284, 75–83.
- Beschorner, R.; Engel, S.; Mittelbronn, M.; Adjodah, D.; Dietz, K.; Schluesener, K.J.; Meyermann, R. Differential regulation of the monocytic calcium-binding peptides macrophage-inhibiting factor related protein-8 (MRP8/S100A8) and allograft inflammatory factor-1 (AIF-1) following human traumatic brain injury. Acta Neuropathol. 2000, 100, 627–634.
- Schwab, J.M.; Eveline Frei, E.; Klusman, I.; Schnell, L.; Schwab, M.E.; Schluesener, H.J. AIF-1 expression defines a proliferating and alert microglialrmacrophage phenotype following spinal cord injury in rats. J. Neuroimmunol. 2001, 119, 214–222.
- Postler, E.; Rimner, A.; Beschorner, R.; Schluesener, H.J.; Meyermann, R. Allograft-Inflammatory-factor-1 is upregulated in microglial cells in human cerebral infarctions. J. Neuroimmunol. 2000, 104, 85–91.
- Kruse, M.; Steffen, R.; Batel, R.; Müller, I.M.; Müller, W.E.G. Differential expression of allograft inflammatory factor 1 and of glutathione peroxidase during auto- and allograft response in marine sponges. J. Cell. Sci. 1999, 112, 4305–4313.
- Müller, W.E.G.; Krasko, A.; Skorokhod, A.; Bünz, C.; Grebenjuk, V.A.; Steffen, R.; Batel, R.; Schröder, H.C. Histocompatibility reaction in tissue and cells of the marine sponge Suberites domuncula in vitro and in vivo: Central role of the allograft inflammatory factor 1. Immunogenetics 2002, 54, 48–58.
- Deininger, M.H.; Meyermann, R.; Schluesener, H.J. The allograft inflammatory factor-1 family of proteins. FEBS Lett. 2002, 514, 115–121.
- Cuttitta, A.; Ragusa, M.A.; Costa, S.; Bennici, C.; Colombo, P.; Mazzola, S.; Gianguzza, F.; Nicosia, A. Evolutionary conserved mechanisms pervade structure and transcriptional modulation of allograft inflammatory factor-1 from sea anemone Anemonia viridis. Fish Shellfish Immunol. 2017, 67, 86–94.
- Wang, K.-J.; Rena, H.-L.; Xua, D.-D.; Caia, L.; Yanga, M. Identification of the up-regulated expression genes in hemocytes of variously colored abalone (Haliotis diversicolor Reeve, 1846) challenged with bacteria. Dev. Comp. Immun. 2008, 32, 1326–1347.
- Zhang, L.; Zhao, J.; Li, C.; Su, X.; Chen, A.; Li, T.; Qin, S. Cloning and characterization of allograft inflammatory factor-1 (AIF-1) from manila clam Venerupis philippinarum. Fish Shellfish Immunol. 2011, 30, 148–153.
- Li, J.; Chen, J.; Zhang, Y.; Yu, Z. Expression of allograft inflammatory factor-1 (AIF-1) in response to bacterial challenge and tissue injury in the pearl oyster, Pinctada martensii. Fish Shellfish Immunol. 2013, 34, 365–371.
- Zhang, Y.; Li, J.; Yu, F.; He, X.; Yu, Z. Allograft inflammatory factor-1 stimulates hemocyte immune activation by enhancing phagocytosis and expression of inflammatory cytokines in Crassostrea gigas. Fish Shellfish Immunol. 2013, 34, 1071–1077.
- Xu, T.; Xie, J.; Zhu, B.; Liu, X.; Wu, X. Allograft inflammatory factor 1 functions as a pro-inflammatory cytokine in the oyster, Crassostrea ariakensis. PLoS ONE 2014, 9, e95859.
- Li, Q.; Bai, Z.; Zhao, L.; Li, J. Characterization of allograft inflammatory factor-1 in Hyriopsis cumingii and its expression in response to immune challenge and pearl sac formation. Fish Shellfish Immunol. 2016, 59, 241–249.
- Wang, J.; Zhang, H.; Wang, L.; Qiu, L.; Yue, F.; Yang, C.; Song, L. Molecular cloning and transcriptional regulation of an allograft inflammatory factor-1 (AIF-1) in Zhikong scallop Chlamys farreri. Gene 2013, 530, 178–184.
- De Zoysa, M.; Nikapitiya, C.; Kim, Y.; Oh, C.; Kang, D.H.; Whang, I.; Kim, S.-J.; Lee, J.-S.; Choi, C.Y.; Lee, J. Allograft inflammatory factor-1 in disk abalone (Haliotis discus discus): Molecular cloning, transcriptional regulation against immune challenge and tissue injury. Fish Shellfish Immunol. 2010, 29, 319–326.
- Park, J.M.; Greten, F.R.; Wong, A.; Westrick, R.J.; Arthur, J.S.; Otsu, K.; Hoffmann, A.; Montminy, M.; Karin, M. Signaling pathways and genes that inhibit pathogen-induced macrophage apoptosis—CREB and NF-kB as key regulators. Immunity 2005, 23, 319–329.
- Tang, X.; Marciano, D.L.; Leeman, S.E.; Amar, S. LPS induces the interaction of a transcription factor, LPS-induced TNF-alpha factor, and STAT6(B) with effects on multiple cytokines. Proc. Natl. Acad. Sci. USA 2005, 102, 5132–5137.
- Kuchel, R.P.; Raftos, D.A.; Birch, D.; Vella, N. Haemocyte morphology and function in the Akoya pearl oyster, Pinctada imbricata. J. Invertebr. Pathol. 2010, 105, 36–48.
- Beltran, C.G.G.; Coyne, V.E. iTRAQ-based quantitative proteomic profiling of the immune response of the South African abalone, Haliotis midae. Fish Shellfish Immunol. 2020, 99, 130–143.
- Gust, M.; Fortier, M.; Garric, J.; Fournier, M.; Gagné, F. Effects of short-term exposure to environmentally relevant concentrations of different pharmaceutical mixtures on the immune response of the pond snail Lymnaea stagnalis. Sci. Total Environ. 2013, 445–446, 210–218.
- Drago, F.; Sautière, P.-E.; Le Marrec-Croq, F.; Accorsi, A.; Van Camp, C.; Salzet, M.; Lefebvre, C.; Vizioli, J. Microglia of medicinal leech (Hirudo medicinalis) express a specific activation marker homologous to vertebrate ionized calcium-binding adapter molecule 1 (Iba1/alias Aif-1). Dev. Neurobiol. 2014, 74, 987–1001.
- Geirsdottir, L.; David, E.; Keren-Shaul, H.; Weiner, A.; Bohlen, S.C.; Neuber, J.; Balic, A.; Giladi, A.; Sheban, F.; Dutertre, C.A.; et al. Cross-Species single-cell analysis reveals divergence of the primate microglia program. Cell 2019, 179, 1609–1622.
- Schorn, T.; Drago, F.; Tettamanti, G.; Valvassori, R.; de Eguileor, M.; Vizioli, J.; Grimaldi, A. Homolog of allograft inflammatory factor-1 induces macrophage migration during innate immunity response in leech. Cell Tissue Res. 2014, 359, 853–864.
- Baranzini, N.; Monti, L.; Vanotti, M.; Orlandi, V.T.; Bolognese, F.; Scaldaferri, D.; Girardello, R.; Tettamanti, G.; de Eguileor, M.; Vizioli, J.; et al. AIF-1 and RNASET2 Play Complementary Roles in the Innate Immune Response of Medicinal Leech. J. Innate Immun. 2019, 11, 150–167.
- Baranzini, N.; Pulze, L.; Acquati, F.; Grimaldi, A. Hirudo verbana as an alternative model to dissect the relationship between innate immunity and regeneration. Invertebr. Surviv. J. 2020, 17, 90–98.
- Pulze, L.; Baranzini, N.; Girardello, R.; Grimaldi, A.; Ibba-Manneschi, L.; Ottaviani, E.; Reguzzoni, M.; Tettamanti, G.; de Eguileor, M. A new cellular type in invertebrates: First evidence of telocytes in leech Hirudo medicinalis. Sci. Rep. 2017, 7, 13580.
- Cammarata, M.; Pagliara, P. Elie Metchnikoff and the multidisciplinary link novelty among Zoology, Embryology and Innate Immunity. Invertebr. Surviv. J. 2018, 15, 234–239.
- Metchnikoff, E. Phagocytosis and Immunity; British Medical Association: London, UK, 1891.
- Hildemann, W.H.; Dix, T.G. Transplantation reactions of tropical Australian echinoderms. Transplantation 1972, 15, 624–633.
- Karp, R.D.; Hildemann, W.H. Specific allograft reactivity in the sea star Dermasterias imbricata. Transplantation 1976, 22, 434–439.
- Coffaro, K.A.; Hinegardner, R.T. Immune response in the sea urchin Lytechinus pictus. Science 1977, 197, 1389–1390.
- Coffaro, K.A. Memory and specificity in the sea urchin Lytechinus pictus. In Phylogeny of Immunological Memory; Manning, M.J., Ed.; Elsevier/North-Holland Biomedical Press: New York, NY, USA, 1980; pp. 77–80.
- Nair, S.V.; Del Valle, H.; Gross, P.S.; Terwilliger, D.P.; Smith, L.C. Microarray analysis of coelomocyte gene expression in response to LPS in the sea urchin. Identification of unexpected immune diversity in an invertebrate. Physiol. Genom. 2005, 22, 33–47.
- Ovando, F.; Gimpel, C.; Cardenas, C.; Da Silva, M.C., Jr.; De Lorgeril, J.; Gonzalez, M. Cloning and expression analysis of allograft inflammatory factor type 1 in coelomocytes of antarctic sea urchin (Sterechinus neumayeri). J. Shellfish Res. 2012, 31, 875–883.
- Barca, A.; Vacca, F.; Vizioli, J.; Drago, F.; Vetrugno, C.; Verri, T.; Pagliara, P. Molecular and expression analysis of the Allograft inflammatory factor 1 (AIF-1) in the coelomocytes of the common sea urchin Paracentrotus lividus. Fish Shell. Immunol. 2017, 71, 136–143.
- Chiaramonte, M.; Arizza, V.; La Rosa, S.; Queiroz, V.; Mauro, M.; Vazzana, M.; Inguglia, L. Allograft Inflammatory Factor AIF-1: Early immune response in the Mediterranean sea urchin Paracentrotus lividus. Zoology 2020, 142, 1–8.
- Ji, N.; Chang, Y.; Zhao, C.; Pang, Z.; He, Z. Cloning and gene expression of allograft inflammatory factor-1 (AIF-1) provide new insights into injury and bacteria response of the sea cucumber Apostichopus japonicus (Selenka, 1867). Fish Shellfish Immunol. 2014, 38, 400–405.
