Natural biopolymers are an interesting resource for edible films production, as they are environmentally friendly packaging materials.
- biopolymers
- edible films and coatings
- dry and wet processes
- mechanical properties
- permeability
1. Introduction
Inferior packaging or its absence causes significant food loss (about 20–25 %) due to microbiological contamination and oxidative processes, which lead to a decrease in the quality of food products and makes them unsuitable for consumption [1].
The development and application of bioactive packaging systems is a relevant field of research. The application of such smart packaging systems is a tool for protecting food from spoilage and reducing the risk of growth of pathogenic microorganisms due to both the creation of a barrier and the action of active components at the border of the product with the packaging [2][3]. The currently used packaging materials are mainly produced from petrochemical products [4][5]. The global problem of environmental pollution makes alternative environmentally friendly and biodegradable polymers be in demand [6].
Every year, the problem of recycling polymer packaging materials becomes more acute [7] due to its accumulation in large quantities, which cause significant harm to the environment [8]. The incineration or pyrolysis of polymer waste, to some extent, solves the problem of their accumulation in landfills, but does not contribute to improving the overall environmental situation [9]. Recycling of polymer waste is more environmentally friendly, but in this case, significant labor and energy costs are required for sorting polymer materials and their subsequent processing [10][11]. It should be noted that the recycling of polymers is carried out a limited number of times, after which the problem of burial or incineration of these materials arises again [12].
Concerning environmental suitability, biopolymers are environmentally friendly packaging materials [13][14]. The main advantage of their use as bioplastics is the closed natural cycle, where the end of one cycle leads to the beginning of the next cycle [6].
Biopolymers can be divided into three main categories depending on the origin and method of production (Figure 1): directly extracted from biomass, synthesized bio-derived monomers, and produced by microorganisms [15]. Polysaccharides and proteins are the most promising biopolymers for the production of packaging materials [16][17]. Proteins are heteropolymers consisting of α-amino acids as monomeric units. The combinations of 20 amino acids to form a protein sequence allow for an almost unlimited number of various polymer chains with different physical and chemical properties. Proteins also contain a large number of functional groups that can be changed enzymatically, chemically, or physically for varying the properties of the films [16]. Polysaccharides are good candidates for replacing oil-based polymers due to their ability to form a film, affinity for paper-based materials, an appropriate barrier to gases and aromas, and good mechanical strength. Moreover, these biopolymers are biodegradable, non-toxic, and are used as a matrix for the inclusion of additives with specific functionality, such as active antimicrobial properties, for example [17].
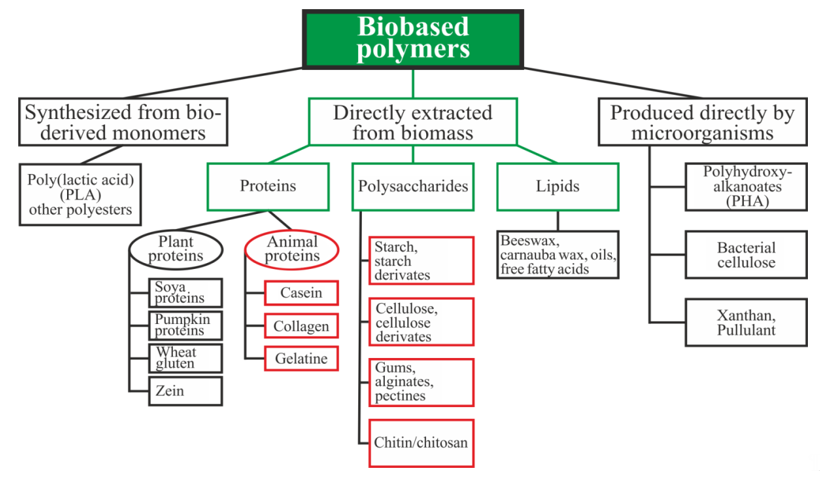
Figure 1. Schematic classification of biopolymer types [15][18][19]. Reproduced with permission from Popović, S.Z. et al., Biopolymers for Food Design; published by Elsevier Inc., 2018.
The prefix "bio" indicates that biopolymers are biodegradable. The word "biodegradable" means that materials can be decomposed by bacteria, fungi, and yeast to the final products of biomass under anaerobic conditions—hydrocarbons and methane [20]. These types of polymers consist of monomers that are covalently connected, forming a chain of the molecule. They are also produced inside the cell as a result of complex metabolic processes. Biopolymers can be used for food packaging as a replacement for oil-based plastics made from petroleum due to their biodegradability, renewability, and wide distribution [21].
Environmental friendliness is a key ideology nowadays. The use of biopolymers from renewable resources could solve global plastic pollution. For many years, researchers have been trying to develop and design packaging materials based on natural biopolymers. However, animal proteins and natural polysaccharides are characterized by some undesirable properties caused by their chemical nature and structure. These disadvantages reduce their competitiveness with oil-based plastics but can be overcome.
2. Biopolymers Used for Food Packaging
Biopolymers are widely implemented and can be used as coatings and films [4][22]. Coating involves the formation of a cover directly on the surface of food products, whereas films are structures that are used separately after formation [23].
Materials based on biopolymers must meet the basic requirements of health safety, mechanical and chemical resistance, and durability [24][25]. Therefore, food packaging should not only be biodegradable, but also functional. Compared to synthetic polymers derived from petroleum, biopolymers have a more complex chemical structure and side chain structure, which provides additional opportunities for the formation of packaging materials with specific characteristics for specific purposes [18][20].
Biopolymers directly extracted from biomass as polysaccharides and animal proteins, which are most often used for the preparation of packaging materials for food products, will be considered in this review [16][26][27].
2.1. Starch
Starch is one of the most readily available polysaccharides on the planet [28]. The plants from which it is obtained grow in almost all temperate climate zones. Corn, wheat, potatoes, and rice are the world leaders: 84 %, 7 %, 4 %, and 1%, respectively [29]. This biopolymer is a mixture of amylose and amylopectin (Figure 2), the ratio of which varies depending on the type of starch.
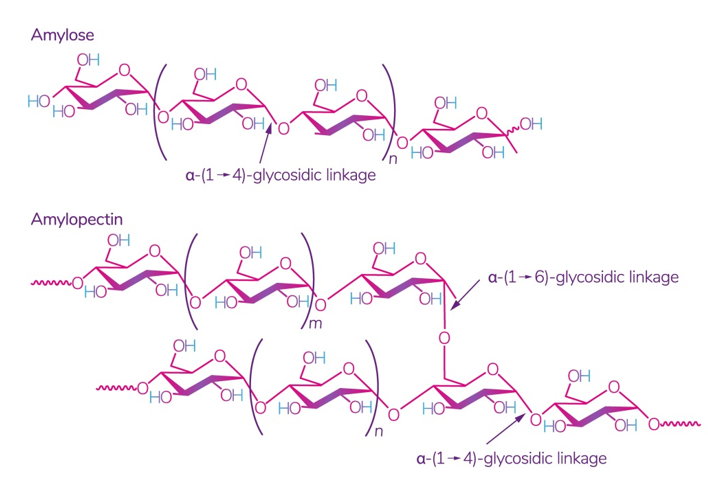
Figure 2. Structure of amylose and amylopectin [30]. Reproduced from Kadokawa, Polymers; published by MDPI, Basel, Switzerland, 2012.
The ratio of amylose:amylopectin varies significantly not only between different plants, but also within a single plant species or plant organ [31]. The conditions and phase of growth also influence this ratio [32][33]. Several studies have shown that a change in this ratio implies a change in the physical and chemical characteristics of starch and its interaction with other molecules, which leads to different swelling capacity [34], solubility in water, microscopic properties [35], and stability and barrier/mechanical properties in starch films [36]. According to some studies, starch with a high content of amylose, for example, from peas, has the best mechanical and gas barrier properties [37]. It was also noted that lentil starch (30% amylose) has a strong tendency to gelatinize at a relatively low concentration compared to corn and potato starch [29].
Cereal (grain) starch is obtained by its physical separation from non-starch components. Various processes of wet grinding of grains have been developed for the production of starch. The main stages of these processes are soaking, grinding, and separation of grain components [38]. Potato starch is extracted from potato tubers in a process called bio-processing: the potatoes are ground, and the contents of the cells are released, including starch and protein [39].
Starch-based polymers have low moisture resistance, which limits their use in packaging. The usual form of natural starch is a crystalline molecular structure that is not flexible [40]. However, an interesting starch derivative is thermoplastic starch (TPS), which is more convenient for films production [41][42], which could be obtained with thermal and physical impacts in the presence of plasticizers [40]. Various physical and chemical reactions are involved in the heat treatment of starch-based polymers, such as water diffusion, granule expansion, gelatinization, melting, crystallization, and extrusion [43].
TPS with improving properties can be used in the field of food packaging since it is economical and available in large quantities. The production of flexible and solid packaging (biofilms, bags, laminated plastic, etc.) is the main sector of TPS application in the food industry [40]. Polymer films derived from starch are biodegradable and have good properties as oxygen barriers. However, the amount of plasticizer, humidity, and amylose content are the limiting factors that determine the mechanical properties of TPS. The type and amount of plasticizer used in the production of thermoplastic starch strongly affect the physical, chemical, and thermal properties of the film [44]. Various structural enhancers, such as microcrystalline cellulose, carboxymethylcellulose, carbon nanotubes, etc., are added to the starch-polymer matrix in order to improve its properties [45]. Various types of such reinforced starch are already successfully used in the packaging of bread, vegetables, and meat products stored in standard conditions [41][46][47].
2.2. Cellulose
Cellulose is the most common natural biopolymer and consists of β-(1–4)-D-glucopyranose monomers (Figure 3) [48]. It is biosynthesized by a number of living organisms, from lower to higher plants, marine animals, bacteria, and fungi [49]. It has been estimated that 1011–1012 tons are synthesized annually by photosynthesis in a fairly pure form, for example, in the seed hairs of the cotton plant, but mainly cellulose combines with lignin and other polysaccharides (hemicelluloses) in the cell wall of woody plants [50]. Although cellulose is primarily found in forests, where wood is the most important source, cellulose-containing materials include agricultural residues, aquatic plants, grasses, and other plant matter [51][52]. Commercial cellulose production is based on harvested sources, such as wood, or on natural sources with high biopolymer content, such as cotton [53]. In contrast with starch, cellulose is a linear polymer without winding and branching [48]. Numerous hydroxyl groups in cellulose form strong hydrogen bonds, which make the material non-fusible. Chemical modification of cellulose is required for the production of flexible materials, which often involves the replacement of hydroxyl groups with acetate or methyl groups (esterification), the purpose of which is to reduce the intensity of the hydrogen bonds [54]. The rate of esterification and type of replacers, as well as the length of the polymer chain, affect the further permeability, mechanical properties, and solubility. Methylcellulose (MC) and carboxymethylcellulose (CMC) are the most common cellulose esters and have good film-forming properties, which allow them to be used as packaging materials for food products [55][56][57][58].
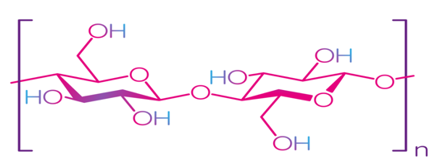
Figure 3. Structure of cellulose [49]. Reproduced with permission from Trache, D. et al., International Journal of Biological Macromolecules; published by Elsevier Ltd., 2016.
Methylcellulose (MC) is formed when one or more hydroxyl groups (-OH) in the anhydroglucose are replaced by a methoxide group (-OCH3). The degree of substitution of MC ranges from 1.4 to 2.0, and it is soluble in cold water [59]. It is reported that MC forms unbreakable, flexible, transparent, tasteless, and non-toxic films that have good barrier properties for oxygen, but bad ones for water vapor [60]. MC has a relatively high tensile strength and elastic modulus, so it demonstrates a reinforcing effect [61] and exhibits improved mechanical properties in mixtures containing proteins, lipids, etc. [62].
Carboxymethylcellulose (CMC) is a cellulose derivative in which some hydroxyl groups of glucopyranose units in cellulose are replaced by carboxymethyl groups. CMC is formed by reaction with chloroacetic acid (ClCH2CO2H) and catalyzed by alkali [63]. The amount of carboxymethyl groups in the cellulose molecule improves the strength of CMC film due to strong intermolecular forces [64]. Initially, CMC was studied as a hydrogel polymer, but it was later discovered that its dry form could be considered a biodegradable alternative to petroleum-based food packaging materials [64]. CMC easily absorbs moisture, dissolves in cold water, and exhibits thermal gelatinization [65]. CMC-based films can be combined with MC, clay, chitosan, etc., which usually improves the mechanical properties of the film: the tensile strength and elastic modulus increase, while the strain at break of the films is reduced [65]. It was noted that such a plasticizer as glycerin significantly increases the flexibility of the film, but also reduces the tensile strength and elastic modulus [66].
Cellulose derivatives are more suitable for packaging that is in direct contact with food products [65]. Studies of the properties of various cellulose derivatives used for film formation are continuing with the aim of developing mixtures of cellulose derivatives with other biopolymers to improve the mechanical, barrier properties and increase the shelf life of packaged food products [46][67][68].
2.3. Pectin
Pectin is one of the main components of the plant cell wall, contributing to the integrity and rigidity of tissues, and is considered one of the most complex macromolecules in nature [69]. Although pectin is ubiquitous in the plant kingdom, pectin derived from apples, citrus fruits, sunflowers, and sugar beets is an undisputed commercial source for the processing industry due to their physical and chemical properties and the availability of biomass [70]. Pectin is a poly–α–1–4–galacturonic acid (Figure 4a) with different degrees of methylation of carboxylic acid residues and/or amidated polygalacturonic acids (Figure 4b) [71]. Carboxyl groups of galacturonic acid are esterificated with methanol, resulting in methoxylated carboxyl groups. On the other hand, amidated carboxyl groups are obtained when galacturonic acid is converted with ammonia to carboxylic acid amide.
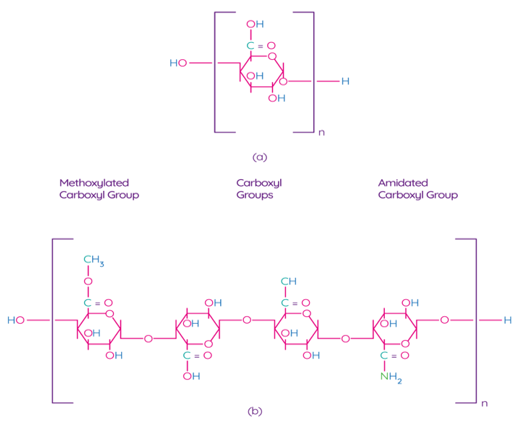
Figure 4. Chemical structure of polygalacturonic acid (a) and representative chemical structure of pectin, showing typical repeating groups (b) [72]. Reproduced with permission from Espitia, P.J.P. et al., Food Hydrocolloids; published by Elsevier Ltd., 2013.
According to the degree of esterification by methanol (the ratio of esterified galacturonic acid groups to its total number), pectin can be classified as high-methoxylpectin (HMP, > 50 % of esterified carboxyl groups) pectin or low-methoxyl pectin (LMP, < 50 % of esterified carboxyl groups) [70]. The degree of esterification affects the gelling properties of pectins. For example, pectin with a low content of methoxyl groups forms a gel in the presence of multivalent ions, which bonds pairs of carboxyl groups of different pectin chains. Pectin with a high content of methoxyl groups forms a gel in acidic solutions with the addition of various sugars, such as sucrose or glucose [72][73]. LMP is used for encapsulation, food packaging film processing, and low-calorie gels in dietary foods [72]. Structurally, pectins are classified as rhamnogalacturonan-I (RG-I; "hairy" pectin), substituted galacturonans (RG-II or SG; "hairy" pectin), and homogalacturonans (HG; smooth pectin). The hairy region of pectins (RG-I and RG-II) has a high probiotic potential [74]. Thus, based on the macromolecular and microstructural characteristics of pectins, the scope of their application in food products is determined.
Pectin is an ingredient used in the food industry without any restrictions other than current good manufacturing practices, is generally recognized as safe (GRAS) by the U.S. Food and Drug Administration (FDA), and is used in food primarily as a gelling agent, stabilizing agent, or thickener in products such as jams, yogurt drinks, fruit milk drinks, and ice cream [75][76]. The ubiquitous presence, low cost, structural flexibility, and polymerization ability of pectin contribute to its use as a matrix for active food packaging materials [70]. Since bioactive packaging films made from pectin have very weak antimicrobial properties, their antimicrobial potential can be enhanced by integrating and combining them with various functional compounds, such as essential oils, phenolic compounds, nanomaterials, free fatty acids, and others [77]. The production of edible films from pectin can be carried out in various ways, such as casting, extrusion, spraying, and coating with a knife [78].
This entry is adapted from the peer-reviewed paper 10.3390/polym13101592
References
- Wohner, B., Pauer, E., Heinrich, V., Tacker, M. Packaging-Related Food Losses and Waste: An Overview of Drivers and Issues. Sustainability 2019, 11, 264. https://doi.org/10.3390/su11010264
- Arkoun, M., Daigle, F., Holley, R.A., Heuzey, M.C., Ajji, A. Chitosan-based nanofibers as bioactive meat packaging materials. Packag. Technol. Sci. 2018, 31, 185–195. https://doi.org/10.1002/pts.2366
- Moreira, D., Gullón, B., Gullón, P., Gomes, A., Tavaria, F. Bioactive packaging using antioxidant extracts for the prevention of microbial food-spoilage. Food Funct. 2016, 7, 3273–3282. https://doi.org/10.1039/C6FO00553E
- Garavand, F., Rouhi, M., Razavi, S.H., Cacciotti, I., Mohammadi, R. Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: A review. Int. J. Biol. Macromol. 2017, 104, 687–707. https://doi.org/10.1016/j.ijbiomac.2017.06.093
- Iwata, T. Biodegradable and Bio-Based Polymers: Future Prospects of Eco-Friendly Plastics. Angew. Chemie Int. Ed. 2015, 54, 3210–3215. https://doi.org/10.1002/anie.201410770
- Kabir, E., Kaur, R., Lee, J., Kim, K.-H., Kwon, E.E. Prospects of biopolymer technology as an alternative option for non-degradable plastics and sustainable management of plastic wastes. J. Clean. Prod. 2020. 258, 120536. https://doi.org/10.1016/j.jclepro.2020.120536
- Datta, J., Kopczyńska, P. From polymer waste to potential main industrial products: Actual state of recycling and recovering. Crit. Rev. Environ. Sci. Technol. 2016, 46, 905–946. https://doi.org/10.1080/10643389.2016.1180227
- Faraca, G., Astrup, T. Plastic waste from recycling centres: Characterisation and evaluation of plastic recyclability. Waste Manag. 2019, 95, 388–398. https://doi.org/10.1016/j.wasman.2019.06.038
- Miandad, R., Barakat, M.A., Aburiazaiza, A.S., Rehan, M., Nizami, A.S. Catalytic pyrolysis of plastic waste: A review. Process Saf. Environ. Prot. 2016, 102, 822–838. https://doi.org/10.1016/j.psep.2016.06.022
- Mwanza, B.G., Mbohwa, C. Drivers to Sustainable Plastic Solid Waste Recycling: A Review. Procedia Manuf. 2017, 8, 649–656. https://doi.org/10.1016/j.promfg.2017.02.083
- Ragaert, K., Delva, L., Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. https://doi.org/10.1016/j.wasman.2017.07.044
- Wróblewska-Krepsztul, J., Rydzkowski, T. Pyrolysis and incineration in polymer waste management system. J. Mech. Energy Eng. 2020, 3, 337–342. https://doi.org/10.30464/jmee.2019.3.4.337
- Jakobek, L. Food packaging materials with polyphenols as active compounds. Meso 2019, 21, 469–474. https://doi.org/10.31727/m.21.5.3
- Stoica, M., Marian Antohi, V., Laura Zlati, M., Stoica, D. The financial impact of replacing plastic packaging by biodegradable biopolymers - A smart solution for the food industry. J. Clean. Prod. 2020, 277, 124013. https://doi.org/10.1016/j.jclepro.2020.124013
- Popović, S.Z., Lazić, V.L., Hromiš, N.M., Šuput, D.Z., Bulut, S.N. Chapter 8 - Biopolymer Packaging Materials for Food Shelf-Life Prolongation. In Biopolymers for Food Design, 1st ed.; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, Massachusetts, USA, 2018; Volume 10, pp. 223–277. https://doi.org/10.1016/B978-0-12-811449-0.00008-6
- Gómez-Estaca, J., Gavara, R., Catalá, R., Hernández-Muñoz, P. The Potential of Proteins for Producing Food Packaging Materials: A Review. Packag. Technol. Sci. 2016, 29, 203–224. https://doi.org/10.1002/pts.2198
- Nechita, P., Roman (Iana-Roman), M. Review on Polysaccharides Used in Coatings for Food Packaging Papers. Coatings 2020, 10, 566. https://doi.org/10.3390/coatings10060566
- Weber, C.J., Haugaard, V., Festersen, R., Bertelsen, G. Production and applications of biobased packaging materials for the food industry. Food Addit. Contam. 2002, 19, 172–177. https://doi.org/10.1080/02652030110087483
- Yadav, A., Mangaraj, S., Singh, R., Das, K., Kumar, N., Arora, S. Biopolymers as packaging material in food and allied industry. Int. J. Chem. Stud. 2018, 6, 2411–2418.
- Adeyeye, O.A., Sadiku, E.R., Babu Reddy, A., Ndamase, A.S., Makgatho, G., Sellamuthu, P.S., Perumal, A.B., Nambiar, R.B., Fasiku, V.O., Ibrahim, I.D., Agboola, O., Kupolati, W.K., Daramola, O.O., Machane, M.J., Jamiru, T., The Use of Biopolymers in Food Packaging. In Green Biopolymers and their Nanocomposites. Materials Horizons: From Nature to Nanomaterials, 1st ed.; Gnanasekaran, D., Eds.; Springer: Singapore, 2019, pp. 137–158. https://doi.org/10.1007/978-981-13-8063-1_6
- Roohi, Srivastava, P., Bano, K., Zaheer, M.R., Kuddus, M. Biodegradable Smart Biopolymers for Food Packaging: Sustainable Approach Toward Green Environment. In Bio-Based Materials for Food Packaging, 1st ed.; Ahmed, S., Eds.; Springer: Singapore, 2018, pp. 197–216. https://doi.org/10.1007/978-981-13-1909-9_9
- Xu, T., Ma, C., Aytac, Z., Hu, X., Ng, K.W., White, J.C., Demokritou, P. Enhancing Agrichemical Delivery and Seedling Development with Biodegradable, Tunable, Biopolymer-Based Nanofiber Seed Coatings. ACS Sustain. Chem. Eng. 2020, 8, 9537–9548. https://doi.org/10.1021/acssuschemeng.0c02696
- Dhumal, C.V., Sarkar, P. Composite edible films and coatings from food-grade biopolymers. J. Food Sci. Technol. 2018, 55, 4369–4383. https://doi.org/10.1007/s13197-018-3402-9
- Ghanbarzadeh, B., Oleyaei, S.A., Almasi, H. Nanostructured Materials Utilized in Biopolymer-based Plastics for Food Packaging Applications. Crit. Rev. Food Sci. Nutr. 2015, 55, 1699–1723. https://doi.org/10.1080/10408398.2012.731023
- Wicochea-Rodríguez, J.D., Chalier, P., Ruiz, T., Gastaldi, E. Active Food Packaging Based on Biopolymers and Aroma Compounds: How to Design and Control the Release. Front. Chem. 2019, 7. https://doi.org/10.3389/fchem.2019.00398
- Cazón, P., Velazquez, G., Ramírez, J.A., Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. https://doi.org/10.1016/j.foodhyd.2016.09.009
- Mohamed, S.A.A., El-Sakhawy, M., El-Sakhawy, M.A.-M. Polysaccharides, Protein and Lipid -Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 238, 116178. https://doi.org/10.1016/j.carbpol.2020.116178
- Thakur, R., Pristijono, P., Scarlett, C.J., Bowyer, M., Singh, S.P., Vuong, Q. V. Starch-based films: Major factors affecting their properties. Int. J. Biol. Macromol. 2019, 132, 1079–1089. https://doi.org/10.1016/j.ijbiomac.2019.03.190
- Basiak, E., Lenart, A., Debeaufort, F. Effect of starch type on the physico-chemical properties of edible films. Int. J. Biol. Macromol. 2017, 98, 348–356. https://doi.org/10.1016/j.ijbiomac.2017.01.122
- Kadokawa, J.-i. Preparation and Applications of Amylose Supramolecules by Means of Phosphorylase-Catalyzed Enzymatic Polymerization. Polymers 2012, 4, 116-133. https://doi.org/10.3390/polym4010116
- Lemos, P.V.F., Barbosa, L.S., Ramos, I.G., Coelho, R.E., Druzian, J.I. Characterization of amylose and amylopectin fractions separated from potato, banana, corn, and cassava starches. Int. J. Biol. Macromol. 2019, 132, 32–42. https://doi.org/10.1016/j.ijbiomac.2019.03.086
- Bertoft, E., Blennow, A. Chapter 3 - Structure of Potato Starch. In Advances in Potato Chemistry and Technology, 2nd ed.; Singh, J., Kaur, L., Eds.; Academic Press: Cambridge, Massachusetts, USA, 2016; pp. 57–73. https://doi.org/10.1016/B978-0-12-800002-1.00003-0
- Zhu, J., Zhang, S., Zhang, B., Qiao, D., Pu, H., Liu, S., Li, L. Structural features and thermal property of propionylated starches with different amylose/amylopectin ratio. Int. J. Biol. Macromol. 2017, 97, 123–130. https://doi.org/10.1016/j.ijbiomac.2017.01.033
- Keeratiburana, T., Hansen, A.R., Soontaranon, S., Blennow, A., Tongta, S. Porous high amylose rice starch modified by amyloglucosidase and maltogenic α-amylase. Carbohydr. Polym. 2020, 230, 115611. https://doi.org/10.1016/j.carbpol.2019.115611
- Singh, J., Colussi, R., McCarthy, O.J., Kaur, L. Chapter 8 - Potato Starch and Its Modification. In Advances in Potato Chemistry and Technology, 2nd ed.; Singh, J., Kaur, L., Eds.; Academic Press: Cambridge, Massachusetts, USA, 2016; pp. 195–247. https://doi.org/10.1016/B978-0-12-800002-1.00008-X
- Li, H., Prakash, S., Nicholson, T.M., Fitzgerald, M.A., Gilbert, R.G. The importance of amylose and amylopectin fine structure for textural properties of cooked rice grains. Food Chem. 2016, 196, 702–711. https://doi.org/10.1016/j.foodchem.2015.09.112
- Palviainen, P., Heinämäki, J., Myllärinen, P., Lahtinen, R., Yliruusi, J., Forssell, P. Corn Starches as Film Formers in Aqueous-Based Film Coating. Pharm. Dev. Technol. 2001. 6, 353–361. https://doi.org/10.1081/PDT-100002617
- Shevkani, K., Singh, N., Bajaj, R., Kaur, A. Wheat starch production, structure, functionality and applications-a review. Int. J. Food Sci. Technol. 2017, 52, 38–58. https://doi.org/10.1111/ijfs.13266
- Semeijn, C., Buwalda, P.L. Chapter 9 - Potato Starch. In Woodhead Publishing Series in Food Science, Technology and Nutrition, 2nd ed.; Sjöö, M., Nilsson, L., Eds.; Woodhead Publishing: Cambridge, United Kingdom, 2018; pp. 353–372. https://doi.org/10.1016/B978-0-08-100868-3.00009-3
- Khan, B., Bilal Khan Niazi, M., Samin, G., Jahan, Z. Thermoplastic Starch: A Possible Biodegradable Food Packaging Material-A Review. J. Food Process Eng. 2017, 40, e12447. https://doi.org/10.1111/jfpe.12447
- Gironès, J., López, J.P., Mutjé, P., Carvalho, A.J.F., Curvelo, A.A.S., Vilaseca, F. Natural fiber-reinforced thermoplastic starch composites obtained by melt processing. Compos. Sci. Technol. 2012, 72, 858–863. https://doi.org/10.1016/j.compscitech.2012.02.019
- Díaz-Galindo, E.P., Nesic, A., Cabrera-Barjas, G., Mardones, C., von Baer, D., Bautista-Baños, S., Dublan Garcia, O. Physical-Chemical Evaluation of Active Food Packaging Material Based on Thermoplastic Starch Loaded with Grape cane Extract. Molecules 2020, 25, 1306. https://doi.org/10.3390/molecules25061306
- Dorigato, A., Perin, D., Pegoretti, A. Effect of the Temperature and of the Drawing Conditions on the Fracture Behaviour of Thermoplastic Starch Films for Packaging Applications. J. Polym. Environ. 2020, 28, 3244–3255. https://doi.org/10.1007/s10924-020-01843-3
- Schmitt, H., Guidez, A., Prashantha, K., Soulestin, J., Lacrampe, M.F., Krawczak, P. Studies on the effect of storage time and plasticizers on the structural variations in thermoplastic starch. Carbohydr. Polym. 2015, 115, 364–372. https://doi.org/10.1016/j.carbpol.2014.09.004
- López, O. V., Castillo, L.A., García, M.A., Villar, M.A., Barbosa, S.E. Food packaging bags based on thermoplastic corn starch reinforced with talc nanoparticles. Food Hydrocoll. 2015, 43, 18–24. https://doi.org/10.1016/j.foodhyd.2014.04.021
- Jabeen, N., Majid, I., Nayik, G.A., Yildiz, F. Bioplastics and food packaging: A review. Cogent Food Agric. 2015, 1, 1117749. https://doi.org/10.1080/23311932.2015.1117749
- Sengupta, T., Han, J.H. Chapter 4 - Surface Chemistry of Food, Packaging, and Biopolymer Materials. In Innovations in Food Packaging, 2nd ed.; Han, J.H., Ed.; Academic Press: Cambridge, Massachusetts, USA, 2014; pp. 51–86. https://doi.org/10.1016/B978-0-12-394601-0.00004-7
- Gardner, K.H., Blackwell, J. The structure of native cellulose. Biopolymers 1974, 13, 1975–2001. https://doi.org/10.1002/bip.1974.360131005
- Trache, D., Hussin, M.H., Hui Chuin, C.T., Sabar, S., Fazita, M.R.N., Taiwo, O.F.A., Hassan, T.M., Haafiz, M.K.M. Microcrystalline cellulose: Isolation, characterization and bio-composites application—A review. Int. J. Biol. Macromol. 2016, 93, 789–804. https://doi.org/10.1016/j.ijbiomac.2016.09.056
- Trache, D., Hussin, M.H., Haafiz, M.K.M., Thakur, V.K. Recent progress in cellulose nanocrystals: sources and production. Nanoscale 2017, 9, 1763–1786. https://doi.org/10.1039/C6NR09494E
- Mishra, S., Kharkar, P.S., Pethe, A.M. Biomass and waste materials as potential sources of nanocrystalline cellulose: Comparative review of preparation methods (2016 – Till date). Carbohydr. Polym. 2019, 207, 418–427. https://doi.org/10.1016/j.carbpol.2018.12.004
- Zhao, Y., Moser, C., Lindström, M.E., Henriksson, G., Li, J. Cellulose Nanofibers from Softwood, Hardwood, and Tunicate: Preparation–Structure–Film Performance Interrelation. ACS Appl. Mater. Interfaces 2017, 9, 13508–13519. https://doi.org/10.1021/acsami.7b01738
- Heinze, T., El Seoud, O.A., Koschella, A. Production and Characteristics of Cellulose from Different Sources. In Cellulose Derivatives. Springer Series on Polymer and Composite Materials, 1st ed.; Heinze, T., El Seoud, O.A., Koschella, A., Eds.; Springer: Cham, Switzerland, 2018; pp. 1–38. https://doi.org/10.1007/978-3-319-73168-1_1
- Zhang, B., Azuma, J., Uyama, H. Preparation and characterization of a transparent amorphous cellulose film. RSC Adv. 2015, 5, 2900–2907. https://doi.org/10.1039/C4RA14090G
- Azeredo, H.M.C., Barud, H., Farinas, C.S., Vasconcellos, V.M., Claro, A.M. Bacterial Cellulose as a Raw Material for Food and Food Packaging Applications. Front. Sustain. Food Syst. 2019, 3. https://doi.org/10.3389/fsufs.2019.00007
- Azeredo, H.M.C., Rosa, M.F., Mattoso, L.H.C. Nanocellulose in bio-based food packaging applications. Ind. Crops Prod. 2017, 97, 664–671. https://doi.org/10.1016/j.indcrop.2016.03.013
- Robertson, G.L. Food Packaging Principles and Practice, 3rd ed.; CRC Press: Boca Raton, Florida, USA, 2012; 733 p.
- Wu, Y., Li, Q., Zhang, X., Li, Y., Li, B., Liu, S. Cellulose-based peptidopolysaccharides as cationic antimicrobial package films. Int. J. Biol. Macromol. 2019, 128, 673–680. https://doi.org/10.1016/j.ijbiomac.2019.01.172
- Da Silva Filipini, G., Romani, V.P., Guimarães Martins, V. Biodegradable and active-intelligent films based on methylcellulose and jambolão (Syzygium cumini) skins extract for food packaging. Food Hydrocoll. 2020, 109, 106139. https://doi.org/10.1016/j.foodhyd.2020.106139
- López de Dicastillo, C., Rodríguez, F., Guarda, A., Galotto, M.J. Antioxidant films based on cross-linked methyl cellulose and native Chilean berry for food packaging applications. Carbohydr. Polym. 2016, 136, 1052–1060. https://doi.org/10.1016/j.carbpol.2015.10.013
- Yoo, S., Krochta, J.M. Starch-methylcellulose-whey protein film properties. Int. J. Food Sci. Technol. 2012, 47, 255–261. https://doi.org/10.1111/j.1365-2621.2011.02833.x
- Debeaufort, F. Lipid hydrophobicity and physical state effects on the properties of bilayer edible films. J. Memb. Sci. 2000, 180, 47–55. https://doi.org/10.1016/S0376-7388(00)00532-9
- Heinze, T., Pfeiffer, K. Studies on the synthesis and characterization of carboxymethylcellulose. Die Angew. Makromol. Chemie 1999, 266, 37–45. https://doi.org/10.1002/(SICI)1522-9505(19990501)266:1<37::AID-APMC37>3.0.CO;2-Z
- Roy, N., Saha, N., Kitano, T., Saha, P. Biodegradation of PVP–CMC hydrogel film: A useful food packaging material. Carbohydr. Polym. 2012, 89, 346–353. https://doi.org/10.1016/j.carbpol.2012.03.008
- Malhotra, B., Keshwani, A., Kharkwal, H. Natural polymer based cling films for food packaging. Int. J. Pharm. Pharm. Sci. 2015, 7, 10–18.
- Rachtanapun, P., Rattanapanone, N. Synthesis and characterization of carboxymethyl cellulose powder and films from Mimosa pigra. J. Appl. Polym. Sci. 2011, 122, 3218–3226. https://doi.org/10.1002/app.34316
- He, X., Lu, W., Sun, C., Khalesi, H., Mata, A., Andaleeb, R., Fang, Y. Cellulose and cellulose derivatives: Different colloidal states and food-related applications. Carbohydr. Polym. 2021, 255, 117334. https://doi.org/10.1016/j.carbpol.2020.117334
- Youssef, A.M., El-Sayed, S.M. Bionanocomposites materials for food packaging applications: Concepts and future outlook. Carbohydr. Polym. 2018, 193, 19–27. https://doi.org/10.1016/j.carbpol.2018.03.088
- Jolie, R.P., Duvetter, T., Van Loey, A.M., Hendrickx, M.E. Pectin methylesterase and its proteinaceous inhibitor: a review. Carbohydr. Res. 2010, 345, 2583–2595. https://doi.org/10.1016/j.carres.2010.10.002
- Kumar, M., Tomar, M., Saurabh, V., Mahajan, T., Punia, S., Contreras, M. del M., Rudra, S.G., Kaur, C., Kennedy, J.F. Emerging trends in pectin extraction and its anti-microbial functionalization using natural bioactives for application in food packaging. Trends Food Sci. Technol. 2020, 105, 223–237. https://doi.org/10.1016/j.tifs.2020.09.009
- Espitia, P., Avena-Bustillos, R.J., Du, W.-X., Teófilo, R.F., Soares, N.F.F., McHugh, T.H. Optimal antimicrobial formulation and physical–mechanical properties of edible films based on açaí and pectin for food preservation. Food Packag. Shelf Life 2014, 2, 38–49. https://doi.org/10.1016/j.fpsl.2014.06.002
- Espitia, P., Du, W.-X., Avena-Bustillos, R. de J., Soares, N. de F.F., McHugh, T.H. Edible films from pectin: Physical-mechanical and antimicrobial properties - A review. Food Hydrocoll. 2014, 35, 287–296. https://doi.org/10.1016/j.foodhyd.2013.06.005
- Mishra, R.K., Banthia, A.K., Majeed, A.B.A. Pectin based formulations for biomedical applications: A review. Asian J. Pharm. Clin. Res. 2012, 5, 1-7.
- Gómez, B., Gullón, B., Yáñez, R., Schols, H., Alonso, J.L. Prebiotic potential of pectins and pectic oligosaccharides derived from lemon peel wastes and sugar beet pulp: A comparative evaluation. J. Funct. Foods 2016, 20, 108–121. https://doi.org/10.1016/j.jff.2015.10.029
- Ciriminna, R., Fidalgo, A., Delisi, R., Ilharco, L.M., Pagliaro, M. Pectin production and global market. Agro Food Ind. Hi Tech 2016, 27, 17–20.
- Raei, M., Shahidi, F., Farhoodi, M., Jafari, S.M., Rafe, A. Application of whey protein-pectin nano-complex carriers for loading of lactoferrin. Int. J. Biol. Macromol. 2017, 105, 281–291. https://doi.org/10.1016/j.ijbiomac.2017.07.037
- Sganzerla, W.G., Rosa, G.B., Ferreira, A.L.A., Rosa, C.G.d., Beling, P.C., Xavier, L.O., Hansen, C.M., Ferrareze, J.P., Nunes, M.R., Barreto, P.L.M., Lima Veeck, A.P.d. Bioactive food packaging based on starch, citric pectin and functionalized with Acca sellowiana waste by-product: Characterization and application in the postharvest conservation of apple. Int. J. Biol. Macromol. 2020, 147, 295–303. https://doi.org/10.1016/j.ijbiomac.2020.01.074
- Valdés, A., Burgos, N., Jiménez, A., Garrigós, M. Natural Pectin Polysaccharides as Edible Coatings. Coatings 2015, 5, 865–886. https://doi.org/10.3390/coatings5040865
