A comparative analysis concerning bio-based gels production, to be used for tissue regeneration, has been performed in this review. These gels are generally applied as scaffolds in the biomedical field, thanks to their morphology, low cytotoxicity, and high biocompatibility.
1. Introduction
In 1931, Kistler [1] produced highly porous materials by removing the liquid part in a gel, working at supercritical conditions. This process avoided shrinkage and solid network deformations during drying, maintaining open porosity and preserving the structure of the gel. In this way, a solid, in which the dispersed phase was air, was obtained, i.e., an aerogel.
In recent years, the research on aerogels production has been widely intensified thanks to the aerogels’ interesting physical and chemical properties [2], such as high porosity (80–99.8%), ultralight weight, thermal resistance (thermal conductivity range from 0.005 to 0.1 W∙mK−1), high specific surface area (between 500 and 1200 m2∙g−1), low dielectric constant (κ value range from 1.0 to 2.0), and low refractive index (≈1.05) [3].
Despite the numerous advantages that can be provided by silica and other inorganic aerogels [4][5][6][7][8], the raw materials of conventional aerogels come from petrochemical-based sources. In response to environmentally friendly requirements, bio/bio-inspired materials have attracted widespread interest thanks to their biocompatibility, biodegradability, and abundance; these are some of the most important requisites for potential biomedical applications, such as tissue engineering (TE), disease diagnosis, drug delivery (DD), and bio-sensing [9][10][11].
The main difference among bio-aerogels, especially polysaccharide aerogels, and other organic (resorcin–formaldehyde, poly(vinyl chloride), and others) or inorganic (silica, alumina, titania, and others) aerogels, is the sequence of steps involved in their preparation. Inorganic aerogel synthesis starts with the hydrolysis and/or polycondensation of alkoxide compounds in the presence of a catalyst [12]; the produced solvogel is formed by a liquid phase embedded in a highly porous solid network. The production of organic aerogels starts with the dissolution of polymers in water or in organic solvents (polysaccharides are generally soluble in aqueous solutions). Then, solution gelation occurs, in which polymer chains rearrange themselves into an open porous network. Gelation can be induced by chemical, enzymatic or physical crosslinking. Chemical cross-linking involves the introduction of permanent linkages by means of a cross-linking agent; for example, glyoxal(dialdehyde), glutaraldehyde (GTA), butane tetracarboxylic acid and citric acid [13][14]. On the other hand, physical cross-linking is characterized by hydrogen bonding, Van der Waals forces, or electrostatic interactions [15][16]. Many polysaccharides can undergo the formation of Van der Waals forces or hydrogen bonding, due to the presence of functional groups localized on their backbones [15].
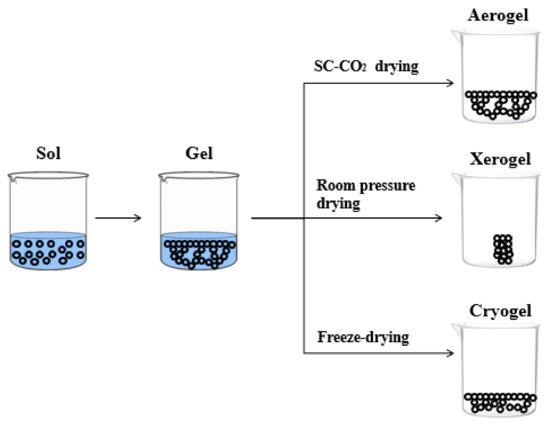
The features of polysaccharide-based aerogels porous structure depend on the drying technique. There are three kinds of solid materials that can be formed after drying, i.e., xerogel, cryogel and aerogel, as shown in . The solid material can be termed as xerogel when drying is carried out under ambient pressure and at room temperature, generally, for several days [17][18]. When water (ice) inside the hydrogel is sublimated by freeze-drying, the resultant materials are called cryogels. However, these samples have a macroporous structure, characterized by large and irregular pores [19][20][21][22][23][24].
Figure 1. Schematic representation of aerogel, xerogel, and cryogel.
Aerogels are generally produced by supercritical CO
2 drying. This technique, whether properly performed by selecting the operative pressure and temperature, avoids the collapse of the native gel nanostructure and preserves the excellent textural properties of the gel
[25][26][27][28][29]. Moreover, unlike many organic solvents, supercritical CO
2 (SC-CO
2) is non-flammable, inert, non-toxic, has a low cost, and moderate critical values (about 31 °C and 73.8 bar). It is worth mentioning that SC-CO
2 can form a supercritical mixture with the organic solvent used to prepare the solvogel at mild pressures and temperatures; therefore, during solvent extraction, liquid and gaseous phases become a homogenous phase, avoiding the formation of a liquid–vapor meniscus. In this context, no capillary forces are exerted on pore walls, because the supercritical mixture presents an almost-zero surface tension, and the polymeric matrix does not collapse
[27][28][29][30][31][32][33][34]. However, SC-CO
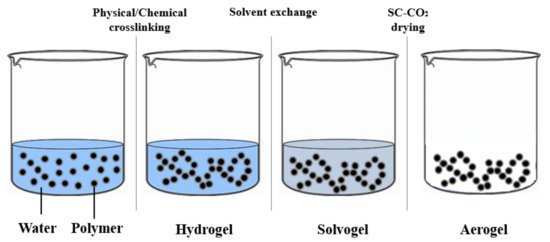
2 can only be directly used for solvogels, because it shows a limited solubility with water at ordinary pressures and temperatures. For this reason, in the case of hydrogels, water has to be replaced with a suitable solvent miscible in both water and CO
2, before carrying out the drying process
[25] ().
Figure 2. Hydrogel–solvogel–aerogel production.
2. Classification
2.1. Inorganic Aerogels
Inorganic aerogels are not the main scope of this work; however, they are relevant to understand the possible processes that can be applied to polysaccharide-based aerogels. Much attention has been given to silica aerogels for their use in several industrial applications, including thermal insulation for skylights and windows
[4][5]. Silica aerogels have also been used as heat storage devices and as acoustic barrier materials
[6]. Thanks to the high porosity and very large surface area, silica aerogels can be also utilized as gas filters, encapsulation media, and hydrogen fuel storage
[35].
Silicon alkoxide precursor is reactive enough to form gel networks with other metal oxides
[36]; therefore, several studies were carried out to synthesize silica aerogel composites; for example, silica–titania (TiO
2), silica–carbon and silica–alumina
[37][38][39][40][41][42][43][44]. These inorganic aerogels are generally prepared using a two-step sol–gel process, starting from an organic solvent, frequently ethanol. Solvogel preparation involves hydrolysis and alkoxide polycondensation. The obtained solvogel is formed by a solid amorphous structure characterized by an open porosity within the range of 90–95% and nanometric pore diameter. Then, to obtain the corresponding aerogel, the alcoholic solvent is removed from the solid skeleton
[45][46]. The main problem during aerogel production is solvent evaporation: when a liquid is evaporated from a fragile and microporous structure, the internal capillary force can lead to a collapse of the pores, with consequent shrinkage of the material. Kistler
[1] found a way to obtain monolith silica gels avoiding structure collapse, operating above the solvent critical point, i.e., using the so-called high temperature supercritical drying (HTSCD). In this way, liquid–vapor transition does not occur, and no capillary tension is generated on pore walls. Silica aerogels produced under supercritical conditions are translucent or transparent with low density (30–500 kg/m
3) and have a considerably high specific surface area (200–1000 m
2/g). Campbell et al.
[47] prepared TiO
2 aerogels in methanol to increase the surface area of this catalytic material and found that, during supercritical drying, performed at 207 bar, temperatures in the range of 210–300 °C were required for complete solvent removal. At the end of the process, TiO
2 samples showed a surface area value (200 m
2/g) three-fold larger than the conventional ones. Kocon et al.
[48] produced ultralow density silica aerogels by HTSCD. Solvent composition and solvent quantity were investigated to keep the silica dissolution as low as possible during the supercritical drying; operating in this way, silica aerogels with densities lower than 3 kg/m
3 were obtained. Moussaoui et al.
[49] synthesized TiO
2 aerogels for photocatalysis applications. In this case, isopropanol was supercritically extracted at 300 °C and 100 bar. A comparison between supercritical drying and ambient pressure drying at 200 °C was performed, and they noted that, under subcritical solvent conditions, the evaporation of the solvent from wet gel led to high stresses in the structure, due to capillary pressure at the gel/solvent interface.
However, HTSCD process may present some problems due to the combination of high temperatures and high pressures, as well as the flammability of the solvents. For this reason, low temperature supercritical drying (LTSCD) has been introduced. During this process, liquid CO
2 is pumped at a temperature lower than 10 °C, until pressure reaches about 100 bar. When the solvent is completely replaced by CO
2, temperature is raised up to 40 °C, and the transition of CO
2 into supercritical conditions is obtained; then, the system is slowly depressurized
[50][51][52][53]. Compared to HTSCD, longer operating times are required for this process. Mizushima et al.
[50] compared the physical properties of alumina solvogels dried using SC-CO
2 and ethanol, operating at 80 °C and 157 bar, with alumina solvogels dried under supercritical condition of ethanol (270 °C and 265 bar). Alumina aerogel prepared by supercritical drying had lower densities than alumina xerogel. Moreover, these authors discovered that alumina aerogels were strengthened by treatment performed at higher temperature and pressure. Deng et al.
[51] and Kinoshita et al.
[52] prepared silica and TiO
2 nanoaerogels, respectively, using LTSCD. Shimoyama et al.
[53] performed SC-CO
2 drying for the preparation of high-surface area TiO
2 aerogels from sol–gel routes. It was found that the drying conditions over the critical pressure for the CO
2 + acetone binary system produced TiO
2 aerogels characterized by needle-like shape and high specific surface area.
2.2. Organic Aerogels
Inorganic aerogels and some organic aerogels are not biocompatible. In order to overcome this drawback, polysaccharide-based aerogels have been widely investigated, frequently starting from water-based solutions. CO
2 shows a low solubility with water at ordinary process conditions; therefore, it is necessary to exchange it with an intermediate solvent that is miscible both in water and CO
2, after the hydrogel preparation
[54].
Yamashita et al.
[55] prepared poly(vinyl chloride) (PVC) aerogels starting from a dimethylformamide (DMF) solution. DMF in the solvogel was firstly exchanged with liquid CO
2, then supercritically dried at 31 °C and 73.8 bar. Alshrah et al.
[56] developed high-porosity resorcinol (RSR)/formaldehyde (FRM) aerogels, through an LTSCD process. After RSR/FRM gelation, the aqueous solution was replaced by acetone. Then, acetone was exchanged with liquid CO
2 for three days and, at the end, pressure and temperature were increased beyond the CO
2 critical point. However, the LTSCD process is questionable because CO
2 exchange is very long and several days are required to obtain a complete solvent removal using liquid CO
2 [56][57][58].
To overcome this drawback, HTSCD was investigated for organic aerogel manufacturing. Wu et al.
[59] prepared an organic aerogel using NaOH-catalyzed polycondensation of RSR/furfural (FRF) and supercritical drying in ethanol, setting pressure at 120 bar and temperature at 255 °C. Chen et al.
[60] synthesized lignin/RSR/FRM aerogels using supercritical ethanol drying. Process conditions were set at 250 °C and 100 bar. However, HTSCD cannot be used to produce polysaccharide-based aerogels, because polymeric chains may undergo thermal decomposition. Therefore, different alternative drying techniques have been explored.
The first step in polysaccharide-based aerogel production is the dissolution of the organic compounds in an aqueous solution. Then, the hydrogel is formed by chemical, enzymatic, or physical cross-linking. During the drying process, the liquid surrounding the polymeric network is carefully removed and replaced with air. Drying carried out under ambient pressure for several days can lead to the formation of a condensed structure with an insufficient porosity and large shrinkage; therefore, cryogels produced by freeze-drying have become a common approach. To fabricate cryogels, the gel-like matrices formation occurs in the frozen systems, using physical or chemical gelation. This typically occurs between −5 °C and −20 °C, because most of the solvents crystallize at these conditions. Solvent crystals act as porogens, while the hydrogel constituents remain in liquid micro-phases
[21][22][23][24]. Freezing rate can have a relevant impact on the cryogel formation: slower rates may result in larger pores with increased interconnectivity, whereas faster freezing rates produce mechanically weaker cryogels, with a low level of interconnectivity
[61]. Mahmoud et al.
[62] prepared norfloxacin-loaded scaffolds for wound treatment by freeze-drying, combining collagen (CLG) with two different types of chitosan (CS). These scaffolds were characterized by pores with an average diameter ranging from 150 to 300 µm, which showed an almost-100% drug release within 24 h. You et al.
[20] produced metallic nanosilver (NAg)–CLG–CS hybrid scaffolds and investigated their potential effects on wound healing. It was demonstrated that NAg–CLG–CS was bactericidal, anti-inflammatory, and promoted wound healing, regulating fibroblast migration and macrophage activation. Both CLG–CS and NAg–CLG–CS had a mean pore size of 136 ± 5 µm and a porosity of 93.6%. Rubio-Elizalde et al.
[21] realized alginate (ALG) cryogels, using polyethylene glycol (PEG)–methyl ether methacrylate (MA) as a plasticizer, with the aim of improving the stability properties of the scaffolds into an aqueous medium. Viability of fibroblasts into the scaffolds was increased with the addition of
Aloe vera and
Moringa oleifera extracts. All the obtained scaffolds showed semispherical pores between 50 and 100 μm. In particular,
M. oleifera significantly increased the scaffold cell proliferation, compared with scaffolds without plant extracts. To mimic the natural structure of tissue extracellular matrix (ECM), Yang et al.
[22] prepared a silk fibroin (SF)–hyaluronic acid (HA)–sodium alginate (SALG) composite scaffold by freeze-drying: SF materials are beneficial for fibroblast proliferation, and HA materials are beneficial for cell adhesion. The average pore size (≈93 μm), the mean porosity (≈92%) and the swelling ratio (≈42%) were suitable for fibroblast infiltration and for skin regeneration (SR). Gupta et al.
[23] fabricated porous scaffolds for bone regeneration (BR), blending keratin (KRT) and ALG. The solution was kept in a freezer at −20 °C for three days. After that, solvent extraction was performed to obtain a porous scaffold. The morphology of the KRT/ALG scaffold indicated the presence of closed pores having a polydisperse distribution, ranging from 10 to 200 μm. Cheng et al.
[24] prepared ALG-based aerogels by ionotropic gelation and freeze-drying, using N,N′-methylenebisacrylamide and carboxy-methylcellulose as reinforcing agents. The final aerogels were characterized by an irregular and closed morphology.
However, the native nanoporous hydrogel morphology is not preserved during freeze-drying. In particular, when an aqueous solution is frozen at an extremely low temperature, the rapid formation of ice nuclei leads to a growth of small ice crystals; although, in any case, not of nanometric dimension
[61]. This effect can be considered a relevant drawback for medical applications, because mesoporosity has an important impact on implant topography and scaffold bioactivity. Other problems associated with this technique are a low structural stability and generally weak mechanical properties of the fabricated materials; moreover, the 3D structure, similar to the tissue to be substituted, is frequently missing
[63][64].
At this point, the question is: “Is there a way to preserve the delicate polysaccharide–hydrogel structure, maintaining the native macro- and nanoporosity?”. An SC-CO
2 drying technique has been tested to generate nanoporous polysaccharide 3D aerogels
[25]. SC-CO
2 can only be used directly with organic solvents; therefore, a solvent exchange is required for polysaccharides that form 3D networks in aqueous solution. As the second step, SC-CO
2-assisted drying is performed using pressures and temperatures that exceed the critical values of the solvent mixture (CO
2 + organic solvent). In this way, the liquid and gaseous phase form a supercritical mixture: no capillary forces are exerted on pore walls and, therefore, there is no collapse of the polymeric matrix. Using SC-CO
2 processing, Munor-Ruiz et al.
[26] developed a highly porous CLG–ALG–graphene oxide (GO)-based aerogel. Scanning electron microscopy (SEM) images showed a porous interconnected network covered by a nonporous external wall. Baldino et al.
[54] produced poly-L-lactid acid (PLLA) aerogels by SC-CO
2 drying. These structures showed a microporous architecture and, at the same time, maintained the native nanostructure of the gel, consisting of a network of nanofilaments. These scaffolds were also loaded with hydroxyapatite (HAp) nanoparticles to improve the mechanical properties of the PLLA structure. Baldino et al.
[27], using the same technique, produced ALG/gelatin (GLT) and CS/GLT
[29] aerogels. The results indicated that both ALG/GLT and CS/GLT mixtures formed uniform gels, and SC-CO
2 drying preserved their delicate nanostructured morphology (≈100 nm), thanks to the near-zero surface tension of the supercritical mixture (CO
2 + organic solvent) formed during the drying process. Baldino et al.
[28] also produced ascorbic acid loaded-SF aerogels by SC-CO
2 drying. Supercritical assisted process allowed preservation of the SF aerogel morphology at nanoscale, and ascorbic acid release rate was controlled, as well as mechanical properties of the produced aerogels, varying the SF concentration in the starting hydrogel. The same authors
[29] prepared CS/GLT aerogels characterized by a Young’s modulus of 181 kPa, larger than that of the single polymers, because a new interpenetrated gel network was obtained.
This entry is adapted from the peer-reviewed paper 10.3390/ma14071631