Multiple interconditioning between photobiomodulation (PBM), probiotics, and the human microbiota, their effects on the human body, and their implications for the management of viral infectious diseases is essential. Coupled complex PBM and probiotic interventions can control the microbiome, improve the activity of the immune system, and save the lives of people with immune imbalances.
- infections
- lung
- microbiome
- SARS-CoV-2
- COVID-19
- immune
- laser
- photobiomodulation
- probiotics
1. Prebiotics, Probiotics, Paraprobiotics, Postbiotics and Synbiotics
The term “probiotic” was adopted in 2001 at an International Meeting of Experts under the auspices of the World Health Organization (WHO) and the Food and Agriculture Organization (FAO) and was subsequently revised in 2014. Definition of “probiotics” includes all micro-organisms that are beneficial to the health of the host when used in the appropriate dose [41], with capacity of survival in the gut without the danger of transferring elements of pathogenicity, antibiotic resistance, and toxicity.
Presently, probiotics are the subject of comprehensive research to design innovative products, effective marketing, regulation, and rigorous control, and to support consumer interest and safety in prescribing the product by healthcare practitioners. Most probiotic strains represented by the species of lactic acid bacteria, bifidobacteria and yeasts, present on the consumer market are considered to be safe for use in food and as supplements.
The differences between “paraprobiotics” and “postbiotics” are that paraprobiotics are considered lifeless or inoperative probiotic cells, while “postbiotics” are tonic metabolites of probiotics, both with common origins that are widely studied in functional biotics. Postbiotics have multiple benefits over conventional probiotics, through the molecular structure, which is already known, are used in purified compounds, have a specific activity, and intervene more easily on microbe-associated molecular pattern (MAMP), i.e., on MAMP-PRR to promote the downstream path. Another difference is that they are easy to make industrially through easy processes of production, packaging, transport, storage, administration etc. [70].
2. Probiotics in the Management of Various Pathologies
2.1. Probiotics in Digestive Tract Pathology
Antibiotics can cause a microbial imbalance in the gut resulting in antibiotic-associated diarrhea (AAD). Probiotics can prevent AAD by rebalancing the intestinal microflora, repairing the intestinal barrier, etc. Probiotics are increasingly used to prevent and treat diarrheal disease more in children than in adults.
2.2. Probiotics in Pulmonary Viral Infections
The pulmonary microbiota plays a particularly important role in preserving the homeostasis of the respiratory system, to promote and preserve a state of immune tolerance, to prevent an unwanted inflammatory reaction after inhalation of harmless environmental agents. This activity is supported by an indestructible and permanent link between the microbiota and the immune cells in the lungs, which through specialized sensors detect invasive micro-organisms [213].
Changes in the lung microbiome through which dysbiosis can occur, will influence the host’s immunity and defense; understanding these complex interactions between the host and the pathogen elucidates the pathogenesis of chronic lung disease [214]. Probiotics can be a valuable alternative for preventing and ameliorating respiratory tract infections with viral agents, which cause so many diseases in children and adults.
2.3. Probiotics and COVID-19
Balancing the intestinal microbiota during and after viral infections can be achieved with the help of probiotics that adhere and line the intestinal mucosa, constituting a strong barrier against pathogens and at the same time, activate the immune system. When an infection occurs in the lungs, the alarm signals are transmitted from the lung to the intestine on the lung-gut axis and from there, the information is transmitted further to the central nervous system (brain) on the gut–brain axis, to stop the inflammatory processes. These data are processed in the cerebral cortex and sent back on the brain–lung–intestine axis, so that the defense processes are implemented; in this way, the microbiota, through its bacterial complexity, mobilizes itself to defend the lung.
Probiotics and their mechanisms of action in the prevention and treatment of respiratory diseases, could bring great benefits in the COVID-19 pandemic. In an experiment conducted by Harata et al. on BALB/c mice infected with influenza virus IFV A/PR/8/34 (H1N1), who were administered intranasally the probiotic LGG, it was found that LGG reduced the respiratory symptoms, increased survival rate compared to the control group and improved the immune responses by increasing the activation of natural killer (NK) lung cells [232].
3. Photobiomodulation Applied on the Gut–Lung–Brain Axis
Scientific basis for the use of light in clinical medical applications originated at the beginning of the last century in Niels Ryberg Finsen’s first successful experiments [240] on smallpox in red light (1893) and further in 1895, on the treatment of Lupus vulgaris (also known as tuberculosis luposa [241]), i.e., painful cutaneous tuberculosis skin lesions with nodular appearance.
Presently, lots of therapeutic techniques that employ low-level laser or LED light limited to a specified set of wavelengths from red to near-infrared, and for some special applications even ultraviolet (UVB), proved to be safe, with no known side effects, used to relieve pain or to heal wounds, ulcers, and to treat many different diseases and disorders under the term of photobiomodulation or PBM, as it stimulates and enhances cell function.
Circadian clocks have proven to be particularly important for human physiology adapted to the light-dark cycle of 24 h, so that a person’s sleep habits, eating patterns and diet can desynchronize the body’s clocks and can contribute to the onset of non-communicable diseases [245,246]. External optical signals captured by optical photoreceptors are processed and activate the expression of circadian genes in the central nervous system, influencing molecular clocks and having major implications for some diseases [247]. The spectral quality of the sun modulates our neurotransmitters, and our health suffers because contemporary life often lacks strong daily circadian stimuli [248].
There is a permanent feedback between probiotics, microbiota, immune system, neuroendocrine, and nerve cells, the intestine–brain axis being significant.
Reasonable dietary fiber consumption and probiotics improve the balance of the intestinal microbiota by stimulating the production of short-chain fatty acids, important for the body’s energy and inflammatory reactions and response, regulating hunger, nutritional status, and body weight, insulin response and energy storage in the liver and muscles [57].
The pioneering of laser medicine (low-level laser applications that is, current photobiomodulation or PBM), is due to the doctor Endre Mester (1903–1984) who immediately after the discovery of the first operational laser, began in 1967 his applications on cutaneous neoplasms; and thus, he discovered the positive biological effects, which were then used successfully in the alternative treatment of various medical conditions [263].
Depending on the dose of radiation (light) applied, the cellular response will be different, so that low doses of energy will have beneficial, positive, stimulating effects; and, exactly the opposite, for high doses, which will be inhibitory; so, this is the biphasic dose response.
PBM can modulate oxidative stress (in certain cells with low ROS levels, it increases ROS synthesis; and in other cases, it reduces the oxidative stress), reduces the reactive nitrogen species, the prostaglandins levels, and it regulates the NF-kB pathway, it decreases the inflammatory markers in activated inflammatory cells, leading to an overall reduction in inflammation, effects particularly important for respiratory dysfunctions, musculoskeletal system disorders, brain and intestinal tract [265].
4. Photobiomodulation and COVID-19
Coupled complex PBM and probiotic interventions can adjust the microbiome, improve the activity of the immune system, and save the lives of people with immune imbalances, as in the model suggested in Figure 1.
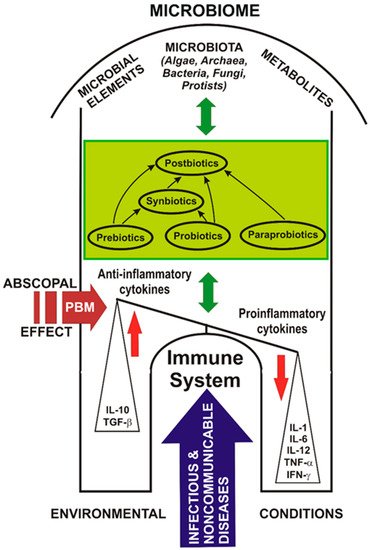
Figure 1. Probiotics, photobiomodulation, and the microbiome in COVID-19.
Figure 1 shows a model of the abscopal effect of PBM on the human microbiome and the relationship between probiotics, the immune system, and diseases affecting the host.
Probiotics may have the ability to modulate exacerbated immune responses, such as the COVID-19 cytokine storm. Targeting the SARS-CoV-2 cytokine storm using PBM and probiotics could be a useful treatment choice.
In the case of COVID-19, PBM could influence the balance between anti-inflammatory and pro-inflammatory cytokines, leading to the resolution of the infectious disease.
Table 5 shows the effects of PBM in the action on probiotics [307], followed by a series of experimental animal studies [266,288,289,308,309], in vitro and/or in vivo cellular models [291,310,311,312], including the latest clinical trials in patients with COVID-19 [313,314], which demonstrates the perspective applications of PBM for the targeting and modulation of the microbiome [266,270,308,309], with also the enrichment of the functional genes [308], the immune system [268,270,288], the auxiliary control of chronic degenerative diseases and viral infections [310,312,313,314], as a challenge for future research in the 21st century (see also Figure 1).
| Type of Study | PBM Parameters and Protocol |
Performed Analysis | PBM Effects | Reference |
|---|---|---|---|---|
| Experimental study with red laser on L. casei NRRL-B-1922 | Red laser 632.7 nm, 40 mW; 3, 6, 12 J/cm2; exposure time 10, 20, 40 min, respectively. | PBM (red laser exposure) applied to L. casei NRRL-B-1922 before the fermentation of skim milk. | Exposure of L. casei NRRL-B-1922 to the dose of 12 J/cm2 before skimmed milk fermentation exhibited a significant improvement of the anti-oxidant capacity, β-galactosidase, antimicrobial, and proteolytic activities. It decreased the cholesterol and lactose levels of fermented skimmed milk, enhancing the fermentation process of skimmed milk prepared with L. casei NRRL-B-1922. It opens the perspective of red laser photobiomodulation of probiotic bacteria during the fermentation process of skimmed milk to improve the quality of fermented milk on an industrial scale, with significant economic benefits, too. |
[307] |
| Animal study on BALB/c mice |
Abdomen irradiated with red (660 nm), output power 75 mW, power density 93.75 mW/cm2; or, infrared (808 nm), output power = 83 mW, power density = 103.75 mW/cm2, either as single or multiple doses, over a two-week period. Spot size = 0.8 cm2 for both lasers, pulse frequency of 250 Hz. Each mouse received a total energy density of 10 J/cm2. Sham treatments were identical. |
Genomic DNA extracted from fecal pellets was pyrosequenced for the 16S rRNA gene. | Allobaculum bacterium, associated with a healthy microbiome, significantly increased (p < 0.001) after infrared (but not red light) PBM by day 14. It is the first experiment proving that PBM can alter microbiome diversity in healthy mice and increase numbers of Allobaculum. If confirmed in humans, it opens avenues for PBMT to be applied as an auxiliary treatment in obesity, cardiovascular and neurodegenerative diseases, as well as other disorders. |
[266] |
| Animal study on C57BL/6 N mice | PBM was performed on the abdomen of the mice at the wavelengths of 630 nm, 730 nm, and 850 nm. Irradiation time was 1000 s (16 min and 40 s), the power density was 10 mW/cm2, and the energy density was 100 J/cm2, once a day, 5 times a week, for 8 weeks. |
Gut flora-targeted PBM (gf-targeted PBM) on Alzheimer’s disease (AD) animal model. Expression levels of 509 proteins, which involved the pathways of hormone synthesis, phagocytosis, and metabolism. The 16 s rRNA gene sequencing of fecal contents. |
Gf-targeted PBM reversed the imbalance of intestinal flora and improved learning ability, amyloid plaque deposition, tau phosphorylation, and microglia inflammation of Aß-induced AD mice. Many proteins in the hippocampus responded to gf-targeted PBM, with mitochondrial respiratory chain complex enzymes as a possible key intermediate target. PBM significantly altered the diversity and abundance of intestinal flora, reversing the typical increase of Helicobacter and uncultured Bacteroidales, and the decreasing the Rikenella seen in AD mice. Gf-targeted PBM has the potential to be a noninvasive microflora regulation method for Alzheimer’s disease patients. Future studies will confirm the effect of gf-targeted PBM on the brain-gut axis, promoting PBM as a potential prevention and treatment method for AD. | [308] |
| Animal study on Sprague-Dawley (SD) rats | PBM with IR (830 nm, 100 mW/cm2) supplementary light irradiation was carried out from 14:00 to 14:30 every day, for three months. Illuminance in the feeding box was 1000 lx. | Concentration of bone metabolism markers, including 1,25-dihydroxyvitamin D3 (1,25-(OH)2-D3), bone-specific alkaline phosphatase (BALP), and tartrate-resistant acid phosphatase (TRACP), were detected from blood samples in four study groups. Whole body, femur and tibia of the rats were scanned with a dual-energy X-ray bone densitometer. Bacterial genomic DNA was extracted from the frozen stool samples with a DNA extraction kit. The V3-V4 region of the 16S rRNA (341F-805R), F: GATCCTACGGGAGGCAGCA; R: GCTTACCGCGGCTGCTGGC) was studied. An open-source R package, Tax4Fun, was first used to analyze the enrichment of functional genes of the microbiome of each group. |
Analysis of the structure and function of gut microbiota in the rats after PBM infrared supplementation significantly reduced the abundance of Saccharibacteria and increased the abundance of Clostridiaceae 1 and Erysipelotrichaceae bacteria. Results proved that changes in the gut microbiome correlate well with bone mass and bone metabolism. Infrared supplementation can have a positive effect on rat bone metabolism by affecting gut microbiota. These findings could be used in the future design of healthy lighting environments that prevent or possibly ameliorate osteoporosis. | [309] |
| Animal study on C57BL/6 mice |
PBM with laser at 660 nm and radiant exposure of 10 J/cm2, was applied six hours after intratracheal inflammation produced with instillation of lipopolysaccharide (LPS) (5 mg/kg) or phosphate buffer saline (PBS). | Inflammatory cells in perivascular and alveolar spaces, and inflammatory mediator secretion. | Increased expression and secretion of cytokines (TNF-α, IL-1β, IL-6,) and chemokine (MCP-1). PBM induced a significant decrease in both inflammatory cell influx and inflammatory mediator secretion. PBM did not affect the mechanical properties of the lungs, nor the strength of the tissue, nor the elasticity. PBM reduced the inflammatory reaction in the lungs exposed to LPS without affecting lung function and recovery. |
[288] |
| Animal study on BALB/c mice |
PBM (830 nm laser, 9 J/cm2, 35 mW, 80s per point, 3 points per application) was applied in direct contact with skin, 1 h after LPS administration. Mice were distributed in control (n = 6; PBS), ARDS IT (n = 7; LPS orotracheally 10 μg/mouse), ARDS IP (n = 7; LPS intra-peritoneally 100 μg/mouse), ARDS IT + Laser (n = 9; LPS intra-tracheally 10 μg/mouse), ARDS IP + Laser (n = 9; LPS intra-peritoneally 100 μg/mouse). |
LPS-induced pulmonary and extrapulmonary acute respiratory distress syndrome (ARDS). 24 h after last LPS administration, mice were studied for pulmonary inflammation by total and differential cell count in bronchoalveolar lavage (BAL), cytokines (IL-1beta, IL-6, KC and TNF-alpha) levels in BAL fluid and by quantitative analysis of neutrophils number in the lung parenchyma. |
PBM significantly reduced pulmonary and extrapulmonary inflammation in LPS-induced ARDS, reduced number of total cells and neutrophils in BAL, reduced levels of IL-1beta, IL-6, KC and TNF-alpha in BAL fluid and in serum, as well as the number of neutrophils in lung parenchyma. PBM was efficient in reducing pulmonary inflammation in both pulmonary and extrapulmonary model of LPS-induced ARDS. |
[289] |
| In vitro study of dermal fibroblast cell line (HFF-1) with premature senescence H2O2-induced |
PBMT: 660 nm, energy density = 3, 4, 5, 6, and 8 J/cm2; power density = 35 mW; time = 10 s, 14 s, 16 s, 20 s, and 28 s. Beam area = 0.035 cm2, beam diameter = 0.21 cm2, frequency 16 Hz, pulsed. Number of points 8. Area of the laser application 9.6 cm2. Contact/No contact—distance of 35 mm. Cellular mortality, proliferation, and the levels of oxidative, inflammatory cytokines, apoptotic markers, and of two growth signaling molecules (FGF-1 and KGF) were compared among treatments. |
Protein quantification of the following markers: DNA 8-deoxyguanosine and cytokines involved in inflammatory response interleukin IL-1β, IL-6, IL-10, tumoral necrosis factor alfa (TNF-α), and interferon-gamma (IFN-γ). Caspase-1, caspase-3, and caspase-8 activities were determined by assay kits, fluorometric. |
Interaction between H2O2 at 50 μM and PBM at 4 J (best dose) showed partially reversion of the higher levels of DNA oxidation, CASP 3, CASP 8, IL-1B, IL-6, and IFN-γ induced by H2O2 exposure. PBM also trigger increase of IL-10 anti-inflammatory cytokine, FGF-1 and KGF levels. PBM on the fibroblast without injury was relative safe and harmless, given its cytogenotoxic potential, oxy-inflammatory, and proliferative effects. However, in the injured H2O2 fibroblast, PBM had significant protection and proliferative effect, partially or totally reversing the negative effects triggered by H2O2. At certain dose ranges, PBM may trigger anti-aging properties. |
[291] |
| In vitro model of human keratinocytes cell line (HaCaT) infected with Herpes Simplex Virus Type-1 (HSV-1) |
HSV-1 were irradiated using a diode laser device (class IV) with the following two protocols: 445 nm, 0.3 W/cm2, 60 J/cm2, CW, or 445 nm, 0.15 W/cm2, 30 J/cm2, 5 Hz. |
After 30 min the virus irradiated and not irradiated was transferred to a HaCaT cells culture and then, after another 24 h HSV-1 quantification was performed on the cell supernatants. Five experimental settings were used and the increase in cell vitality and the decrease in HSV-1 viral load in supernatants of previously irradiated virus-infected cells were measured comparatively with non-irradiated virus-infected cells. |
Experimental results proved that the blue laser has antiviral activity against HSV-1, and it is more effective against virus irradiated alone, suggesting that PBMT inactivates the virus prior to cell entry. In contrast, when the virus is already inside the cells, the effect of PBMT is less evident and does not increase cells’ resistance to infection. Blue PBM had a direct inhibitory effect on the virus itself. Further studies are necessary to determine how blue PBM exerts its antiviral effect, the aim being to move from an in vitro to a clinical setting, thus promoting its use on HSV-1 infected patients. | [310] |
| In vitro cellular model of hidradenitis suppurativa (HS) on human keratinocyte cell line (HaCaT) |
Two irradiation protocols with near-infrared (NIR) and Blue PBM: 970 nm, 0.3 W/cm2, 20 J/cm2, continuous wave (CW) and 445 nm, 0.2 W/cm2, 10 J/cm2, CW, using fluency at 10–30–50 J/cm2. |
Effect of PBM on IL1B gene (encoding for interleukin-1β [IL-1β]) expression in immortalized human keratinocyte cell line using a wild-type line and a knockout cell model mimicking genetic-driven Hidradenitis Suppurativa (HS). |
Based on the hypothesis that increased production of pro-inflammatory cytokines would promote a dysbiosis of resident skin microbes and so, the perpetuation of skin inflammation in HS, it was shown that PBM decreased IL1B gene expression, which could block the up-mentioned vicious mechanism. PBM could be a useful tool in the management of skin lesions in patients with HS. | [311] |
| In vitro model of SARS-CoV-2 infection | PBMT using LEDs at 450 nm with 12.5 J/cm2; 454 nm with 10 J/cm2; 470 nm with 20 J/cm2; irradiance of 40 mW/cm2, continuous waves. | Experiments were performed on Vero E6 epithelial normal cell line derived from the kidney of Cercopithecus aethiops (ATCC CRL-1586), with three experimental settings: SARS-CoV-2 was irradiated and then transferred to cells; already infected cells were irradiated; cells were irradiated prior to infection. | Results may support the possible exploitation of blue light to meet the challenges of SARS-CoV-2, as blue wavelengths have stopped SARS-CoV-2 replication. The antiviral activity of PBMT against SARS-CoV-2 on human cell lines is intended to propose translatability for this new approach to support individuals affected by COVID-19, also considering that PBMT is largely safe, with no side effects, and well tolerated by patients. | [312] |
| Systematic review | PBM using red to infrared light (λ = 600–1070 nm) has been analyzed in several pre-clinical models of Alzheimer’s and Parkinson’s disease, as an emerging putative neuroprotective therapy. | Tissue stressed by hypoxia, toxic insult, genetic mutation and mitochondrial dysfunction. | Analysis proved important reductions in β-amyloid plaques, neurofibrillary tangles of hyperphosphorylated tau protein, inflammation and oxidative stress, together with increased ATP levels and improved overall mitochondrial function as follows: increase (↑) Cell survival (striatal and cortical cells), ↑T-Helper + cells, ↑ATP content, ↑Complex IV-dependent respiration, decrease (↓) Oxidative stress, ↓Inflammation, ↑Mitochondrial function, ↑Heat shock proteins, ↓Amyloid aggregates, ↓Hyperphosphorylated tau. In addition, PBM reduced the characteristic cognitive deficits in transgenic mouse models. |
[268] |
| Systematic review | A literature search was conducted for published reports on the effect of PBM [visible or near-infrared (NIR)] on the microbiome, red (630–680 nm) or in the NIR region (780–940 nm) and (980 and 1064 nm). Power densities: 10–100 mW/cm2, energy densities in the region of 4–50 J/cm2. |
Subcellular, cellular (neurons, epithelial cells, keratinocytes, fibroblasts etc.) and tissue levels. Organ level: brain (oscillation patterns), gut etc. Microbiome. |
The following conclusions can be drawn: A. Light can affect the microbiome indirectly through the daily circadian rhythm. B. Light has an indirect effect on the microbiome through vitamin D, produced by the action of sunlight on keratinocytes. C. PBM effects on cytochrome c oxidase (CCO): ↑CCO, ↑Mitochondrial membrane potential, ↑ATP production, brief burst of reactive oxygen species, ↑nitric oxide, ↑cyclic AMP, ↑movements in intracellular calcium, ↑transcription factors, ↑expression of a multitude of gene, ↑structural proteins, ↑enzymes, ↑cell division, ↑cell migration. D. PBM effects at cellular and tissue level: ↑nonvisual phototransduction cascades involving opsins. Blue (415 nm) and green (540 nm) light absorbed by opsins, trigger opening of transient receptor potential (TRP) calcium ion channels. E. PBM on inflammatory pathways: ↓pro-inflammatory cytokines (IL-6, TNF-α, IFN-γ). F. PBM on immune system: ↑circulating immune cells (mast cells, macrophages, etc.), transduce protective signals from distal tissues to sites of injury (brain, heart, or gut). E. PBM on microbiome: ↑Akkermansia muciniphila, ↑Bifidobacterium spp., ↑Faecalibacterium spp., and ↓Firmicutes/Bacteroidetes ratio. |
[270] |
| Randomized clinical trial with COVID-19 pneumonia | Two laser sources (808 nm and 905 nm), working simultaneously and synchronously as follows: 1. Three GaAlAs laser diodes, 808 nm, peak power of 1 W, average power 500 mW each diode, in total 1.5 W, power density 75 mW/cm2, 1500 Hz, duty cycle of 50%, pulse duration of 330 µs, spot size of 19.6 cm2. 2. Three superpulsed GaAs laser diodes, 905 nm, peak power 75 W, average power 203 mW each diode, in total 610 mW, power density 31 mW/cm2, 1500 Hz (train pulses 90 kHz modulated at 1 Hz ÷ 2 kHz), pulse duration of 100 ns, spot size of 19.6 cm2. Each lung was scanned for 14 min, from apex to base, over an area of 250 cm2 of the posterior thorax, resulting in 28 min of PBMT with a dosage of 7.18 J/cm2 and a total energy of 3590 J. |
PBMT group received standard medical care plus adjunctive PBMT, four daily sessions of near-infrared light treatment targeting the lung tissue. Control group received only standard medical care. Patient outcomes were measured via blood work, chest x-rays, pulse oximetry and validated scoring tools for pneumonia. |
PBMT-treated patients showed rapid recovery, did not require ICU admission or mechanical ventilation, and reported no long-term sequelae at 5 months after treatment. In the control group, 60% of patients were admitted to the ICU for mechanical ventilation. The control group had an overall mortality of 40%. At a 5-month follow-up, 40% of the control group experienced long-term sequelae. PBMT is a safe and effective potential treatment for COVID-19 pneumonia and improves clinical status in COVID-19 pneumonia. |
[313] |
| PDT clinical trial with COVID-19 in the early stage of infection | Laser light watch with 4 red laser diodes (658 nm), 2 blue (447 nm), 2 green (532 nm) and 2 yellow (589 nm) LEDs for systemic treatment of blood via the wrist arteries for 60 min; one nose treatment applicator with 1 blue LED (447 nm) and 1 UVA LED (375 nm), 10 min each nostril with blue and UVA light (sides switched after 10 min); one mouth treatment applicator with 14 blue LEDs (447 nm) and 14 UVA LEDs (375 nm) for 20 min inside the mouth and throat. As photosensitizer for photodynamic therapy (PDT): 2 capsules Riboflavin-5phosphate 100 mg/each treatment, as follows: one capsule for systemic application taken 1 h before starting the PDT, and the second one (100 mg) dissolved into a glass of 200 mL water (for local application in nose, mouth and throat). |
Two groups with 20 patients each: one group receiving PDT and daily testing, and a control group receiving conventional care plus testing. All patients in both groups had positive Covid-19 test results at the beginning of the study being in an early infection stage with mild symptoms such as fever, dry cough, headache, hard breathing, fatigue etc. QPCR tests with CT-viral load were performed on day 1, 2, 3, 4, 5 and 7 in the PDT group, and on day 1, 3, 5 and 7 in the control group. |
All 20 patients in the PDT group showed significant improvement in clinical symptoms and viral load assessment within the 5 days of PDT. 14 out of 20 patients had a negative QPCR test after 5 days of PDT, while the other 6 patients also showed significantly reduced viral load. All 20 patients in the control group were tested 3 times within 5 days and no significant improvement could be seen clinically or in the viral load assessment. |
This entry is adapted from the peer-reviewed paper 10.3390/ijms22094942
