The biological features of prostate cancer as a tumor with a low alpha beta ratio have led clinicians to consider the use of higher doses per fraction, thus gaining an advantage both in terms of clinical outcomes and of logistic opportunities. In this setting, the recent introduction of MR-Linac will provide clinicians an attractive tool for the treatment of prostate cancer, by exploiting the superior soft tissue visualization combined with the possibility to daily adapt the treatment plan with the real-time anatomy of the patient.
1. Introduction
Prostate cancer (PC) is the most frequently diagnosed tumor in the male population in Europe [
1], with high survival rates. Besides surgery, radiotherapy (RT) represents the best non-invasive alternative in the curative setting and plays a key role in the post-operative scenario [
2,
3,
4].
There are several data supporting PC as a tumor with a low alpha-beta ratio that is more sensitive to higher doses per fraction [
5,
6,
7].
This biological characteristic is the main basis for the worldwide propagation of hypofractionated schedules, which were initially supported by a large number of studies and are currently implemented in several international clinical practice guidelines [
8,
9,
10,
11].
The excellent outcomes in terms of toxicity and disease control and the constant technological advances have led clinicians to investigate the use of extreme hypofractionation, which combines a superior biological effect with non-negligible logistic advantages [
12,
13]. To date, very promising results are available in the literature and the role of extreme hypofractionation is expected to gain more attractiveness with the recent introduction of Magnetic Resonance (MR)-guided RT performed with Linacs equipped with on-board MR-imaging [
14,
15,
16,
17].
The advent of these hybrid machines may represent a game-changer for the radiation oncology community, aiming to improve the accuracy in target volume and organs at risk (OARs) delineation, based on a better anatomy visualization due to the improved soft tissue contrast provided by MR. Because the prostate can be clearly identified using MRI, it is expected that target volumes will decrease, also inter-observer variability will be reduced in accordance with ESTRO-ACROP guidelines [
18,
19].
Moreover, MR-Linacs allow a daily online treatment plan adaptation based on the ability to recalculate the plan prior to each fraction, taking into account changes in shape and size of the target and surrounding healthy structures [
20].
These advantages could significantly reduce the inter-fraction variability, which is a major problem in extreme hypofractionated schedules [
21,
22].
In contrast, the longer duration of the treatment session can potentially affect intra-fraction motion, although cine-MR sequences allow clinicians to constantly monitor organ motion during the beam-on-time and apply automated beam gating features, where available [
23].
However, as recently reported by Hehakaya et al. [
24], the setting of PC is a congenial field for the development of MR-guided RT, given the opportunity to improve treatment tolerability with a potentially lower incidence of toxicity and a consequently favorable outcome in terms of patient-reported outcomes (PROMs). Moreover, the implementation of these hybrid devices represents a theoretical opportunity that also has positive socio-economic implications, both in terms of professional developments and for logistical reasons. Furthermore, specifically in the setting of prostate cancer, but also generally speaking, the improved accuracy in target volume delineation and the possibility to daily-adapt the target based on real-time anatomy will increase clinicians’ confidence in proposing extremely hypofractionated schedules with a reduced length of the treatment and decreased accesses to the facility. Indeed, this device is expected to reinforce the multi-disciplinary nature of RT by involving multiple professional groups, such as radiologists, physicists and Radiation Therapy Technologist (RTTs), and leading to a new dynamic in daily clinical activity [
25,
26,
27].
Given the relative novelty of this technology, several diagnostic and therapeutic opportunities can be explored, especially in the setting of PC, such as radiomics or focal boost investigational studies in order to further tailor the oncologic treatment. Nevertheless, to date, the published evidence remains quite sparse [
28,
29].
2. MR-Guided Radiotherapy: Present Evidence
To date, two MR-Linac devices are commercially available, Unity Elekta (Elekta, Stockholm, Sweden) and MRIdian Viewray (Viewray Inc., Cleveland, OH, USA) [
30,
31].
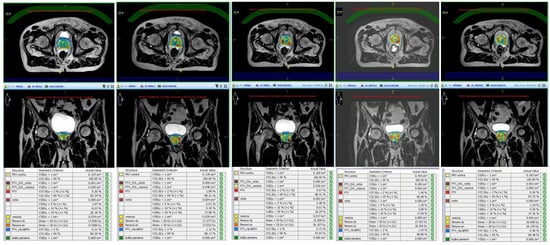
Unity Elekta conjugates a 1.5 T magnetic resonance system with a 7 MV linear accelerator and it allows daily-adapted radiotherapy by means of two different workflows: the adapt-to-position (ATP) procedure is based on a daily update of the iso-center position, with no need for re-contouring, while in the adapt-to-shape (ATS) workflow, the daily treatment plan is re-calculated on the re-contoured volumes of the real-time anatomy of the patient ().
Figure 1. Daily replanning for Magnetic Resonance-guided Stereotactic Body Radiotherapy (MR-guided SBRT).
The MRIdian Viewray combines a 0.35 T split magnetic resonance scanner with a circular ring-gantry in which all 6 MV Linac components are shielded to avoid magnetic field interferences. This hybrid machine enables also different types of plan adaptation ranging from simple re-optimization to a full online-adaptive workflow with re-contouring and dose re-optimization. Moreover, it allows real-time soft tissue tracking and gating.
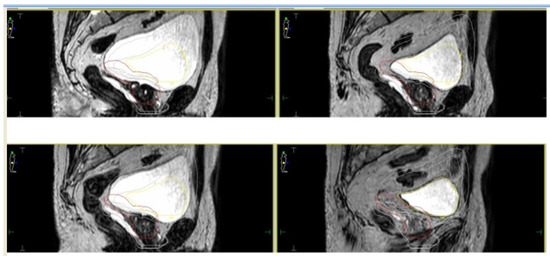
For both devices, given the relatively longer treatment time per session, the simulation process is a crucial factor in order to perform an accurate and refined treatment delivery. Based on available literature, most experiences reported a similar protocol in terms of bladder filling and rectal emptying ([
32,
33,
34,
35,
36,
37,
38]—see ). For both the CT scan (performed for dose calculation purposes) and the MRI scan, patients were educated to have a half-full bladder in order to take into account residual volume changes during the plan adaptation phase ().
Figure 2. Daily interfraction variability of Planning Target Volume (PTV) and bladder.
Table 1. Literature experiences of MR-guided daily adaptive SBRT for prostate cancer.
|
Author
|
N° of Patients
|
MR-Linac Device
|
SBRT Schedule
|
Main Endpoint of the Study
|
Results
|
|
Alongi et al. [36]
|
20
|
Elekta Unity
|
35 Gy/5 fractions
|
Dosimetric analysis and preliminary PROMs report
|
Hydrogel improves rectal sparing with minimal impact on QoL
|
|
Bruynzeel et al. [32]
|
101
|
Viewray MRIdian
|
36.25 Gy/5 fractions
|
Early toxicity analysis
|
G ≥ 2 GU = 23.8% (including 5.9% of G3 according to RTOG criteria); ≥2 GI = 5.0%
|
|
Cuccia et al. [34]
|
20
|
Elekta Unity
|
35 Gy/5 fractions
|
Assessment of the impact of rectal spacer on prostate motion
|
Significant impact on rotational antero-posterior shifts with consequently reduced prostate motion
|
|
Tetar et al. [33]
|
101
|
Viewray MRIdian
|
36.25 Gy/5 fractions
|
PROMs analysis
|
After one year, only 2.2% of cases reported a relevant impact on daily activities due to GI toxicity
|
|
Nicosia et al. [39]
|
10
|
Elekta Unity
|
35 Gy/5 fractions
|
Dosimetric comparison between MR-guided SBRT and conventional Linacs SBRT
|
MR-guided SBRT resulted in lower constraint violation rates
|
|
Sahin et al. [37]
|
24
|
Viewray MRIdian
|
36.25 Gy/5 fractions
|
Preliminary report of feasibility
|
Substantial feasibility of MR-adaptive SBRT with acceptable time schedules
|
|
Ugurluer et al. [38]
|
50
|
Viewray MRIdian
|
36.25 Gy/5 fractions
|
Early toxicity analysis
|
Acute G2 GU = 28%; Late G2 GU = 6%; Late GI GU = 2%
|
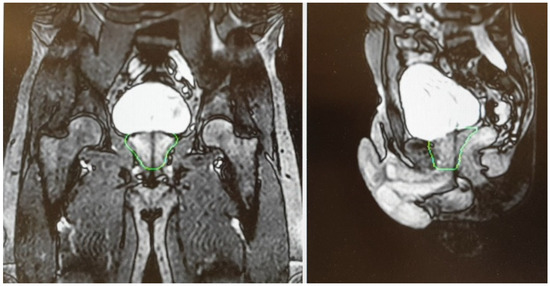
A T2-weighted gradient-echo sequence is acquired for a better visualization of the prostate gland. After the re-optimization of the plan, a further cine MR, usually acquired on sagittal and coronal planes, is performed to check organ motion during the beam-on time ().
Figure 3. Cine Magnetic Resonance (CineMR) sequence before the delivery of MR-guided SBRT.
The Viewray system, in addition to T2-weighted imaging, currently has a True Fast Imaging with steady-state-free precession (TRUFI) sequence. The system enables simple couch shifts, as well as more elaborated online plan adaptation strategies [
40].
Currently available evidence reports MR-guided SBRT as a safe and feasible treatment option. Alongi et al. [
36] reported excellent preliminary results in terms of PROMs in a cohort of 25 patients who received 35 Gy in 5 fractions, with no evidence of acute G ≥ 3 adverse events. Interestingly, the favorable results in terms of quality of life (QoL) outcomes after a median treatment time of 56 min (range, 34–86) per fraction, indicate the tolerability of MR-guided SBRT for prostate cancer and show that the longer treatment time per session has only a minimal impact on QoL. In agreement with these findings, also the study by Bruynzeel et al. [
32], performed using a 0.35 T MR-Linac, reported early promising results in a phase II study enrolling 104 patients, with only 5.9% of grade 3 genitourinary toxicity according to RTOG criteria. Similarly, on QoL evaluation, no relevant differences were detected at any time point of the study, with the exception of role functioning. These data were recently updated with a final PROMs analysis after one year of follow-up, which confirmed the absence of G ≥ 3 adverse events. Furthermore, at 12 months after the end of treatment, QoL returned to baseline conditions, with only 2% of patients reporting persistent bowel symptoms [
33].
A further recent paper has been published by Uguerler et al. [
38] reporting in a series of 50 patients with a median follow-up of 10 months with no evidence of G3 acute or late toxicity. Although observing a 36% rate of G2 GU adverse events, when available, late GI and GU toxicity rates were respectively 2% and 6%.
In this scenario, the use of rectal spacers for mitigating prostate motion represents a helpful tool to maximize the safety and accuracy of extremely hypofractionated treatments for prostate cancer [
41,
42]. To date, the use of this device has been safely reported by Alongi et al. in a series of 20 patients who received MR-guided prostate SBRT using rectal hydrogel spacer. Interestingly, the authors recorded a significant advantage in terms of rectal sparing and target coverage, in comparison with a cohort of patients who did not receive the administration of the rectal spacer. In addition, despite the invasive procedure, no adverse impact on QoL was observed using PROMs assessment [
43].
The same sample of patients was also analyzed in a subsequent study with the aim of evaluating a potential positive effect in terms of intra-fraction motion mitigation. The authors recorded a statistically significant effect of the rectal hydrogel spacer on rotational antero-posterior displacements compared to patients without spacers. Although these data are preliminary, they suggest a potential effect of prostate fixation due to the squeezing effect towards the pubic bone, but mature evidences is still needed to support a potential clinical impact of these dosimetric advantages [
44,
45,
46].
Consistent with this, the study by Nicosia et al. [
39] also highlights the beneficial impact of a superior anatomy visualization provided by MR-guided radiotherapy. In a dosimetric comparison between 40 patients receiving prostate SBRT using MR-Linac or a Volumetric Modulated Arc Therapy (VMAT) technique with or without fiducials, the authors recorded a significantly lower rate of constraints violation in the MR-Linac cohort compared to Volumetric Modulated Arc Therapy - Image Guided Radiation Therapy (VMAT-IGRT) patients treated without fiducials. Thus, the authors suggest that in VMAT-IGRT, only the implementation of fiducials can lead to a comparable quality in terms of real dose-distribution, which consequently highlights the advantages of MR-Linacs as a fiducial-free technique for extreme prostate hypofractionation.
3. MR-Guided Radiotherapy: Future Directions
3.1. Boost of the Dominant Intraprostatic Lesion
Despite the limited literature currently available, MR-guided SBRT in PC leads the way to several therapeutic opportunities to be explored. Among these, the administration of a boost to the dominant intraprostatic lesion (DIL), defined as the largest radiologically detected nodule in a milieu of a multifocal disease, is a critical issue for the RT scientific community [
47,
48]
Sparse emerging evidence suggests that the administration of doses ≥90 Gy
EQD2 to the dominant macroscopic node has a potentially favorable impact on biochemical control and biochemical disease-free survival. Furthermore, the administration of a higher dose to the DIL is thought to improve biochemical and local control, based on evidence reporting the macroscopic dominant nodule as the first site of local relapse after curative radiotherapy [
49,
50,
51,
52].
In these series, the boost delivery was performed using a variety of techniques, including External Beam Radiotherapy (EBRT), SBRT and brachytherapy. Interestingly, only the ASCENDE-RT trial reported an increased incidence of genito-urinary effects [
53].
In contrast, the recently published primary endpoint analysis of the multicenter prospective HYPO-FLAME trial reported acceptable acute GI and GU toxicity rates in a population of 100 men with intermediate and high-risk prostate cancer [
54]. More mature data now provide further evidence in terms of clinical benefits, including the currently ongoing FLAME phase III trial [
55].
In this scenario, the ability to rely on daily MR-guided imaging allows clinicians to improve the quality of IGRT and increase the confidence in the delivery of a focal boost based on daily re-calculation of the plan that accounts for inter-fraction variability. Of note, the correct visualization of the DIL may be difficult, for example in the case of concomitant androgen deprivation therapy, also because diagnostic MRI is still superior in terms of soft tissue contrast compared with the on-board MRI of hybrid machines [
56]. Moreover, as reported by van Schie et al., the T2-signal of healthy prostate decreases during radiotherapy, making the identification of the DIL more complex [
57].
3.2. Margin Reduction/Single Shot Treatments
The exploration of hypofractionation in recent years has led clinicians to consider the possibility of introducing single fraction regimens. As this option has been preliminary reported for SBRT of oligometastases [
58], it is currently under investigation also in the setting of PC SBRT. The prospective multicenter phase I/II study ONE SHOT is investigating the feasibility and efficacy of 19 Gy single fraction SBRT with urethral sparing in patients with low and intermediate risk prostate cancer [
59].
To date, only phase I results have recently been published with no acute grade ≥3 toxicity reported. The study recruitment is ongoing, and phase 2 results are eagerly awaited [
60].
As recently hypothesized in a dosimetric study by Dunlop et al. [
61], MR-Linacs may represent the best device for the delivery of single fraction PC SBRT. The authors investigated the technical feasibility of MR-guided prostate SBRT in 5, 2 or 1 fractions and reported no constraints violations in 30 plans. Only in 4 out of 10 plans of the 2- and 1-fraction regimens, target coverage criteria in terms of PTV D95% were not met in order to comply with Organs At Risk (OARs) constraints. On this basis, the authors planned to conduct a study to evaluate the clinical feasibility of a two fraction schedule. In this dosimetric study, an isotropic margin of 2 mm was applied to generate the PTV.
As mentioned above, it remains a matter of debate whether the use of a rectal spacer can lead to a margin reduction strategy. As recently reported by Mannerberg et al. [
62], the daily volume changes of the bladder and rectum result in a large displacement of the prostate, which increases the risk of a potential target underdosing. Combined with the time-consuming procedure of daily online adaptive treatments, a margin reduction in the absence of a stable immobilization of the prostate appears to be unwise at the moment.
3.3. Sexual Function Preservation
Given the ability of MRI to better visualize pelvic structures, in the context of prostate SBRT, there is an increased attention being paid on preserving sexual function. The refined quality of the diagnostic process has led to an earlier detection of the disease, prompting the scientific community to reflect on the optimal balance between safety and efficacy, including the occurrence of erectile dysfunction [
63]. The onset of this side effect is based on a multifactorial pathogenesis that includes both organic and psychological factors [
64].
To date, the biological rationale for erectile dysfunction after RT is thought to be based on a mechanism of vascular sclerosis; however, it is unclear which healthy structure is directly involved in the development of this late sequela [
65].
Moreover, the radiation-induced inflammatory response of the prostate gland may potentially contribute to facilitate this injury, along with eventually concurrent ADT or the injection of rectal immobilization devices, for which conflicting data in terms of pro-inflammatory effects have been reported [
66,
67].
The study by Spratt et al. [
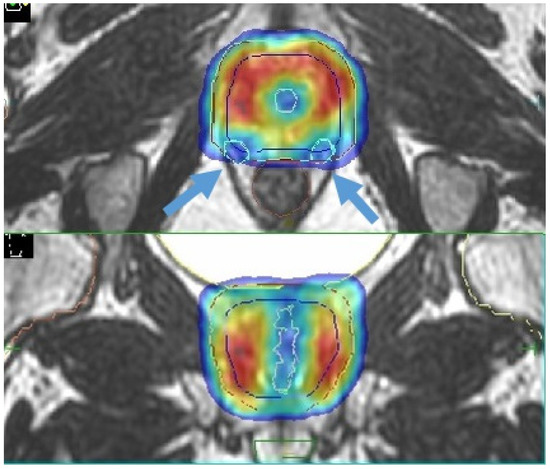
68] focused attention on sparing of the internal pudendal arteries, with encouraging results. A recent review by Ramirez-Fort et al. highlights the role of the ejaculatory ducts and the neurovascular plexus; the latter is adherent to the posterior part of the prostate gland and is therefore difficult to avoid with current image-guided radiotherapy modalities. Assuming an anatomic similarity to the brachial plexus, the authors hypothesize a similar dose constraint in conventional fractionation with a Dmax<75 Gy to 2 cc ([
64,
65,
69]—).
Figure 4. Sexual preservation during MR-guided prostate SBRT (blue arrows indicate the prostate neurovascular plexus).
Although longer treatment sessions in this setting may theoretically result in greater organ displacement with a consequent major inflammatory exposure, MR-guided RT may help clinicians in identifying these pelvic substructures with the aim of reducing the dose exposure and consequently preserve sexual function. However, further studies are needed to confirm this approach.
3.4. Re-Irradiation
Another potential area of interest for MR-guided prostate SBRT is local re-irradiation after curative or post-operative RT [
70].
Furthermore, in this setting, solid evidence is currently lacking and generally consists of small and mono-institutional retrospective series [
71,
72,
73,
74,
75,
76,
77,
78,
79,
80].
Nevertheless, preliminary data are very promising in terms of toxicity, biochemical control and ADT-free survival, prompting clinicians to consider this therapeutic alternative in a scenario in which there is a lack of consensus regarding clinical management [
81].
In addition, the availability of refined imaging modalities such as PET-CT with more sensitive tracers and multiparametric-MR has increased the accuracy in identifying the site of local relapse, improving the confidence in proposing a more tailored treatment [
82].
Specifically for MR imaging, it should be noted that local recurrence detection can potentially be hampered by T2-signal distortions induced by the previous RT treatment; nevertheless, the integration of diffusion-weighted imaging (DWI) and dynamic contrast-enhanced (DCE) imaging is expected to overcome these limitations [
83,
84].
Through an online adaptive workflow, MR-guided SBRT treatments can provide a better sparing of healthy structures, which is a crucial issue especially in the setting of re-irradiation. Compared to conventional CT-based image-guidance, the MRI-based IGRT may be the optimal choice for prostate re-irradiation SBRT.
This entry is adapted from the peer-reviewed paper 10.3390/cancers13081791