Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Plant Sciences
Microalgal triacylglycerols (TAGs) are a good feedstock for liquid biofuel production. Improving the expression and/or function of transcription factors (TFs) involved in TAG accumulation may increase TAG content; however, information on microalgae is still lacking. In this study, 14 TFs in the unicellular red alga Cyanidioschyzon merolae were identified as candidate TFs regulating TAG accumulation using available transcriptome and phosphoproteome data under conditions driving TAG accumulation.
- algal biofuel
- lysophosphatidic acid acyltransferase
- red alga
- transcription factor
1. Introduction
Microalgae store energy in the form of storage molecules, such as neutral lipids that form cytoplasmic lipid droplets (LDs). The accumulated lipid is mainly in the form of triacylglycerols (TAGs), which can be harnessed for biofuel production, and the carbons of TAGs originate from the fixed CO2 via photosynthesis. Therefore, algal-based biofuels represent an important potential renewable energy system that could contribute to solving global warming [1][2][3]. However, large-scale industrial biofuel production systems using algae have not been constructed. A major reason for this is a lack of understanding of the underlying molecular mechanisms that control TAG accumulation in microalgae.
2. Previous Work
In our previous work, we had sought to clarify the mechanisms that regulate TAG biosynthesis using the unicellular model red alga Cyanidioschyzon merolae [4]. As observed with most microalgae, TAG content in these cells was enhanced after exposure to nitrogen depletion (–N) conditions [5]. Furthermore, we have elucidated that the TOR signaling pathway governs the accumulation of TAGs under –N conditions. TOR is a highly conserved protein kinase among eukaryotes, and it plays central roles in cell growth and stress responses by sensing environmental conditions, including available nutrients [6]. TOR kinase activity can be specifically inhibited by the TOR inhibitor rapamycin [7]. When we added rapamycin to the rapamycin-susceptible C. merolae F12 or SF12 strains [8][9] grown under standard growth conditions, the TAG content was increased to almost the same levels observed under –N conditions [5][9]. TAG accumulation after TOR inactivation was also observed for Chlamydomonas reinhardtii [10], Euglena gracilis [11], and Phaeodactylum tricornutum [12], indicating that the function of the TOR signaling pathway in modulating TAG accumulation is conserved among divergent eukaryotic algae [13].
Several studies have utilized enhanced expression of genes involved in TAG biosynthesis as a method for increasing TAG accumulation [14][15]. Driving the overexpression of transcription factors (TFs) that control the expression of TAG biosynthesis genes represents an efficient method for enhancing TAG accumulation, as each TF generally regulates sets of genes whose functions are interconnected [16]. Only a few TFs that control the accumulation of TAGs have so far been identified. For example, Boyle et al. identified NRR1, which encodes a SQUAMOSA promoter-binding protein domain TF, as induced under –N conditions. NRR1 is considered an important regulator of TAG synthesis under –N because the nrr1 mutant produces only ~50% TAG compared with the wild type [17]. Goncalves et al. revealed that ROC40, a MYB-related TF, also has a role in –N-induced TAG accumulation, as the roc40 mutant was impaired in its ability to increase the accumulation of TAG after exposure to –N [18].
In the present study, we used available transcriptome and phosphoproteome data obtained under –N and/or TOR inactivation conditions and identified four TFs and their regulatory genes that are involved in TAG accumulation in C. merolae. Based on these data, we demonstrate that one of the rate-limiting steps for TAG accumulation in this alga is catalyzed by endoplasmic reticulum (ER)-localized lysophosphatidic acid acyltransferase, which is regulated by the TFs.
3. Development and Findings
In this study, we identified four TFs involved in TAG accumulation; via the analysis of their target genes, we demonstrated that overexpression of ER-localized LPAT1 leads to TAG accumulation under normal growth conditions in C. merolae. Figure 1 illustrates a potential pathway for TAG accumulation through LPAT1. Under the –N or TOR inactivation conditions, the expression of four TFs (BRD1, HSF1, MYB3, and MYB4) increases, upregulating LPAT1 expression, and finally the resultant LPAT1 leads to TAG accumulation. TFs generally regulate sets of genes with interconnected functions, and thus, the identification of TFs involved in TAG accumulation is a valuable strategy for increasing TAG content. In this paper, we demonstrate the practicality and usefulness of this approach and elucidate fundamental knowledge regarding the regulation of TAG synthesis in microalgae.
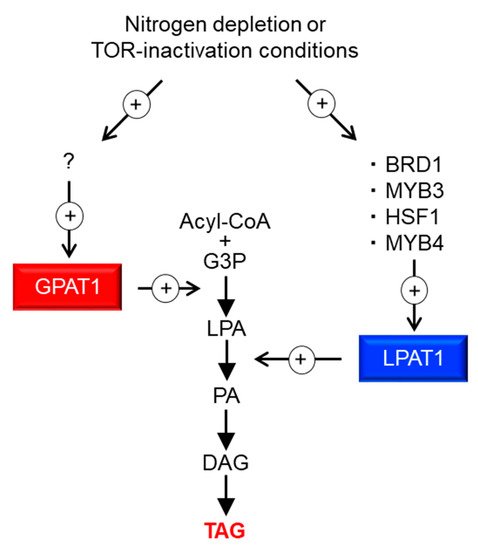
Figure 1. Possible model for the regulation of TAG synthesis in C. merolae. + with circle denotes positive effects. ? denotes an unidentified TF that regulates GPAT1 transcripts. G3P, glycerol-3-phosphate; LPA, lysophosphatidic acid; PA, phosphatidic acid; DAG, diacylglycerol; TAG, triacylglycerol.
Of the four identified TFs, MYB3 and MYB4 are single MYB-type TFs, signifying that they possess only one MYB domain that is involved in DNA-binding. It has been previously reported that ROC40, a single MYB-type TF, contributes to –N-induced lipid accumulation, including TAG, in Chlamydomonas [18]. Together, these findings suggest that single MYB-type TFs are involved in TAG accumulation in microalgae. In addition to the MYB-type TFs, two different types of TFs were identified as positive regulators of TAG accumulation in this study. HSF1 is annotated as a heat-shock TF in the C. merolae database (http://czon.jp, accessed on 5 May 2021). However, no induction of the HSF1 transcript could be confirmed by microarray analysis using RNA isolated after a 1 h 50 °C treatment (Kanesaki et al., unpublished data). It is conceivable that HSF1 is a stress-responsive TF that is regulated depending on environmental conditions, such as nutritional deficiency, since HSF1 transcripts were induced under –N and TOR inactivation conditions by rapamycin [5]. The other TF we identified is BRD1, which is annotated as similar to bromodomain-containing TF in the database. Our previous study revealed that BRD1 is phosphorylated and its phosphorylation status is reduced after TOR inhibition by rapamycin, indicating that it is regulated by the TOR signaling pathway [19]. This suggests that BRD1 functions in response to changes in the external environment. The bromodomain is a domain that recognizes acetylated histones [20]; hence, states of histone modification could influence the expression of the gene targeted by BRD1, including C. merolae LPAT1. However, this process is unclear and should be addressed in future studies. Furthermore, examining the generality of the function of HSF1 and BRD1 homologs on TAG accumulation in other microalgae will elucidate fundamental TAG regulation mechanisms in microalgae.
The TAG fatty acid composition varied among the four TF-overexpressing strains. This finding indicates that the functions of these TFs are similar (i.e., positively impacting TAG accumulation and LPAT1 expression) but not identical. In fact, the four identified TFs were not upregulated in common under –N and TOR inactivation conditions. Therefore, it is possible to identify new genes regulated by each TF involved in TAG biosynthesis with the purpose of accumulating high amounts of TAG by upregulating the genes’ functions. These points are the next objectives to be investigated regarding the control of TAG biosynthesis and accumulation. This step-by-step approach based on the fundamental molecular regulation of TAG synthesis should lead to successful biofuel production using microalgae.
In this study, we demonstrated that the reaction catalyzed by LPAT1 is one of the rate-limiting steps in TAG synthesis in C. merolae. Recently, Muñoz et al. [21] investigated the ER-localized LPAT function of Acutodesmus obliquus in Neochloris oleoabundans cells. The authors showed that TAGs were significantly accumulated compared with the wild type under nitrogen-deficient conditions. These observations indicate that this step, which is catalyzed by ER-localized LPAT, is a good target for increasing the TAG content in microalgae in general. Furthermore, our recent study on C. merolae revealed that ER-localized GPAT1, which encodes glycerol-3-phosphate acyltransferase, is the major rate-limiting step of TAG biosynthesis. We observed an increase in TAG contents in the GPAT1-overexpression strain to approximately 2.1% of the dry matter (0.2% of dry matter in the case of LPAT1ox), which is comparable to that observed after 72 h under –N (3.1% of dry matter), even under normal growth conditions [15]. Therefore, at least two rate-limiting steps with different magnitudes are involved in TAG synthesis in C. merolae (Figure 1). As such, inducing the overexpression of LPAT1 or TFs that regulate LPAT1 expression (BRD1, HSF1, MYB3, or MYB4) in addition to GPAT1 would be a good strategy to increase the amount of TAG accumulation. Moreover, TF(s) that regulate GPAT1 transcripts are currently unknown (“?” in Figure 1), but another strategy to increase intracellular TAG content could be via the overexpression of GPAT1-regulating TF(s) and/or the other positive regulators identified in this study. By contrast, in some green algae, it is generally accepted that the rate-limiting step of TAG synthesis is catalyzed by ER-localized diacylglycerol acyltransferase (DGAT) [22][23][24]; thus, the rate-limiting step in TAG synthesis is complex and varies depending on the microalgal strain. For further improvement of TAG accumulation in microalga cells, we should revalidate the established theory for the regulation of TAG synthesis in microalgae.
This entry is adapted from the peer-reviewed paper 10.3390/plants10050971
References
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306.
- Chisti, Y. Biodiesel from microalgae beats bioethanol. Trends Biotechnol. 2008, 26, 126–131.
- Parmar, A.; Singh, N.K.; Pandey, A.; Gnansounou, E.; Madamwar, D. Cyanobacteria and microalgae: A positive prospect for biofuels. Bioresour. Technol. 2011, 102, 10163–10172.
- Matsuzaki, M.; Misumi, O.; Shin-I, T.; Maruyama, S.; Takahara, M.; Miyagishima, S.Y.; Mori, T.; Nishida, K.; Yagisawa, F.; Nishida, K.; et al. Genome sequence of the ultrasmall unicellular red alga Cyanidioschyzon merolae 10D. Nature 2014, 428, 653–657.
- Imamura, S.; Kawase, Y.; Kobayashi, I.; Sone, T.; Era, A.; Miyagishima, S.Y.; Shimojima, M.; Ohta, H.; Tanaka, K. Target of rapamycin (TOR) plays a critical role in triacylglycerol accumulation in microalgae. Plant Mol. Biol. 2015, 89, 309–318.
- Saxton, R.A.; Sabatini, D.M. mTOR signaling in growth, metabolism, and disease. Cell 2017, 168, 960–976.
- Heitman, J.; Movva, N.R.; Hall, M.N. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science 1991, 253, 905–909.
- Imamura, S.; Ishiwata, A.; Watanabe, S.; Yoshikawa, H.; Tanaka, K. Expression of budding yeast FKBP12 confers rapamycin susceptibility to the unicellular red alga Cyanidioschyzon merolae. Biochem. Biophys. Res. Commun. 2013, 439, 264–269.
- Imamura, S.; Taki, K.; Tanaka, K. Construction of a rapamycin-susceptible strain of the unicellular red alga Cyanidioschyzon merolae for analysis of the target of rapamycin (TOR) function. J. Gen. Appl. Microbiol. 2017, 63, 305–309.
- Imamura, S.; Kawase, Y.; Kobayashi, I.; Shimojima, M.; Ohta, H.; Tanaka, K. TOR (target of rapamycin) is a key regulator of triacylglycerol accumulation in microalgae. Plant Signal. Behav. 2016, 11, e1149285.
- Mukaida, S.; Ogawa, T.; Ohishi, K.; Tanizawa, Y.; Ohta, D.; Arita, M. The effect of rapamycin on biodiesel-producing protist Euglena gracilis. Biosci. Biotechnol. Biochem. 2016, 80, 1223–1229.
- Prioretti, L.; Carriere, F.; Field, B.; Avilan, L.; Montané, M.H.; Menand, B.; Gontero, B. Targeting TOR signaling for enhanced lipid productivity in algae. Biochimie 2019, 169, 12–17.
- Pancha, I.; Chokshi, K.; Tanaka, K.; Imamura, S. Microalgal Target of Rapamycin (TOR): A Central Regulatory Hub for Growth, Stress Response and Biomass Production. Plant Cell Physiol. 2020, 61, 675–684.
- Du, Z.Y.; Benning, C. Triacylglycerol Accumulation in Photosynthetic Cells in Plants and Algae. Subcell. Biochem. 2016, 86, 179–205.
- Fukuda, S.; Hirasawa, E.; Takemura, T.; Takahashi, S.; Chokshi, K.; Pancha, I.; Tanaka, K.; Imamura, S. Accelerated triacylglycerol production without growth inhibition by overexpression of a glycerol-3-phosphate acyltransferase in the unicellular red alga Cyanidioschyzon merolae. Sci. Rep. 2018, 8, 12410.
- de Nadal, E.; Ammerer, G.; Posas, F. Controlling gene expression in response to stress. Nat. Rev. Genet. 2011, 12, 833–845.
- Boyle, N.R.; Page, M.D.; Liu, B.; Blaby, I.K.; Casero, D.; Kropat, J.; Cokus, S.J.; Hong-Hermesdorf, A.; Shaw, J.; Karpowicz, S.J.; et al. Three acyltransferases and nitrogen-responsive regulator are implicated in nitrogen starvation-induced triacylglycerol accumulation in Chlamydomonas. J. Biol. Chem. 2012, 287, 15811–15825.
- Goncalves, E.C.; Koh, J.; Zhu, N.; Yoo, M.J.; Chen, S.; Matsuo, T.; Johnson, J.V.; Rathinasabapathi, B. Nitrogen starvation-induced accumulation of triacylglycerol in the green algae: Evidence for a role for ROC40, a transcription factor involved in circadian rhythm. Plant J. 2016, 85, 743–757.
- Pancha, I.; Shima, H.; Higashitani, N.; Igarashi, K.; Higashitani, A.; Tanaka, K.; Imamura, S. Target of rapamycin-signaling modulates starch accumulation via glycogenin phosphorylation status in the unicellular red alga Cyanidioschyzon merolae. Plant J. 2019, 97, 485–499.
- Musselman, C.A.; Lalonde, M.E.; Côté, J.; Kutateladze, T.G. Perceiving the epigenetic landscape through histone readers. Nat. Struct. Mol. Biol. 2012, 19, 1218–1227.
- Muñoz, C.F.; Weusthuis, R.A.; D’Adamo, S.; Wijffels, R.H. Effect of Single and Combined Expression of Lysophosphatidic Acid Acyltransferase, Glycerol-3-Phosphate Acyltransferase, and Diacylglycerol Acyltransferase on Lipid Accumulation and Composition in Neochloris oleoabundans. Front. Plant Sci. 2019, 10, 1573.
- Iwai, M.; Ikeda, K.; Shimojima, M.; Ohta, H. Enhancement of extraplastidic oil synthesis in Chlamydomonas reinhardtii using a type-2 diacylglycerol acyltransferase with a phosphorus starvation-inducible promoter. Plant Biotechnol. J. 2014, 12, 808–819.
- Li, D.W.; Cen, S.Y.; Liu, Y.H.; Balamurugan, S.; Zheng, X.Y.; Alimujiang, A.; Yang, W.D.; Liu, J.S.; Li, H.Y. A type 2 diacylglycerol acyltransferase accelerates the triacylglycerol biosynthesis in heterokont oleaginous microalga Nannochloropsis oceanica. J. Biotechnol. 2016, 229, 65–71.
- Klaitong, P.; Fa-Aroonsawat, S.; Chungjatupornchai, W. Accelerated triacylglycerol production and altered fatty acid composition in oleaginous microalga Neochloris oleoabundans by overexpression of diacylglycerol acyltransferase 2. Microb. Cell Fact. 2017, 16, 61.
This entry is offline, you can click here to edit this entry!
