Postprandial hyperglycemia (PPHG) is strongly linked with the future development of cardiovascular complications in type 2 diabetes (T2D). . Hence, reducing postprandial glycemic excursions is essential in T2D treatment to slow progressive deficiency of β-cell function and prevent cardiovascular complications. Most of the metabolic processes involved in PPHG, i.e., β-cell secretory function, GLP-1 secretion, insulin sensitivity, muscular glucose uptake, and hepatic glucose production, are controlled by the circadian clock and display daily oscillation. Consequently, postprandial glycemia displays diurnal variation with a higher glycemic response after meals with the same carbohydrate content, consumed at dusk compared to the morning. T2D and meal timing schedule not synchronized with the circadian clock (i.e., skipping breakfast) are associated with disrupted clock gene expression and are linked to PPHG. In contrast, greater intake in the morning (i.e., high energy breakfast) than in the evening has a resetting effect on clock gene oscillations and beneficial effects on weight loss, appetite, and reduction of PPHG, independently of total energy intake. Therefore, resetting clock gene expression through a diet intervention consisting of meal timing aligned to the circadian clock, i.e., shifting most calories and carbohydrates to the early hours of the day, is a promising therapeutic approach to improve PPHG in T2D. This review will focus on recent studies, showing how a high-energy breakfast diet (Bdiet) has resetting and synchronizing actions on circadian clock genes expression, improving glucose metabolism, postprandial glycemic excursions along weight loss in T2D.
- clock genes 2
- big breakfast 3
- PPHG 4
- T2D 5
- circadian rhythms
Role of High Energy Breakfast “Big Breakfast Diet” in Clock Gene Regulation of Postprandial Hyperglycemia and Weight Loss in Type 2 Diabetes
1. Introduction
Postprandial hyperglycemia (PPHG) in type 2 diabetes (T2D) strongly contributes to glycated hemoglobin (HbA1c) values [1]. It is linked to increased risk for the development of cardiovascular complications, even when glycemic control is restored [2][3]. Further, PPHG leads to a progressive decline of β-cell function and deficient and delayed early postprandial insulin response [4][5][6]. Hence, the reduction of glycemic peaks is an essential “target” in the treatment of T2D to mitigate the decline of β-cell secretion and prevent cardiovascular complications [2][6][7].
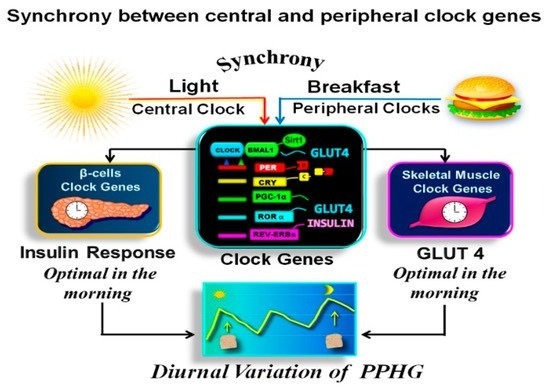
The circadian clock temporally coordinates the metabolism over a 24-h period to anticipate daily recurring feeding-fasting cycles and optimize the metabolic efficiency at an appropriate time of the day, thereby preventing metabolic dysregulation [8][9][10][11][12][13][14][15][16]. Most of the hormonal and enzymatic functions controlling PPHG, i.e., secretion of insulin [17][18][19], glucagon-like peptide-1 (GLP-1) [20], GLUT-4 expression in skeletal muscle [21][22], and hepatic glucose production [23][24]; are regulated by the circadian clock and display diurnal variations.
The circadian clock is controlled by light/dark signals and other external inputs such as meal timing or food availability [12][13][14]. The insulin sensitivity, β-cell responsiveness, GLUT-4 activity, and muscular glucose uptake are all enhanced in the early hours of the day compared to dusk or evening [8][16][17][18][19][20][21][22][23][25][26][27][28][29][30][31][32][33]. Therefore, the metabolism is optimized for food intake in breakfast, while the evening and nighttime are optimal for fasting and sleep [9][34][35][36][37]. Indeed, postprandial glycemia displays a clear circadian pattern, with the higher glycemic response after meals with the same carbohydrate content, consumed at dusk compared to the morning, both in healthy [23][27][29][33][38][39], and T2D individuals [14][15][25][35][40].
Meal timing exerts a critical influence on peripheral clocks involved in postprandial glycemia [14][15][41][42][43][44].
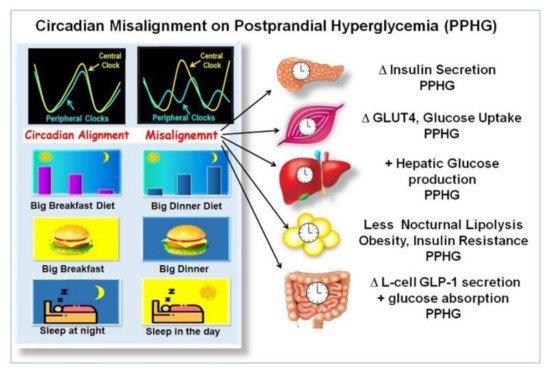
Circadian misalignment, with day/night cycle, often imposed in modern society, like shift workers, skipping breakfast, snacking all day, including in the evening hours, are associated with disrupted clock gene expression and linked with aberrant metabolic responses, weight gain, PPHG, increased risk for T2D [12][27][34][37][42][45][46][47][48][49][50][51][52], and cardiovascular complications [53]. Breakfast skipping is also linked to a significant increase in HbA1c even without overeating in the evening [47].
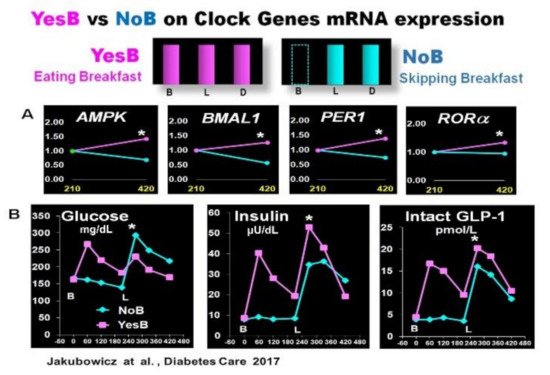
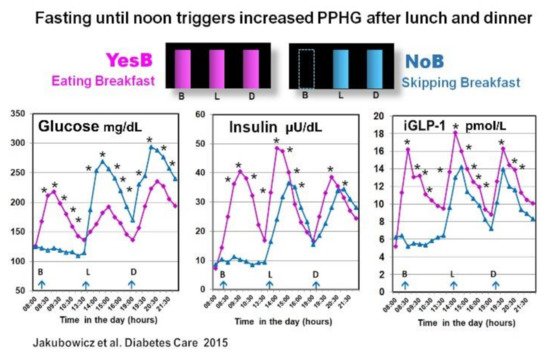
Asynchrony of the circadian clock is central in the pathophysiology of T2D [12][13][54]. Lower transcripts of clock gene expression in T2D are linked to insulin resistance, delayed β-cell secretion and reduced β-cell proliferation [12][27][34][37][48][55], PPHG, and increased HbA1c [15][36][43][54]. Moreover, breakfast’s omission in T2D patients causes further disruption in clock gene expression, and it is linked to PPHG and delayed and deficient early insulin and GLP-1 responses after subsequent meals [15][43][49]. In contrast, meal-timing pattern, aligned with the circadian clock, consuming high in energy breakfast, exerts a powerful effect on the clock network temporal synchronization, thereby improving the postprandial glycemic responses across the day in healthy and T2D patients [15][35][39][43][56].
Therefore, breakfast consumption might be critical in T2D for the achievement of metabolic homeostasis and improvement of PPHG [14][15][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][43][44][45][46][47][48][49][50][51][52][53][54][55][56]. Hence, resetting clock gene expression through a diet intervention consisting of meal timing aligned to the circadian clock is a promising therapeutic intervention approach to improve PPHG [12][15][27][43].
2. Circadian Clock Regulation of Metabolism
2.1. Central and Peripheral Clocks
2.2. Molecular Mechanism of the Circadian Clock-Driven Metabolism
2.3. Circadian Clock Regulation of Glucose Metabolism and Postprandial Glycemia
2.4. Disrupted Clock Genes Expression in Type 2 Diabetes
2.5. Synchronization between Central and Peripheral Clocks
2.6. Asynchrony between Central and Peripheral Clocks
3. Effect of High Energy Breakfast “Big Breakfast Diet” on Resetting Clock Gene Expression and Reduction of PPHG in T2D
The circadian clock regulation of PPHG is influenced by the meal timing schedule [14][41][42][44]. Breakfast skipping and over-eating in the evening led to asynchrony of the circadian clock and is linked to weight gain, PPHG, and diabetes [43][48][49][50].
Several recent reports suggest that eating in synchrony with the circadian clock by shifting more energy and CH to the morning hours (i.e., high energy and CH breakfast), and reducing energy and CH consumption in the evening hours, facilitate weight loss, improve postprandial glycemia, and reduce appetite and craving in metabolic syndrome and in T2D, compared to the inverse pattern, i.e., “high in energy and CH dinner” and reduced breakfast [15][27][30][35][39][40][61][73][74][75][76][77]. Clinical and epidemiological studies have shown that late meals are linked to obesity and T2D [39][40][47]. A diet intervention not aligned with the circadian clock by shifting calories and CH to later hours of the day is associated with less weight loss and higher postprandial and overall glycemia among obese [39][40] and in T2D individuals [15][35].
3.1. Effect Skipping Versus Eating Breakfast, on Clock Gene Expression and PPHG
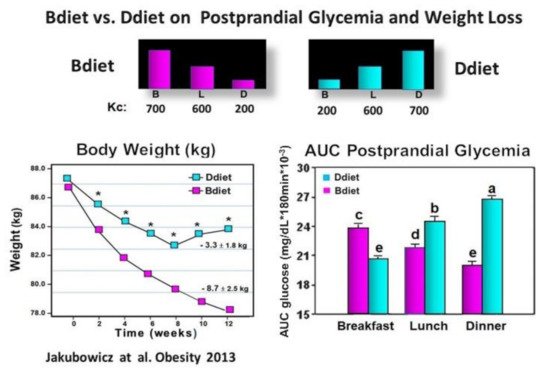
3.2. High Energy Breakfast Diet “Breakfast Diet” (Bdiet) Reduces overall Postprandial Glycemia and Body Weight in Metabolic Syndrome
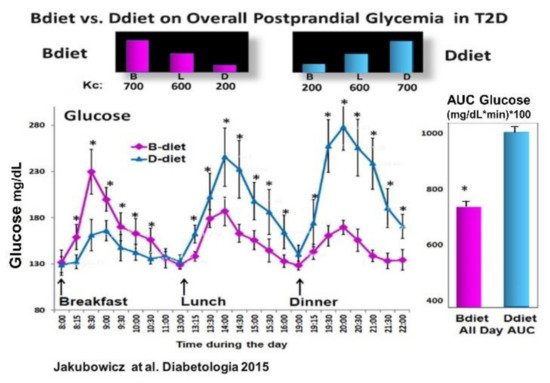
3.3. High Energy Breakfast Diet “Breakfast Diet” (Bdiet) Versus High Energy Dinner Diet (Ddiet) Reduces overall PPHG in Type 2 Diabetes
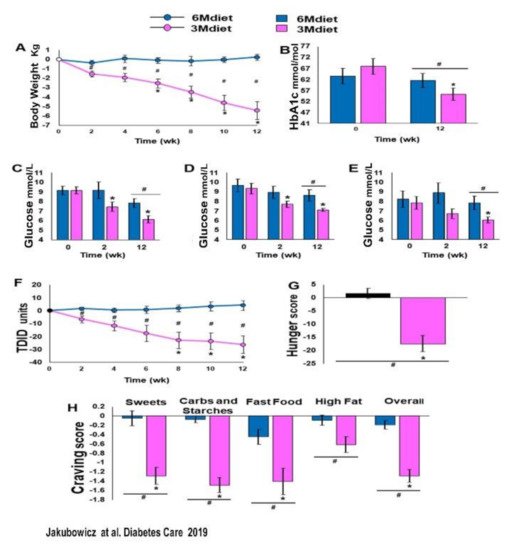
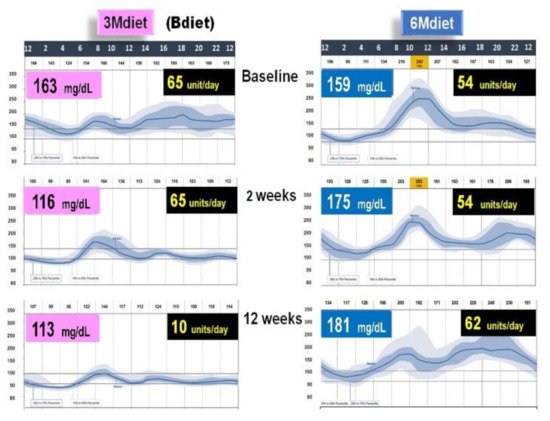
3.4. High Energy Breakfast Diet “Breakfast Diet” (3Mdiet-Bdiet) Versus Traditional Six Meals Diet (6Mdiet) Reduces overall Glycemia, Body Weight and Insulin Dose Requirements in Type 2 Diabetes
3.5. Addition of Whey Protein to High Energy Breakfast (Bdiet), Enhance the Reduction Postprandial Hyperglycemia and Body Weight in Type 2 Diabetes
As we described above, the Bdiet schedule resulted in a significant reduction in overall postprandial glycemia and weight reduction in obese non-diabetics [35][40] and T2D individuals [15][40][56]. Bdiet also led in T2D individuals to a significant decrease in HbA1c [74]. The lowering effect of the Bdiet on the overall PPHG was associated with a greater and earlier increase in GLP-1 and insulin responses after breakfast, lunch, and dinner, suggesting a day-long effect of Bdiet [35][56][75].
It has been reported that increased protein (>35 g) intake in the breakfast leads to the reduction of all-day postprandial glycemic excursions [85][86] and greater GLP-1 and insulin and response after breakfast, lunch, and dinner [35][56].
In addition to the protein load, the source and quality of the protein ingested in the breakfast are critical for its lowering effect on the glycemic postprandial response [87][88]. It was showed in previous studies that Whey protein exerts a greater lowering effect on postprandial glucose compared to other proteins such as eggs, soy, gluten, fish, or casein in healthy [89][90][91] and T2D individuals [76][92][93][94].
Particularly, Whey milk protein that accounts for 20% of whole milk protein has insulinotropic/β-cell-stimulating and glucose-lowering effects through bioactive peptides and amino acids generated during its gastrointestinal digestion [89][90].
These bioactive peptides can stimulate several gut hormones, including GLP-1, which stimulate β-cells insulin secretion [89][92][93][94][95], and reduce the activity of DPP-4, attenuating the degradation of GLP-1 in the proximal gut [94]. Hence, Whey protein pre-load breakfast may be a potential mode to stimulate GLP-1 secretion, thereby augmenting the early insulin response and reducing postprandial glucose.
In an acute crossover study, T2D patients consumed a high glycemic index breakfast, one day preloaded with a drink containing 50 g of Whey protein and other day preloaded with water. Breakfast preloaded with Whey protein vs. water displayed a significant reduction in postprandial glucose response [76] (Figure 9). Furthermore, the addition of Whey pre-load before breakfast led to a substantial increase of postprandial GLP-1 response, predominantly during the early interval, and significantly stimulated the early insulin response and the insulin post-breakfast peak [76] (Figure 9).
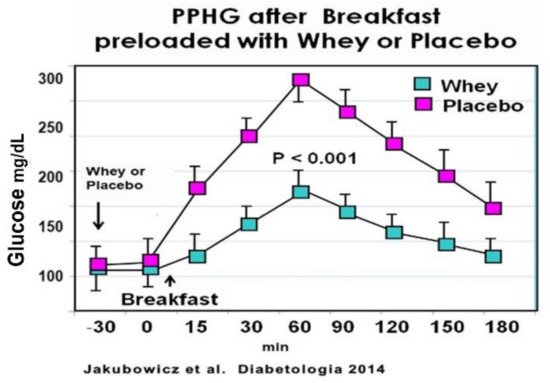
These results are in line with previous reports showing that Whey protein pre-load exerts a potent stimulatory effect on β-cell secretion, reducing postprandial glycemia in healthy [79][80][93][94] and T2D patients [92][94]. This enhanced and almost restored early insulin secretion after Whey pre-load is important since a deficiency or loss of this early insulin response is a key abnormality contributing to hyperglycemia and T2D [4][5][6].
The increase of the postprandial GLP-1 after Whey pre-load occurred in a parallel fashion with the insulinotropic effect. This correlation supports that the higher incretin response is the mechanism subserving the more rapid and higher insulin response in the Whey protein group.
Whey protein pre-load of high glycemic index breakfast stimulated the postprandial total and intact GLP-1 responses, the early prandial insulin secretion, and significantly reduced the PPHG in T2D patients. Therefore, Whey protein may represent a novel glucose-lowering strategy in T2D [76].
We further explored in T2D patients the long-term effect of Whey protein. In this study in T2D, we tested during 12 weeks the long-term influence of Whey protein on weight loss, reduction of overall PPHG, and HbA1c. We used a well-established feeding regimen (Bdiet) consisting of a high-calorie and protein breakfast, medium-sized lunch, and low-calorie dinner [35][39][56][74] (Figure 10).
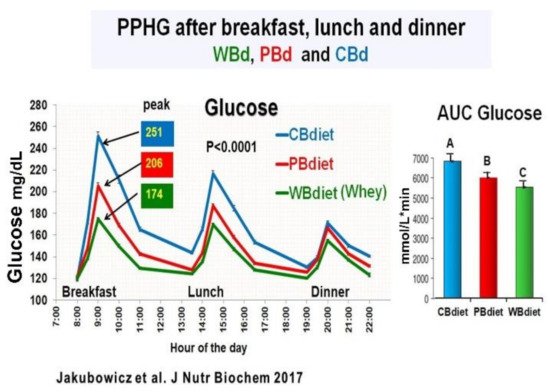
The participants were randomly assigned to one of the three diet intervention groups The only difference among the three diet interventions was the breakfast composition: (1) Whey protein breakfast diet (WBdiet), with high protein content at breakfast, containing Whey as the primary source of protein; (2) Protein breakfast diet (PBdiet), with high protein content at breakfast from other sources, i.e., eggs, tuna, soy; and (3) Carbohydrate breakfast diet (CBdiet), with low protein and high CH content in the breakfast [75]. All patients underwent 3 all-day meal challenges testing WBdiet, PBdiet, and CBdiet (Figure 10).
Although the three diet interventions (CBdiet, PBdiet, WBdiet) had similar lunch and dinner composition, the effect during a meal challenge on postprandial glycemia, insulin, GLP-1, ghrelin, glucagon, and appetite scores was not limited to breakfast but extended to subsequent meals, i.e., lunch and dinner [75].
Compared to PBdiet and CBdiet, the Whey in the breakfast group (WBdiet) showed the lowest overall PPHG and overall AUC for postprandial plasma glucose, ghrelin, and hunger, and highest overall AUC for postprandial plasma insulin, C-peptide, intact GLP-1, and satiety scores. PBdiet showed similar benefits than WBdiet but less pronounced. WBdiet also led to a greater reduction of HbA1c and body weight compared to the other groups. The greatest reduction of PPHG was achieved after breakfast containing Whey (WBdiet) is supported by the increased GLP-1 and its insulinotropic effect, as reported in healthy and T2D individuals [25][80][81][89][94] (Figure 10).
Whey protein and its main components, i.e., alpha-lactalbumin, β-lactoglobulin, and the bioactive peptides generated during its gastrointestinal digestion, are potent secretagogues of L-cells, increasing GLP-1 intracellular levels and production [25][94][95][96]. Whey protein also activates mTOR in the intestinal L cells, increasing prandial GLP-1 secretion, further stimulating β-cell postprandial response [94][95][96]. The increased GLP-1 release in WBdiet, might explain the beneficial effect of Whey consumption on PPHG and enhanced insulin release at subsequent meals after breakfast [25][80][81][94]. Whey protein ingestion is also associated with a rapid increase in plasma amino acid concentrations, specifically leucine, isoleucine, and valine, exerting a potent direct insulinotropic/β-cell-stimulating effect [89][94].
This study showed that in T2D individuals, a diet consisting of high-energy breakfast, medium-sized lunch, and reduced energy dinner is more beneficial in reducing overall PPHG, body weight, and HbA1c levels, when the primary protein source at breakfast is Whey, indicating that Whey protein at breakfast might be a potent adjuvant for the management of type 2 diabetes [75].
4. Conclusions Remarks
Postprandial hyperglycemia in T2D leads to a progressive decline of β-cell function and increases the cardiovascular risk in T2D [2,6,7]. Therefore, DI meal timing and composition should focus on mitigating glycemic peaks to reduce the decline of β-cell function and prevent cardiovascular complications [4].
Almost all hormonal and enzymatic processes influencing the PPHG, like muscular glucose uptake, the secretion of GLP-1, and β-cell insulin production, are regulated by the circadian clock and display circadian rhythms [17–24]. The circadian clock is synchronized to the day/night cycle and food cues, namely, meal timing and food availability [13,14].
As a consequence of circadian clock regulation, the β-cell secretion and responsiveness, insulin sensitivity, and muscular glucose uptake are enhanced in the early hours of the day compared to afternoon and night [8,16–23,25–33]. Therefore, the metabolism is optimal for food intake in the morning (i.e., breakfast). In contrast, the evening and nighttime are optimal for fasting and sleep [9,34–37].
Meal timing, independently of the total energy intake, exerts a critical influence on peripheral clocks genes involved in regulating metabolic processes and PPHG [41–47]. Several recent reports suggested metabolic disadvantages of reduced energy breakfast and high energy and CH consumption in evening hours. While high energy and CH consumption shifted into morning hours, “high energy breakfast” (Bdiet) increased the weight loss, insulin sensitivity and reduced the overall postprandial glycemia in obese and prediabetics [39,40,77], and substantially decrease the PPHG and HbA1c in diabetic individuals [15,35,74,75]. Moreover, in T2D, the omission of breakfast disrupts circadian clock gene expression and is linked to worsening of PPHG and delayed and deficient early insulin and GLP-1 response after subsequent meals [15,43,49].
In contrast, meal-timing pattern, aligned with the circadian clock, consuming high energy and CH breakfast (Bdiet) exerts a powerful synchronizing effect on pivotal clock gene expression. It leads to a significant reduction of postprandial glycemic peaks across the day and enhanced insulin, C-peptide, and GLP-1 postprandial responses in healthy and T2D patients. Therefore, breakfast consumption is critical for achieving metabolic homeostasis and improving PPHG in T2D [15,30,35,73,74].
Synchronization of the clock gene expression through a diet intervention consisting of meal timing aligned to the circadian clock by shifting more calories and CH to the early hours of the day (Bdiet) is a promising strategy for therapeutic interventions to improve PPHG, weight loss, and to prevent cardiometabolic complication in type 2 diabetes.
This entry is adapted from the peer-reviewed paper 10.3390/nu13051558
References
- Monnier, L.; Lapinski, H.; Colette, C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: Variations with increasing levels of HbA1c. Diabetes Care 2003, 26, 881–885.
- Ceriello, A.; Colagiuri, S.; Gerich, J.; Tuomilehto, J. Guideline for management of postmeal glucose. Nutr. Metab. Cardiovasc. Dis. 2008, 18, S17–S33.
- Szuszkiewicz-Garcia, M.M.; Davidson, J.A. Cardiovascular disease in diabetes mellitus: Risk factors and medical therapy. Endocrinol. Metab. Clin. N. Am. 2014, 43, 25–40.
- Bruce, D.G.; Chisholm, D.J.; Storlien, L.H.; Kraegen, E.W. Physiological importance of deficiency in early prandial insulin secretion in non-insulin-dependent diabetes. Diabetes 1988, 37, 736–744.
- Kahn, S.E. The Importance of β-Cell Failure in the Development and Progression of Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2001, 86, 4047–4058.
- Del Prato, S. Loss of early insulin secretion leads to postprandial hyperglycaemia. Diabetologia 2003, 46.
- Fonseca, V. Clinical significance of targeting postprandial and fasting hyperglycemia in managing type 2 diabetes mellitus. Curr. Med. Res. Opin. 2003, 19, 631–635.
- Oike, H. Modulation of circadian clocks by nutrients and food factors. Biosci. Biotechnol. Biochem. 2017, 81, 863–870.
- Poggiogalle, E.; Jamshed, H.; Peterson, C.M. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism 2018, 84, 11–27.
- Panda, S. Circadian physiology of metabolism. Science 2016, 354, 1008–1015.
- Oosterman, J.E.; Kalsbeek, A.; La Fleur, S.E.; Belsham, D.D. Impact of nutrients on circadian rhythmicity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R337–R350.
- Koronowski, K.B.; Sassone-Corsi, P. Communicating clocks shape circadian homeostasis. Science 2021, 371, eabd0951.
- Javeed, N.; Matveyenko, A.V. Circadian etiology of type 2 diabetes mellitus. Physiology 2018, 33, 138–150.
- Froy, O.; Garaulet, M. The circadian clock in white and brown adipose tissue: Mechanistic, endocrine, and clinical aspects. Endocr. Rev. 2018, 39, 261–273.
- Jakubowicz, D.; Landau, Z.; Tsameret, S.; Wainstein, J.; Raz, I.; Ahren, B.; Chapnik, N.; Barnea, M.; Ganz, T.; Menaged, M.; et al. Reduction in Glycated Hemoglobin and Daily Insulin Dose Alongside Circadian Clock Upregulation in Patients with Type 2 Diabetes Consuming a Three-Meal Diet: A Randomized Clinical Trial. Diabetes Care 2019, dc191142.
- Jordan, S.D.; Lamia, K.A. AMPK at the crossroads of circadian clocks and metabolism. Mol. Cell. Endocrinol. 2013, 366, 163–169.
- Yoshino, J.; Imai, S.I. A clock ticks in pancreatic β cells. Cell Metab. 2010, 12, 107–108.
- Sadacca, L.A.; Lamia, K.A.; DeLemos, A.S.; Blum, B.; Weitz, C.J. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia 2011, 54, 120–124.
- Rakshit, K.; Qian, J.; Ernst, J.; Matveyenko, A.V. Circadian variation of the pancreatic islet transcriptome. Physiol. Genom. 2016, 48, 677–687.
- Gil-Lozano, M.; Mingomataj, E.L.; Wu, W.K.; Ridout, S.A.; Brubaker, P.L. Circadian secretion of the intestinal hormone GLP-1 by the rodent L cell. Diabetes 2014, 63, 3674–3685.
- Dyar, K.A.; Ciciliot, S.; Wright, L.E.; Biensø, R.S.; Tagliazucchi, G.M.; Patel, V.R.; Forcato, M.; Paz, M.I.P.; Gudiksen, A.; Solagna, F.; et al. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol. Metab. 2014, 3, 29–41.
- Prasai, M.J.; Mughal, R.S.; Wheatcroft, S.B.; Kearney, M.T.; Grant, P.J.; Scott, E.M. Diurnal variation in vascular and metabolic function in diet-induced obesity: Divergence of insulin resistance and loss of clock rhythm. Diabetes 2013, 62, 1981–1989.
- Saad, A.; Man, C.D.; Nandy, D.K.; Levine, J.A.; Bharucha, A.E.; Rizza, R.A.; Basu, R.; Carter, R.E.; Cobelli, C.; Kudva, Y.C.; et al. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes 2012, 61, 2691–2700.
- Basu, A.; Joshi, N.; Miles, J.; Carter, R.E.; Rizza, R.A.; Basu, R. Paradigm shifts in nocturnal glucose control in type 2 diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 3801–3809.
- Lindgren, O.; Mari, A.; Deacon, C.F.; Carr, R.D.; Winzell, M.S.; Vikman, J.; Ahrén, B. Differential islet and incretin hormone responses in morning versus afternoon after standardized meal in healthy men. J. Clin. Endocrinol. Metab. 2009, 94, 2887–2892.
- Ruddick-Collins, L.C.; Johnston, J.D.; Morgan, P.J.; Johnstone, A.M. The Big Breakfast Study: Chrono-nutrition influence on energy expenditure and bodyweight. Nutr. Bull. 2018, 43, 174–183.
- Jamshed, H.; Beyl, R.A.; Manna, D.L.D.; Yang, E.S.; Ravussin, E.; Peterson, C.M. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients 2019, 11, 1234.
- Bo, S.; Fadda, M.; Castiglione, A.; Ciccone, G.; De Francesco, A.; Fedele, D.; Guggino, A.; Parasiliti Caprino, M.; Ferrara, S.; Vezio Boggio, M.; et al. Is the timing of caloric intake associated with variation in diet-induced thermogenesis and in the metabolic pattern? A randomized cross-over study. Int. J. Obes. 2015, 39, 1689–1695.
- Morgan, L.M.; Shi, J.W.; Hampton, S.M.; Frost, G. Effect of meal timing and glycaemic index on glucose control and insulin secretion in healthy volunteers. Br. J. Nutr. 2012, 108, 1286–1291.
- Ravussin, E.; Beyl, R.A.; Poggiogalle, E.; Hsia, D.S.; Peterson, C.M. Early Time-Restricted Feeding Reduces Appetite and Increases Fat Oxidation but Does Not Affect Energy Expenditure in Humans. Obesity 2019, 27, 1244–1254.
- Gibbs, M.; Harrington, D.; Starkey, S.; Williams, P.; Hampton, S. Diurnal postprandial responses to low and high glycaemic index mixed meals. Clin. Nutr. 2014, 33, 889–894.
- Nitta, A.; Imai, S.; Kajiyama, S.; Miyawaki, T.; Matsumoto, S.; Ozasa, N.; Hashimoto, Y.; Tanaka, M.; Fukui, M. Impact of different timing of consuming sweet snack on postprandial glucose excursions in healthy women. Diabetes Metab. 2019, 45, 369–374.
- Van Cauter, E.; Shapiro, E.T.; Tillil, H.; Polonsky, K.S. Circadian modulation of glucose and insulin responses to meals: Relationship to cortisol rhythm. Am. J. Physiol. Endocrinol. Metab. 1992, 262.
- Scheer, F.A.J.L.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458.
- Jakubowicz, D.; Wainstein, J.; Ahrén, B.; Bar-Dayan, Y.; Landau, Z.; Rabinovitz, H.R.; Froy, O. High-energy breakfast with low-energy dinner decreases overall daily hyperglycaemia in type 2 diabetic patients: A randomised clinical trial. Diabetologia 2015, 58, 912–919.
- Stenvers, D.J.; Jongejan, A.; Atiqi, S.; Vreijling, J.P.; Limonard, E.J.; Endert, E.; Baas, F.; Moerland, P.D.; Fliers, E.; Kalsbeek, A.; et al. Diurnal rhythms in the white adipose tissue transcriptome are disturbed in obese individuals with type 2 diabetes compared with lean control individuals. Diabetologia 2019, 62, 704–716.
- Morris, C.J.; Yang, J.N.; Garcia, J.I.; Myers, S.; Bozzi, I.; Wang, W.; Buxton, O.M.; Shea, S.A.; Scheer, F.A.J.L. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc. Natl. Acad. Sci. USA 2015, 112, E2225–E2234.
- Wehrens, S.M.T.; Christou, S.; Isherwood, C.; Middleton, B.; Gibbs, M.A.; Archer, S.N.; Skene, D.J.; Johnston, J.D. Meal Timing Regulates the Human Circadian System. Curr. Biol. 2017, 27, 1768–1775.e3.
- Jakubowicz, D.; Barnea, M.; Wainstein, J.; Froy, O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity 2013, 21, 2504–2512.
- Jakubowicz, D.; Froy, O.; Wainstein, J.; Boaz, M. Meal timing and composition influence ghrelin levels, appetite scores and weight loss maintenance in overweight and obese adults. Steroids 2012, 77, 323–331.
- Sherman, H.; Frumin, I.; Gutman, R.; Chapnik, N.; Lorentz, A.; Meylan, J.; le Coutre, J.; Froy, O. Long-term restricted feeding alters circadian expression and reduces the level of inflammatory and disease markers. J. Cell. Mol. Med. 2011, 15, 2745–2759.
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.J.; et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012, 15, 848–860.
- Jakubowicz, D.; Wainstein, J.; Landau, Z.; Raz, I.; Ahren, B.; Chapnik, N.; Ganz, T.; Menaged, M.; Barnea, M.; Bar-Dayan, Y.; et al. Influences of Breakfast on Clock Gene Expression and Postprandial Glycemia in Healthy Individuals and Individuals with Diabetes: A Randomized Clinical Trial. Diabetes Care 2017, 40, 1573–1579.
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014, 20, 991–1005.
- Mekary, R.A.; Giovannucci, E.; Willett, W.C.; Van Dam, R.M.; Hu, F.B. Eating patterns and type 2 diabetes risk in men: Breakfast omission, eating frequency, and snacking. Am. J. Clin. Nutr. 2012, 95, 1182–1189.
- Nimitphong, H.; Siwasaranond, N.; Saetung, S.; Thakkinstian, A.; Ongphiphadhanakul, B.; Reutrakul, S. The relationship among breakfast time, morningness–eveningness preference and body mass index in Type 2 diabetes. Diabet. Med. 2018, 35, 964–971.
- Reutrakul, S.; Hood, M.M.; Crowley, S.J.; Morgan, M.K.; Teodori, M.; Knutson, K.L. The relationship between breakfast skipping, chronotype, and glycemic control in type 2 diabetes. Chronobiol. Int. 2014, 31, 64–71.
- Arble, D.M.; Bass, J.; Laposky, A.D.; Vitaterna, M.H.; Turek, F.W. Circadian timing of food intake contributes to weight gain. Obesity 2009, 17, 2100–2102.
- Wu, T.; Sun, L.; Zhuge, F.; Guo, X.; Zhao, Z.; Tang, R.; Chen, Q.; Chen, L.; Kato, H.; Fu, Z. Differential roles of breakfast and supper in rats of a daily three-meal schedule upon circadian regulation and physiology. Chronobiol. Int. 2011, 28, 890–903.
- Fuse, Y.; Hirao, A.; Kuroda, H.; Otsuka, M.; Tahara, Y.; Shibata, S. Differential roles of breakfast only (one meal per day) and a bigger breakfast with a small dinner (two meals per day) in mice fed a high-fat diet with regard to induced obesity and lipid metabolism. J. Circadian Rhythms 2012, 10.
- Sherman, H.; Genzer, Y.; Cohen, R.; Chapnik, N.; Madar, Z.; Froy, O. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J. 2012, 26, 3493–3502.
- Reinke, H.; Asher, G. Crosstalk between metabolism and circadian clocks. Nat. Rev. Mol. Cell Biol. 2019, 20, 227–241.
- St-Onge, M.P.; Ard, J.; Baskin, M.L.; Chiuve, S.E.; Johnson, H.M.; Kris-Etherton, P.; Varady, K. Meal Timing and Frequency: Implications for Cardiovascular Disease Prevention: A Scientific Statement from the American Heart Association. Circulation 2017, 135, e96–e121.
- Ando, H.; Ushijima, K.; Yanagihara, H.; Hayashi, Y.; Takamura, T.; Kaneko, S.; Fujimura, A. Clock gene expression in the liver and adipose tissues of non-obese type 2 diabetic Goto-Kakizaki rats. Clin. Exp. Hypertens. 2009, 31, 201–207.
- Vieira, E.; Burris, T.P.; Quesada, I. Clock genes, pancreatic function, and diabetes. Trends Mol. Med. 2014, 20, 685–693.
- Jakubowicz, D.; Wainstein, J.; Ahren, B.; Landau, Z.; Bar-Dayan, Y.; Froy, O. Fasting Until Noon Triggers Increased Postprandial Hyperglycemia and Impaired Insulin Response after Lunch and Dinner in Individuals with Type 2 Diabetes: A Randomized Clinical Trial. Diabetes Care 2015, 38, 1820–1826.
- Shimizu, H.; Hanzawa, F.; Kim, D.; Sun, S.; Laurent, T.; Umeki, M.; Ikeda, S.; Mochizuki, S.; Oda, H. Delayed first active-phase meal, a breakfastskipping model, led to increased body weight and shifted the circadian oscillation of the hepatic clock and lipid metabolism-related genes in rats fed a high-fat diet. PLoS ONE 2018, 13, e0206669.
- Kim, Y.H.; Lazar, M.A. Transcriptional control of circadian rhythms and metabolism: A matter of time and space. Endocr. Rev. 2021, 41, 707–732.
- Pilorz, V.; Astiz, M.; Heinen, K.O.; Rawashdeh, O.; Oster, H. The Concept of Coupling in the Mammalian Circadian Clock Network. J. Mol. Biol. 2020, 432, 3618–3638.
- Kuang, J.; Hou, X.; Zhang, J.; Chen, Y.; Su, Z. Identification of insulin as a novel retinoic acid receptor-related orphan receptor α target gene. FEBS Lett. 2014, 588, 1071–1079.
- Johnston, J.D. Physiological responses to food intake throughout the day. Nutr. Res. Rev. 2014, 27, 107–118.
- Sun, C.; Zhang, F.; Ge, X.; Yan, T.; Chen, X.; Shi, X.; Zhai, Q. SIRT1 Improves Insulin Sensitivity under Insulin-Resistant Conditions by Repressing PTP1B. Cell Metab. 2007, 6, 307–319.
- Biancolin, A.D.; Martchenko, A.; Mitova, E.; Gurges, P.; Michalchyshyn, E.; Chalmers, J.A.; Doria, A.; Mychaleckyj, J.C.; Adriaenssens, A.E.; Reimann, F.; et al. The core clock gene, Bmal1, and its downstream target, the SNARE regulatory protein secretagogin, are necessary for circadian secretion of glucagon-like peptide-1. Mol. Metab. 2020, 31, 124–137.
- Taira, A.; Arita, E.; Matsumoto, E.; Oohira, A.; Iwase, K.; Hiwasa, T.; Yokote, K.; Shibata, S.; Takiguchi, M. Systemic oscillator-driven and nutrient-responsive hormonal regulation of daily expression rhythms for gluconeogenic enzyme genes in the mouse liver. Chronobiol. Int. 2019, 36, 591–615.
- Pérez-Mendoza, M.; Rivera-Zavala, J.B.; Díaz-Muñoz, M. Daytime restricted feeding modifies the daily variations of liver gluconeogenesis: Adaptations in biochemical and endocrine regulators. Chronobiol. Int. 2014, 31, 815–828.
- Zhang, E.E.; Liu, Y.; Dentin, R.; Pongsawakul, P.Y.; Liu, A.C.; Hirota, T.; Nusinow, D.A.; Sun, X.; Landais, S.; Kodama, Y.; et al. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat. Med. 2010, 16, 1152–1156.
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262.
- Asher, G.; Gatfield, D.; Stratmann, M.; Reinke, H.; Dibner, C.; Kreppel, F.; Mostoslavsky, R.; Alt, F.W.; Schibler, U. SIRT1 Regulates Circadian Clock Gene Expression through PER2 Deacetylation. Cell 2008, 134, 317–328.
- Pinho, A.V.; Bensellam, M.; Wauters, E.; Rees, M.; Giry-Laterriere, M.; Mawson, A.; Ly, L.Q.; Biankin, A.V.; Wu, J.; Laybutt, D.R.; et al. Pancreas-specific Sirt1-deficiency in mice compromises β-cell function without development of hyperglycemia. PLoS ONE 2015, 10, e0128012.
- Nakahata, Y.; Sahar, S.; Astarita, G.; Kaluzova, M.; Sassone-Corsi, P. Circadian Control of the NAD+ Salvage Pathway by CLOCK-SIRT1. Science 2009, 324, 654–657.
- Wefers, J.; Van Moorsel, D.; Hansen, J.; Connell, N.J.; Havekes, B.; Hoeks, J.; Van Marken Lichtenbelt, W.D.; Duez, H.; Phielix, E.; Kalsbeek, A.; et al. Circadian misalignment induces fatty acid metabolism gene profiles and compromises insulin sensitivity in human skeletal muscle. Proc. Natl. Acad. Sci. USA 2018, 115.
- Vetter, C.; Devore, E.E.; Ramin, C.A.; Speizer, F.E.; Willett, W.C.; Schernhammer, E.S. Mismatch of sleep and work timing and risk of type 2 diabetes. Diabetes Care 2015, 38, 1707–1713.
- Kahleova, H.; Belinova, L.; Malinska, H.; Oliyarnyk, O.; Trnovska, J.; Skop, V.; Kazdova, L.; Dezortova, M.; Hajek, M.; Tura, A.; et al. Eating two larger meals a day (breakfast and lunch) is more effective than six smaller meals in a reduced-energy regimen for patients with type 2 diabetes: A randomised crossover study. Diabetologia 2014, 57, 1552–1560.
- Rabinovitz, H.R.; Boaz, M.; Ganz, T.; Jakubowicz, D.; Matas, Z.; Madar, Z.; Wainstein, J. Big breakfast rich in protein and fat improves glycemic control in type 2 diabetics. Obesity 2014, 22, E46–E54.
- Jakubowicz, D.; Wainstein, J.; Landau, Z.; Ahren, B.; Barnea, M.; Bar-Dayan, Y.; Froy, O. High-energy breakfast based on whey protein reduces body weight, postprandial glycemia and HbA 1C in Type 2 diabetes. J. Nutr. Biochem. 2017, 49, 1–7.
- Jakubowicz, D.; Froy, O.; Ahrén, B.; Boaz, M.; Landau, Z.; Bar-Dayan, Y.; Ganz, T.; Barnea, M.; Wainstein, J. Incretin, insulinotropic and glucose-lowering effects of whey protein pre-load in type 2 diabetes: A randomised clinical trial. Diabetologia 2014, 57, 1807–1811.
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221e.3.
- Lee, S.H.; Tura, A.; Mari, A.; Ko, S.H.; Kwon, H.S.; Song, K.H.; Yoon, K.H.; Lee, K.W.; Ahn, Y.B. Potentiation of the early-phase insulin response by a prior meal contributes to the second-meal phenomenon in type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2011, 301.
- Jovanovic, A.; Gerrard, J.; Taylor, R. The second-meal phenomenon in type 2 diabetes. Diabetes Care 2009, 32, 1199–1201.
- Korsgaard, T.V.; Colding-Jørgensen, M. Time-dependent mechanisms in β-cells glucose sensing. J. Biol. Phys. 2006, 32, 289–306.
- Goginashvili, A.; Zhang, Z.; Erbs, E.; Spiegelhalter, C.; Kessler, P.; Mihlan, M.; Pasquier, A.; Krupina, K.; Schieber, N.; Cinque, L.; et al. Insulin granules. Insulin secretory granules control autophagy in pancreatic β cells. Science 2015, 347, 878–882.
- Efendic, S.; Portwood, N. Overview of incretin hormones. Horm. Metab. Res. 2004, 36, 742–746.
- Richards, J.; Diaz, A.N.; Gumz, M.L. Clock genes in hypertension: Novel insights from rodent models. Blood Press Monit. 2014, 19, 249–254.
- Garaulet, M.; Gómez-Abellán, P.; Alburquerque-Béjar, J.J.; Lee, Y.C.; Ordovás, J.M.; Scheer, F.A.J.L. Timing of food intake predicts weight loss effectiveness. Int. J. Obes. 2013, 37, 604–611.
- Rains, T.M.; Leidy, H.J.; Sanoshy, K.D.; Lawless, A.L.; Maki, K.C. A randomized, controlled, crossover trial to assess the acute appetitive and metabolic effects of sausage and egg-based convenience breakfast meals in overweight premenopausal women. Nutr. J. 2015, 14.
- Park, Y.M.; Heden, T.D.; Liu, Y.; Nyhoff, L.M.; Thyfault, J.P.; Leidy, H.J.; Kanaley, J.A. A high-protein breakfast induces greater insulin and glucose-dependent insulinotropic peptide responses to a subsequent lunch meal in individuals with type 2 diabetes. J. Nutr. 2015, 145, 452–458.
- Bendtsen, L.Q.; Lorenzen, J.K.; Larsen, T.M.; van Baak, M.; Papadaki, A.; Martinez, J.A.; Handjieva-Darlenska, T.; Jebb, S.A.; Kunešová, M.; Pfeiffer, A.F.; et al. Associations between dairy protein intake and body weight and risk markers of diabetes and CVD during weight maintenance. Br. J. Nutr. 2014, 111, 944–953.
- Pasiakos, S.M. Metabolic advantages of higher protein diets and benefits of dairy foods on weight management, glycemic regulation, and bone. J. Food Sci. 2015, 80, A2–A7.
- Gunnerud, U.J.; Heinzle, C.; Holst, J.J.; Östman, E.M.; Björck, I.M.E. Effects of pre-meal drinks with protein and amino acids on glycemic and metabolic responses at a subsequent composite meal. PLoS ONE 2012, 7, e44731.
- Nilsson, M.; Holst, J.J.; Björck, I.M. Metabolic effects of amino acid mixtures and whey protein in healthy subjects: Studies using glucose-equivalent drinks. Am. J. Clin. Nutr. 2007, 85, 996–1004.
- Frid, A.H.; Nilsson, M.; Holst, J.J.; Björck, I.M.E. Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects. Am. J. Clin. Nutr. 2005, 82, 69–75.
- Ma, J.; Stevens, J.E.; Cukier, K.; Maddox, A.F.; Wishart, J.M.; Jones, K.L.; Clifton, P.M.; Horowitz, M.; Rayner, C.K. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care 2009, 32, 1600–1602.
- Mignone, L.E. Whey protein: The “whey” forward for treatment of type 2 diabetes? World J. Diabetes 2015, 6, 1274.
- Jakubowicz, D.; Froy, O. Biochemical and metabolic mechanisms by which dietary whey protein may combat obesity and Type 2 diabetes. J. Nutr. Biochem. 2013, 24, 1–5.
- Gillespie, A.L.; Calderwood, D.; Hobson, L.; Green, B.D. Whey proteins have beneficial effects on intestinal enteroendocrine cells stimulating cell growth and increasing the production and secretion of incretin hormones. Food Chem. 2015, 189, 120–128.
- Xu, G.; Hong, X.; Tang, H.; Jiang, S.; Liu, F.; Shen, Z.; Li, Z.; Zhang, W. Ghrelin regulates GLP-1 production through mTOR signaling in L cells. Mol. Cell. Endocrinol. 2015, 416, 9–18.
