The southern boreal forests of North America are susceptible to large changes in composition as temperate forests or grasslands may replace them as the climate warms.
- climate change
- compound disturbance
- fire
- insect infestation
- phenological disturbance
- wind
1. Introduction
Poleward displacement of climate zones due to global warming is expected to cause major changes in vegetation, especially along equatorward biome ecotones [1]. Forests near the southern ecotone of the boreal biome have globally important carbon pools, wildlife and timber resources that are vulnerable to degradation due to climate change [2][3][4]. Climate change can trigger a variety of mechanisms of change in the southern boreal forest, including effects of heat stress, drought, increasing fire frequency, and insect outbreaks [4][5][6][7][8]. The timing, extent and spatial scale at which the varied mechanisms of change may operate during the 21st century cannot be predicted with current knowledge. However, thus far, no one has assembled and organized the potential mechanisms of change to inform monitoring of climate change impacts, climate adaptation planning by natural resource managers and as a basis for future research efforts [9][10][11][12].
Here, we examine potential changes caused by climate change in the central North American and Great Lakes region of North America. This region includes boreal forests of jack pine (Pinus banksiana Lambert), black spruce (Picea mariana (Mill.) Britton, Sterns and Poggenb.), white spruce (P. glauca (Moench) Voss), balsam fir (Abies balsamea (L.) Mill.), red pine (Pinus resinosa Aiton), quaking aspen (Populus tremuloides Michx.), and paper birch (Betula papyrifera Marsh.). To the south and east (northeastern Minnesota, Wisconsin, Michigan, USA, and parts of Ontario, Canada), lie temperate forests of sugar maple (Acer saccharum Marsh.), red maple (A. rubrum L.), hemlock (Tsuga Canadensis (L.) Carr.), yellow birch (Betula alleghaniensis Britton), beech (Fagus grandifolia Ehrh.), northern red oak (Quercus rubra L.), American basswood (Tilia Americana L.) and white pine (Pinus strobus L.). To the south and west (northwestern Minnesota and westward across Manitoba and Saskatchewan, Canada) lie oak savannas with bur oak (Quercus macrocarpa Michx.) and northern pin oak (Q. ellipsoidalis E.J. Hill), and/or quaking aspen parklands, and prairies that have mostly been converted to agricultural fields.
The southern boreal forest boundary in this region has already experienced significant warming of >1.5 °C [13] and is expected to experience a relatively rapid velocity of climate change for the duration of the 21st century [14]. Clearly, this region will be subject to large changes in vegetation as the climate warms over the coming decades, with climates suitable for boreal forests receding northwards by 100–500 km by 2070, depending on scenarios for CO2 emissions and sensitivity of the General Circulation Model (GCM) used [15][16][17].
However, the complexities of transient dynamics of vegetation during rapid change in the southern boreal forest are poorly described. Frelich and Reich [7] proposed that multiple factors (droughts, windstorms, fires, large herbivores, insects) would lead to changes occurring faster and of larger magnitude than direct impacts of climate change due to increasing temperature; here we take the concept further than the cited study was able to, because new details are available from subsequent research. Therefore, the objective of this synthesis is to identify and examine several potential mechanisms of change in the southern boreal forest due to the warming that has already occurred, as well as continuing changes projected as warming progresses throughout the 21st century. Furthermore, some or all of these mechanisms are likely to be important along the southern margin of the boreal biome in Europe and Asia [16][18].
All of the mechanisms of change identified here have been observed locally or regionally in central North America with the relatively small magnitude of warming that has already occurred (relevant literature cited for each mechanism below). With larger magnitudes of climate change, these mechanisms can potentially exceed the resilience of boreal forests, converting them into temperate forest, savanna or grassland vegetation types by the late 21st century [12]. Note that although some mechanisms can kill individual trees within minutes, for purposes of this synthesis we are interested in changes at landscape to regional spatial extents. Gap dynamics via individual tree deaths may take decades to gradually transform the forest canopy of a landscape, while very large disturbances could synchronously kill millions of trees to accomplish a similar transformation in a few days or weeks.
2. Mechanisms Transforming Boreal Forest Vegetation in a Warming Climate
M1. Gradual replacement of boreal trees by temperate trees through gap dynamics. The increase in temperate species would occur over several decades so that they replace boreal tree species through gap-phase replacements as individual boreal trees die of old age [19][20]. Although this was not occurring in the study region during the 1950s [21], it has started to occur between 2010 and 2020, due to warming that has already happened (Figure 1, [22]). Two changes in temperature could allow this to occur. First, summers are now warmer in locations where in the past cool summer temperatures and abbreviated length of the warm season limited the growth of temperate tree species and made them less competitive. Recent studies of understory sapling abundance and growth across a summer temperature gradient in the Great Lakes region have shown that temperate tree species are expanding into nearby boreal stands and that this expansion is related to warmer summer temperatures in recent years (Figure 2, [22][23]). These observational findings are corroborated by experiments in which field plots in the temperate-boreal ecotone with planted seedlings were artificially warmed, resulting in increasing growth rates of temperate seedlings and decreasing growth rates of boreal seedlings [24]. Second, extreme winter minimum temperatures, which were historically lethal to temperate tree species, are rare in the region (<−42 to −44 °C, [25]). ‘Summer boreal’ and ‘winter boreal’ forests (where temperate tree species were previously kept out by cool summers or winter minimum temperature, respectively) would thus be affected by different aspects of the changing temperature regime. We note that many temperate tree species have historically been present in the region, but at low numbers and frequencies (e.g., red maple, bur oak, northern red oak, yellow birch), while the northern range limits of many other temperate species lie to the south and would need to migrate into the region from elsewhere.

Figure 1. Sugar maple saplings (right) within a boreal balsam fir forest in northern Wisconsin USA. Balsam fir saplings on left. Photo by Lee Frelich.
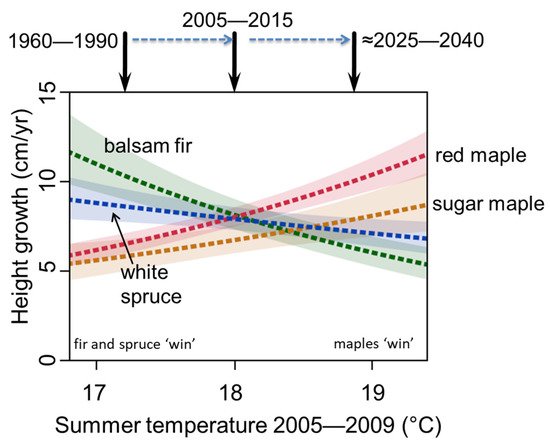
Figure 2. Height growth of understory saplings across a range of regional mean summer temperatures in ecotonal mixed temperate-boreal forest in northern Minnesota, USA. After [23].
M2. Sudden replacement of boreal overstory trees after gradual understory invasion by temperate tree species. In boreal stands with an established temperate understory, for example red maple under boreal spruce-fir-birch-aspen forests (Figure 3), a large event that kills larger boreal trees, releasing the temperate understory, would lead to a rapid transition rather than a gradual transition as in M1. This would occur at medium spatial extents when derechos (straight-line wind events associated with severe thunderstorms) level the canopy over 10 s to 1000 s of km2. Such storms are expected to become more common as the climate warms [26]. Windstorms commonly kill large trees, while leaving most saplings alive [27]. This is a well-known mechanism termed disturbance-mediated accelerated succession [28]. Usually this simply accelerates the normal successional process from shade-intolerant mature trees to shade-tolerant understory trees within a single biome (e.g., paper birch to spruce and fir in the boreal forest, or to hemlock and sugar maple in the temperate forest). However, in the context of a warming climate along the southern boundary of the boreal forest, combined with the observed increased abundance of temperate saplings in the understory [22], the mechanism could cause biome conversion.

Figure 3. A boreal paper birch, aspen, white spruce and balsam fir forest in northern Minnesota with red maple (red foliage in this autumn picture) in the understory. Photo by Dave Hansen.
M3. Trophic cascades causing delayed invasion by temperate species, followed by moderately sudden change from boreal to temperate forest. This is similar to M1, but large herbivores cause delays in establishment of shade-tolerant temperate tree species in boreal forest understories. The two large herbivores in the region, moose (Alces alces L.) and white-tailed deer (Odocoileus virginianus Zimmerman), prefer to eat seedlings of temperate species (sugar maple, red maple, northern red oak, yellow birch and hemlock, Figure 4), more than boreal species (spruce, fir and pine, [29]). White-tailed deer can also change the growth rates of temperate and boreal tree saplings relative to each other, raising the threshold temperature at which temperate trees can replace boreal trees [23]. The consumption of temperate seedlings in nearby boreal stands thus prevents establishment of a temperate understory until either the boreal trees die of old age or the climate becomes so warm that it exceeds the tolerance of boreal trees, which then die without having a temperate understory in place. At some point during decline in the boreal overstory, understory light levels become high enough so that seedlings of temperate tree species can outgrow the impacts of large herbivores [30], causing a change that is somewhat faster than in M1.

Figure 4. Northern red oak sapling height growth reduced to zero by deer browsing in northern Minnesota USA. Photo by Nick Fisichelli.
Note that trophic cascade effects vary in a mosaic pattern across the landscape, due to the locations of gray wolf (Canis lupus L.) packs. In areas with high wolf densities, deer populations are low and unhindered expansion of temperate tree species into adjacent boreal stands can occur, whereas in other areas with low wolf populations, deer populations are higher and prevent the establishment of temperate tree seedlings [31][32]. Deer densities can also interact with other factors to create complex responses of temperate and boreal tree species to changing climate [33][34].
M4. Compound disturbances: wind and fire combination. A warmer climate is expected to lead to more days per year with conditions that will support extreme convective windstorms at the latitudes of the southern boreal forest [26], and the resulting slash is very flammable, therefore this combination of events is expected to increase dramatically as the climate warms [35]. Compound wind plus fire disturbances can convert tracts of conifers (over spatial extents of 10 s to 100 s km2 similar to M2) to aspen and birch within boreal forests (Figure 5). However, it can also increase abundances of temperate tree species such as bur oak, red oak and red maple [35].

Figure 5. Conifer forest converted to paper birch after a wind and fire combination of disturbances in Minnesota USA. Windstorm was in 1999, the fire in 2002, and photo in 2007. Photo by Dave Hansen.
Wind-felled pine, spruce and fir stands have their cones on the ground along with dense slash, leading to consumption of seeds and seedlings in any fire that follows the windstorm within one-two decades. Fires in windfall slash have unusually high intensities and severities [32]. This leaves the forest without a replacement layer, so that ruderal species of herbs, shrubs and trees can take over, possibly temperate grassland or temperate forest species close to the southern boreal forest, depending on how warm the regional climate has become. Succession to temperate species like red maple and northern red oak after combined wind and fire disturbance in the boreal biome would be an ecological surprise compared to historical successional patterns [36].
M5. Long, warm summers and increased drought stress. Much of the boreal forest lies adjacent to grasslands. The transition zone is coincident with a change in climate from one with a positive difference between precipitation and potential evapotranspiration (PPT minus PET > 0, which supports forest) to one where PPT minus PET is less than zero (which supports grasslands, [37][38][39]) that occurs over relatively short spatial distances of 10–100 km [40]. Warming-exacerbated declines in soil moisture are likely to negatively impact tree photosynthesis in this region. In addition, drier soils can also flip the response of photosynthesis to modestly higher temperatures from positive to negative [41]. This results in drier soils from heightened stomatal closure in response to warming outweighing increasing biochemical capacity for photosynthesis; whereas the latter dominates in moist soils. Therefore, a pronounced shift in the zero balance line for precipitation and evaporation could lead to widespread forest death, especially during a run of several unusually warm and dry years that could occur decades before the mean climate shifts to one that favors grasslands. Runs of several warm dry years have already led to mortality and slowed growth of several boreal species [42][43][44] (Figure 6), and conversion from boreal forest to grassland/savanna in some parts of Canada [8]. This trend is predicted to continue during the 21st Century [45][46]. However, the drying of the climate could also lead to conversion from boreal forest to drought-tolerant temperate tree species. Bur oak, northern pin oak, and northern red oak are already present near the southern boreal forest on dry sites, and white oak (Quercus alba L.) and black oak (Q. velutina Lambert) could migrate there [47]. Due to the high landscape diversity of the Canadian Shield that underlies northern Minnesota, Ontario and Manitoba, with soils of greatly varying water holding capacity, a mosaic of grassland, savanna and woodland could form [7][10][12]. Transformation of boreal forests could be gradual (with gradually increasing drought stress) or moderately sudden (with a run of several hot, dry years) [38].

Figure 6. Dying boreal paper birch forest after severe drought in 8 of the 10 most recent summers, North Shore Lake Superior, Minnesota, USA. Photo by David Hansen, September 2008.
M6. Insect infestation due to lack of extreme winter cold. Populations of many potentially lethal insect species are kept at bay by occasional (ca once per decade) winter cold spells [5], which in the central North American boreal forest can be −40 to −55 °C. For example, mountain pine beetle (Dendroctonus ponderosae Hopkins), although native in western North America, has exploded in population density in recent years after a run of several warm winters, and has killed millions of ha of lodgepole pine (Pinus contorta Douglas ex Loudon) [48]. This insect pest has not been able to reach the study region, due to lack of trees across the Great Plains, and extremely cold winters across the southern boreal forest to the north, where it has been shown that native jack pine is a suitable host [48]. With a run of several very warm winters, this insect could move into the study region. Many other potential insect pests that have remained at low populations for decades or centuries could become problems, and there are likely to be surprises as to which species have tree-killing outbreaks in the future. Numerous insect pests and diseases have also been introduced to North America from elsewhere (471 species, [49]). If climate change caused nothing other than warmer nights at mid-winter, it could still kill large swaths of the boreal forest in the study region. Any one insect would be likely to kill monodominant stands of its target species on a regional scale, creating a patchy mosaic of dead stands at the regional scale.
M7. Phenological disturbance. This is a new concept as a disturbance. It would be manifested as late-winter warm spells causing boreal conifers to prematurely lose frost hardiness, followed by foliar damage during subsequent frost [50]. This happened in springs of 2007 and 2012 in northern Minnesota and adjacent Ontario. There is evidence that boreal species respond more rapidly to forcing temperatures and less to other cues (e.g., winter chilling or photoperiod) and as a result have the potential to lose frost hardiness in mid-winter and early spring [51][52][53]. During March 2012, temperatures with daytime maxima of 20–25 °C occurred in northern Minnesota and adjacent Ontario from March 15–22 (note that daily maximum temperatures would normally be below freezing during this period), and trees came out of dormancy, followed by freezing temperatures that led to needle reddening and loss of most of their foliage ([54], Figure 7). Similarly, an unusually warm March followed by extreme cold in April led to significant foliar tissue loss in a warming experiment in boreal peatlands. Trees in the warmest treatments de-hardened in the March warm spell only to have tissues killed by the April frost [53]. Many trees recover after these types of events, following several winters with normal late-winter temperatures [55]. However, occurrences of extremely warm late-winter weather 2–3 years in a row could kill boreal conifers, which have needle life spans of 4–7 years, and do not have the same ability to recover from defoliation as deciduous tree species. Although we do not have observations of more than single years of defoliation due to late-winter warm spells, we do know from other studies of defoliation, that the greater the percent of foliage lost, and the greater the number of consecutive years of defoliation occurs, the greater the mortality rates for boreal conifers [56][57]. The extreme weather of March 2012 is close to an average March projected by some of the GCMs for a business as usual (RPC 8.5) climate for 2070 [17]; thus, sometime between now and then, it is very likely that a run of several consecutive very warm springs will occur. Very large areas could be affected by phenological disturbance, since persistent mid-latitude ridges in the jet stream that lead to long periods of anomalously warm weather, are becoming more common as the arctic warms, and can occur at subcontinental spatial extents [58].

Figure 7. Phenological disturbance—needle reddening of boreal jack pine and balsam fir forest in Minnesota, USA after the anomalously warm March of 2012. Photo by Elias Anoszko.
This entry is adapted from the peer-reviewed paper 10.3390/f12050560
References
- Batllori, E.; Parisien, M.A.; Parks, S.A.; Moritz, M.A.; Miller, C. Potential relocation of climatic environments suggests high rates of climate displacement within the North American protection network. Glob. Chang. Biol. 2017, 23, 3219–3230.
- Kuusela, K. The boreal forests: An overview. Unasylva 1992, 43, 3–13.
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993.
- Stralberg, D.; Arsenault, D.; Baltzer, J.L.; Barber, Q.E.; Bayne, E.M.; Boulanger, Y.; Brown, C.D.; Cooke, H.A.; Devito, K.; Edwards, J.; et al. Climate-change refugia in Boreal North America: What, where, and for how long? Front. Ecol. Environ. 2020, 18, 261–270.
- Logan, J.A.; Regniere, J.; Powell, J.A. Assessing the impacts of global warming on forest pest dynamics. Front. Ecol. Environ. 2003, 1, 130–137.
- Flannigan, M.; Stocks, B.J.; Turetsky, M.; Wotton, M. Impacts of climate change on fire activity fire management in the circumboreal forest. Glob. Chang. Biol. 2009, 15, 549–560.
- Frelich, L.E.; Reich, P.B. Will environmental changes reinforce the impact of global warming on the prairie-forest border of central North America? Front. Ecol. Environ. 2010, 8, 371–378.
- Michaelian, M.; Hogg, E.H.; Hall, R.J.; Arsenault, E. Massive mortality of aspen following severe drought along the southern edge of the Canadian boreal forest. Glob. Chang. Biol. 2011, 17, 2081–2094.
- Millar, C.I.; Stephenson, N.L.; Stephens, S.L. Climate change and forests of the future: Managing in the face of uncertainty. Ecol. Appl. 2007, 17, 2145–2151.
- Galatowitsch, S.; Frelich, L.E.; Phillips-Mao, L. Regional climate change adaptation strategies for biodiversity conservation in a midcontinental region of North America. Biol. Cons. 2009, 142, 2012–2022.
- Wilson, D.C.; Morin, R.; Frelich, L.E.; Ek, A.R. Monitoring Disturbance Intervals in Forests: A Case Study of Increasing Forest Disturbance in Minnesota. Ann. For. Sci. 2019, 76, 78.
- Frelich, L.E.; Jõgiste, K.; Stanturf, J.; Jansons, A.; Vodde, F. Are secondary forests ready for climate change? It depends on magnitude of climate change, landscape diversity and ecosystem legacies. Forests 2020, 11, 965.
- USGCRP. Climate Science Special Report: Fourth National Climate Assessment; Wuebbles, D.J., Fahey, D.W., Hibbard, K.A., Dokken, D.J., Stewart, B.C., Maycock, T.K., Eds.; U.S. Global Change Research Program: Washington, DC, USA, 2017; Volume I, p. 470.
- Loarie, S.R.; Duffy, P.B.; Hamilton, H.; Asner, G.P.; Field, C.B.; Ackerly, D.D. The velocity of climate change. Nature 2009, 462, 1052–1055.
- Batchelet, D.; Neilson, R.P.; Lenihan, J.M.; Drapek, R.J. Climate change effect on vegetation distribution and carbon budget in the United States. Ecosystems 2001, 4, 164–185.
- Gonzalez, P.; Neilson, R.P.; Lenihan, J.M.; Drapek, R.J. Global patterns in the vulnerability of ecosystems to vegetation shifts due to climate change. Glob. Ecol. Biogeogr. 2010, 1, 755–768.
- Toot, R.K.; Frelich, L.E.; Butler, E.; Reich, P.B. Climate-biome envelope shifts create enormous challenges and novel opportunities for conservation. Forests 2020, 11, 1015.
- Liu, Z.; Yang, J.; Chang, Y.; Weisberg, P.J.; He, H.S. Spatial patterns and drivers of fire occurrence and its future trend under climate change in a boreal forest of Northeast China. Glob. Chang. Biol. 2012, 18, 2041–2056.
- Leithead, M.; Silva, L.C.R.; Anand, M. Recruitment patterns and northward tree migration through gap dynamics in an old-growth white pine forest in northern Ontario. Plant Ecol. 2012, 213, 1699–1714.
- Montgomery, R.; Frelich, L.E. Forest succession and gap dynamics. In Handbook of Forest Ecology; Peh, K., Corlett, R., Bergeron, Y., Eds.; Routledge Press: Oxfordshire, UK, 2015; Chapter 10; pp. 141–153.
- Curtis, J.T. The Vegetation of Wisconsin; The University of Wisconsin Press: Madison, WI, USA, 1959.
- Fisichelli, N.A.; Frelich, L.E.; Reich, P.B. Temperate tree expansion into adjacent boreal forest patches facilitated by warmer temperatures. Ecography 2014, 37, 152–161.
- Fisichelli, N.A.; Frelich, L.E.; Reich, P.B. Sapling growth responses to warmer temperatures ‘cooled’ by browse pressure. Glob. Chang. Biol. 2012, 18, 3455–3463.
- Reich, P.B.; Sendall, K.M.; Rice, K.; Rich, R.L.; Stefanski, A.; Hobbie, S.E.; Montgomery, R.A. Geographic range predicts photosynthetic and growth response to warming in co-occurring tree species. Nat. Clim. Chang. 2015, 5, 148–152.
- George, M.F.; Burke, M.J.; Pellett, H.M.; Johnson, A.G. Low temperature exotherms and woody plant distribution. Hort. Sci. 1974, 9, 519–522.
- Diffenbaugh, N.S.; Scherer, M.; Trapp, R.J. Robust increases in severe thunderstorm environments in response to greenhouse forcing. Proc. Natl. Acad. Sci. USA 2013, 110, 16361–16366.
- Rich, R.L.; Frelich, L.E.; Reich, P.B. Wind-throw mortality in the southern boreal forest: Effects of species, diameter and stand age. J. Ecol. 2007, 95, 1261–1273.
- Abrams, M.D.; Scott, M.L. Disturbance-mediated accelerated succession in two Michigan forest types. For. Sci. 1989, 35, 42–49.
- Frelich, L.E.; Peterson, R.O.; Dovciak, M.; Reich, P.B.; Vucetich, J.A.; Eisenhauer, N. Trophic cascades, invasive species, and body-size hierarchies interactively modulate climate change responses of ecotonal temperate-boreal forest. Philos. Trans. R. Soc. B 2012, 367, 2955–2961.
- Povak, N.A.; Lorimer, C.G.; Guries, R.P. Altering successional trends in oak forests: 19 year experimental results of low- and moderate-intensity silvicultural treatments. Can. J. For. Res. 2008, 3, 2880–2895.
- Callan, R.; Nibbelink, N.P.; Rooney, T.P.; Wiedenhoeft, J.E.; Wydeven, A.P. Recolonizing wolves trigger a trophic cascade in Wisconsin (USA). J. Ecol. 2013, 101, 837–845.
- Frelich, L.E. Forest Dynamics and Disturbance Regimes; Cambridge University Press: Cambridge, UK, 2002.
- Fisichelli, N.A.; Frelich, L.E.; Reich, P.B.; Eisenhauer, N. Linking direct and indirect pathways mediating earthworms, deer, and understory composition in Great Lakes forests. Biol. Invasions 2013, 15, 1057–1066.
- Fisichelli, N.A.; Frelich, L.E.; Reich, P.B. Climate and interrelated tree regeneration drivers in mixed temperate-boreal forests. Land. Ecol. 2013, 28, 149–159.
- Anoszko, E.; Frelich, L.E.; Rich, R.L.; Reich, P.B. Wind and fire: Rapid shifts in tree community composition following multiple disturbances in the southern boreal forest. Ecol. Monog. 2021. under review.
- Paine, R.T.; Tegner, M.J.; Johnson, E.A. 1998. Compounded perturbations yield ecological surprises. Ecosystems 1998, 1, 535–545.
- Hogg, E.H. Climate and the southern limit of the western Canadian boreal forest. Can. J. For. Res. 1994, 24, 1835–1845.
- Hogg, E.H. Temporal scaling of moisture and the forest-grassland boundary in western Canada. Agric. For. Meteorol. 1997, 84, 115–122.
- Danz, N.P.; Reich, P.B.; Frelich, L.E.; Niemi, G.J. Vegetation controls vary across space and spatial scale in a historic grassland-forest biome boundary. Ecography 2011, 32, 402–414.
- Danz, N.P.; Frelich, L.E.; Reich, P.B.; Niemi, G.J. Abrupt prairie-forest transition across a smooth climate gradient in presettlement Minnesota, USA. J. Veg. Sci. 2013, 24, 1129–1140.
- Reich, P.B.; Sendall, K.M.; Stefanski, A.; Rich, R.L.; Hobbie, S.E.; Montgomery, R.A. Effects of climate warming on photosynthesis in boreal tree species depend on soil moisture. Nature 2018, 562, 263–267.
- Jones, E.A.; Reed, D.D.; Mroz, G.D.; Leichty, H.O.; Cattelino, P.J. Climate stress as a precursor to forest decline: Paper birch in northern Michigan, 1985–1990. Can. J. For. Res. 1993, 23, 229–233.
- Peng, C.; Ma, Z.; Lei, X.; Zhu, Q.; Chen, H.; Wang, W.; Liu, S.; Li, W.; Fang, X.; Zhou, X. A drought-induced pervasive increase in tree mortality across Canada’s boreal forests. Nat. Clim. Chang. 2011, 1, 467–471.
- Ma, Z.; Peng, C.; Zhu, Q.; Chen, H.; Yu, G.; Li, W.; Zhou, X.; Wang, W.; Zhang, W. Regional drought-induced reduction in the biomass carbon sink of Canada’s boreal forests. Proc. Natl. Acad. Sci. USA 2012, 109, 2423–2427.
- Dai, A. Drought under global warming: A review. Wiley Interdiscip. Rev. Clim. Chang. 2011, 2, 45–65.
- Cook, B.I.; Smerdon, J.E.; Seager, R.; Coats, S. Global warming and 21st century drying. Clim. Dyn. 2014, 43, 2607–2627.
- Frelich, L.E.; Reich, P.B. Wilderness conservation in an era of global warming and invasive species: A case study from Minnesota’s Boundary Waters Canoe Area Wilderness. Nat. Areas J. 2009, 29, 385–393.
- Rosenberger, D.; Venette, R.C.; Maddox, M.P.; Aukema, B.H. Colonization behaviors of mountain pine beetle on novel hosts: Implications for range expansion into eastern North America. PLoS ONE 2017, 12, e0176269.
- Roy, B.A.; Alexander, H.M.; Davidson, J.; Campbell, F.T.; Burdon, J.J.; Sniezko, R.; Brasier, C. Increasing forest loss worldwide from invasive pests requires new trade regulations. Front. Ecol. Environ. 2014, 12, 457–465.
- Hänninen, H. Climate warming and the risk of frost damage to boreal forest trees: Identification of critical ecophysiological traits. Tree Phys. 2006, 26, 889–898.
- Montgomery, R.A.; Rice, K.E.; Stefanski, A.; Rich, R.L.; Reich, P.B. Phenological responses of temperate and boreal trees to warming depend on ambient spring temperatures, leaf habit and geographic range. Proc. Natl. Acad. Sci. USA 2020, 11, 10397–10405.
- Zohner, C.M.; Benito, B.M.; Svenning, J.-C.; Renner, S.S. Day length unlikely to constrain climate-driven shifts in leaf-out times of northern woody plants. Nat. Clim. Chang. 2016, 6, 1120–1123.
- Richardson, A.D.; Hufkens, K.; Milliman, T.; Aubrecht, D.M.; Furze, M.E.; Seyednasrollah, B.; Krassovski, M.B.; Latimer, J.M.; Nettles, W.R.; Heiderman, R.R.; et al. Ecosystem warming extends vegetation activity but heightens vulnerability to cold temperatures. Nature 2018, 560, 368–371.
- Man, R.; Colombo, S.; Kayahara, G.J.; Duckett, S.; Velasquez, R.; Dang, Q.-L. A case of extensive conifer needle browning in northwestern Ontario in 2012: Winter drying or freezing damage? For. Chron. 2013, 89, 675–680.
- Man, R.; Kayahara, G.J.; Foley, S.; Wiseman, C. Survival and growth of eastern larch, balsam fir, and black spruce six years after winter browning in northeastern Ontario, Canada. For. Chron. 2013, 89, 777–782.
- Kulman, H.M. Effects of insect defoliation on growth and mortality of trees. Ann. Rev. Entom. 1971, 16, 289–324.
- Methven, I.R. Prescribed Fire, Crown Scorch and Mortality: Field and Laboratory Studies on Red and White Pine; Information Report PS-X-31; Canadian Forest Service, Petawawa Forest Experiment Station: Chalk River, ON, Canada, 1971.
- Francis, J.A.; Vavrus, S.J. Evidence for a wavier jet stream in response to rapid arctic warming. Environ. Res. Lett. 2015, 10, 014005.
