Sex-reversed females (SRFs; masculinised females, neomales) are genetically females; however, owing to masculinisation, they are capable of producing spermatozoa.
- cryopreservation
- masculinisation
- maturation
- salmonids
- sex-reversed females
- sperm quality
1. Introduction
1.1. Definition
Sex-reversed females (SRFs; masculinised females, neomales) are genetically females; however, owing to masculinisation, they are capable of producing spermatozoa [1]. Females are masculinised by the hormonal treatment of fertilised eggs or larvae with androgens or their analogues before sex differentiation [2]. However, SRFs usually have less developed testes and there is typically a lack of spermatic ducts [3]. Therefore, it is necessary to sacrifice the fish to collect the semen. The sex determination model of salmonid fish is of the XY type; due to masculinisation, the spermatozoa produced in the testes of SRF possess an X chromosome [3]. Therefore, SRF semen is purely used in the creation of all-female stocks when eggs are fertilised using an X-chromosome-bearing spermatozoon [4] (Figure 1).
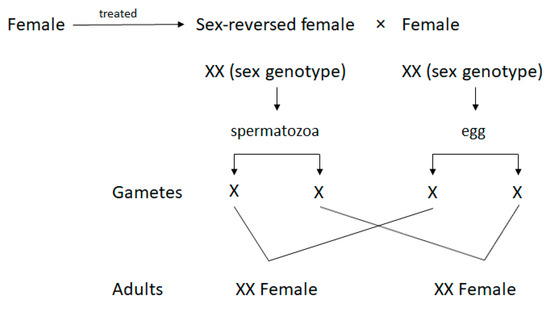
Figure 1. Mating of sex-reversed female with normal female, showing all-female fish.
1.2. The Use of SRFs in Aquaculture
The formation of female monoculture stocks of salmonids is of interest in commercial aquaculture because of its several advantages [1][2][5][6]. It reduces early maturation and limits the number of broodstock fish required [7]. Moreover, since salmonid females reach their commercial size before becoming sexually mature, the nourishment is used only for somatic growth and not for the development of the reproductive system [1]. The low quality of male meat, for example, that of Atlantic salmon (Salmo salar), has also been indicated [8]. The production of all-female fish also has great importance for the creation of monosex female triploids of salmonid fish, which are valued for their sterility, lack of female maturation, and larger commercial size [1][9]. Nowadays, among salmonids, the majority of rainbow trout (Oncorhynchus mykiss) production is based on all-female stocks, with a high proportion of all-female triploid fish occurring in Europe [10].
1.3. Justification
To the best of our knowledge, the sex reversal of salmonid fish has previously been discussed by several excellent reviews [2][11][12][13]. Those reviews mainly focused on the sex control and hormonal induction of sex reversal in fish. The present review distinguishes itself from the mentioned previous studies by focusing on presenting the more recent knowledge concerning salmonid fish SRFs. We discuss the methods of sex reversal and its effect on the morphology and histology of the reproductive system. We focus on the characteristics of SRF semen, as well as the variability of its quality resulting in a need for artificial semen maturation. Most importantly, the methods of semen storage—both short-term and long-term (cryopreservation)—that can improve hatchery operations are presented with the special emphasis on recent progress in development of efficient cryopreservation procedures and use of cryopreserved semen in hatchery practice. Moreover, we also address the emerging knowledge concerning the proteomic investigations of salmonid sperm, focusing primarily on the proteomic comparison of normal male and SRF testicular semen and presenting changes in SRF rainbow trout sperm proteome after in vitro incubation in artificial seminal plasma.
2. Hormonal Induction of Sex Reversal
2.1. Historic
Sexual development in gonochoristic teleosts is protracted and plastic, which makes it possible to reverse sex both toward the masculinisation of females and feminisation of males [2][13][14]. The use of synthetic hormones to sex reverse salmon was initiated as early as 1937 [2][15]. The masculinisation of Salmo sp. with testosterone or its analogue was conducted in the 1950s [11][16]. Major progress in female masculinisation leading to the implementation of this technique into hatchery practice was achieved in 1970 for several salmonid species, such as rainbow trout, salmon, coho salmon (Oncorhynchus kisutch), and chinook salmon (Oncorhynchus tshawytscha) [12].
2.2. Masculinisation—Type of Hormones
There are two potential approaches for the production of monosex female groups of fish: Direct, based on the use of oestrogens to feminise genetic males [17] and indirect, based on the use of androgens to induce the sex reversal of homogametic females and their subsequent use as broodstock with the use of their spermatozoa to fertilise ova from untreated females, which results in the production of 100% female progenies [18]. The method for indirect feminisation appears to be very efficient for homogametic female fish species, which is characteristic for salmonids [19]. Therefore, indirect feminisation was found to be superior and is now a commonly used in hatchery practice. Major androgens that are being used for female masculinisation include synthetic 17 α-methyltestosterone (MT) and 17 α-methyldihydrotestosterone (MDHT), which represent aromatisable and nonaromatisable androgens, respectively [20][21]. Another important androgen used under practical hatchery conditions is naturally occurring 17 β-hydroxyandrostenedione (OHA). OHA was found to be superior to MT in comparative studies [22][23]. It was also recommended to select OHA if functional males are desired [24][25].
It has to be underlined that due to the commercial use of sex reversal, the detailed methods used to produce SRFs are proprietary to the producers and not available publicly, which restricts our knowledge (to an unknown extent) regarding the usefulness of specific protocols [26].
2.3. Important Factors for Sex Reversal
There are a few major factors that need to be considered for the sex reversal of particular salmonid species: (i) The timing of sexual differentiation, (ii) methods of steroid administration and their dose, and (iii) species-specific characteristics important for the efficacy of sex reversal. The multi-factorial determination of sex reversal efficacy makes its control difficult and leads to different extents of male reproductive system development, such as sterile, bisexual, dysfunctional, and normal males [23] (Table 1). A more detailed classification was proposed [6], where sterile or immature males or females, males spermiating or females ready to ovulate, males or females in development, males without functional ducts, intersex, males spermiating (one testis), females ovulating (one ovary), and undifferentiated individuals were distinguished.
Table 1. Examples of the development of the reproductive system in a salmonid sex-reversed female population after hormonal treatment.
| Species | Hormone/Method of Application | Functional Male (%) | Dysfunctional Male (%) | Female (%) | Bisexual (%) | Sterile/ Immature (%) |
Reference |
|---|---|---|---|---|---|---|---|
| Rainbow trout (Oncorhynchus mykiss) |
MT/(6 ppm) feed | 5.3 | 54.4 | 36.7 | 1.8 | 1.8 | [23] |
| OHA/(20 ppm) feed | 1.6 | 93.4 | 1.7 | 1.6 | 1.7 | ||
| OHA/(20 ppm) feed | 3.0 | 89.6 | – | 7.4 | – | [27] | |
| Brook trout (Salvelinus fontinalis) |
MT/(400 µg·L−1) immersion | 17.0 | – | – | – | 83.0 | [28] |
| MT/(400 µg·L−1) immersion and (3 mg·kg−1) feed |
31.0 | – | – | 8.0 | 61.0 | ||
| MT/(400 µg·L−1) immersion and (3 mg·kg−1) feed |
14.0 | 1.0 | 85.0 | [6] |
MT, 17 α-methyltestosterone, OHA, 17 β-hydroxyandrostenedione, –, not obtained.
2.4. Timing of Sexual Differentiation
The timing of sexual differentiation during ontogenic development is critical for the advancement of the sex reversal procedure because the efficacy of steroids is at its highest while it is administrated during the so called “labile period”, i.e., starting when the gonads are undifferentiated and continuing through gonadal differentiation. Any deviations from the labile period decreases the efficacy of sex reversal and elevated doses of hormones and longer application times may be required to counteract these losses [13]. Although sexual differentiation mostly starts around the time of hatching, some species-specific differences do occur; for example, in brook trout (Salvelinus fontinalis), the pre-hatch period was found as a potential target period for sex reversal using androgen treatment [29]. Moreover, the temperature around the time of hatching has an important modulation effect because embryo development is temperature-dependent; this is being used in hatchery practices to control the time of hatching and growth of larvae. For some rainbow trout strains, such as “Mal” populations, high temperatures alone (up to 18 °C) can induce masculinisation [30]. To compensate for the temperature factor commonly used in fish breeding, degree-days (usually °C-days) are often used for the definition of hormone administration (see Table 2).
Table 2. Examples of masculinisation protocols in salmonid fish.
| Species | Hormone/Dose | Treatment | Main Outcome | Reference |
|---|---|---|---|---|
| Arctic charr (Salvelinus alpinus) |
MDHT/10 mg L−1 | Weekly immersion 140 °C-days post-hatch | 90% males | [5] |
| MDHT/0.5 mg kg−1 | Feeding 140–600 °C-days post-hatch | 100% males | ||
| Atlantic salmon (Salmo salar) |
MT/1 or 3 mg kg−1 | Feeding 800 °C-days | 100% males | [31] |
| MDHT/1 mg kg−1 | Feeding 800 °C-days | 100% males | ||
| MDHT/0.4 mg L−1 | Immersion 7 and 14 d after hatch | 100% males | ||
| Brook trout (Salvelinus fontinalis) |
MDHT/0.5 mg L−1 | Immersion 10 d after hatch | up to 45% males | [32] |
| MDHT/0.5 mg kg−1 | Feeding 60 d beginning at first feeding | 100% fem. progeny | ||
| MT/0.4 mg L−1 | Immersion 4 weekly starting 1 week | 100% males | [6] | |
| MT/3 mg kg−1 | before hatching and feeding 800 °C-days from the first feeding | |||
| MT/0.4 mg L−1 | Immersion on 6th and 4th day pre hatching | 75% males | [28] | |
| Brown trout (Salmo trutta) |
MT/3 mg kg−1 | Feeding for 800 °C-days | >90% males | [33] |
| Chinook salmon (Oncorhynchus tshawytscha) |
MDHT/0.4 mg L−1 | Immersion on 3 days after 50% hatch | 100% males | [20] |
| MT/0.4 mg L−1 | Immersion on 520 °C-days and 620 °C-days after hatching | 71% functional males | [34] | |
| Coho salmon (Oncorhynchus kisutch) |
MT/0.2 mg L−1 | Two immersions weekly, starting during final hatching | 46% functional males | [7] |
| Rainbow trout (Oncorhynchus mykiss) |
OHA/20 mg kg−1 | 60 days from the first feeding | 96.6% males | [23] |
| OHA/0.4 mg L−1 | Immersion 20 dpf for 2 h and | 100% functional males | [24] | |
| OHA/3 mg kg−1 | 60 days from the first feeding | |||
| OHA/10 mg kg−1 | 3 months from the first feeding | 100% functional males | [35][36] | |
| MT/3 mg kg−1 | 60 days from the first feeding | 87% males | [37] | |
| MT/0.25 mg kg−1 | 80 days from the first feeding 1024 °C-days | functional males | [38] | |
| MT/0.4 mg L−1 | 2 h at 1 week post-hatching and weekly for 5 weeks, starting at the onset of first feeding | ~50% functional males | [39] |
MDHT, 17 α-methyldihydrotestosterone; MT, 17 α-methyltestosterone; OHA, 17 β-hydroxyandrostenedione.
2.5. Methods of Steroid Administration and Their Doses
There are three methods for steroid administration: (i) Injection (either intramuscular or intraperitoneal), (ii) immersion in a static bath, and (iii) hormone supplementation in the diet. The injection of embryos or larvae is clearly not practical; consequently, the latter two methods are being adopted in hatchery practices, both separately and in combination (Table 2). The selection of the applied method is often clearly related to the labile period for particular species; immersion is preferred when sexual differentiation occurs during the embryonic or larval stages, whereas the dietary treatment is suited for species with differentiation during and/or after the initiation of larval feeding [13]. It also needs to be considered that steroids concentrations may decrease with storage time, especially at room temperature [21][40]. Optimising the dose and duration of treatment with masculinising agents is of the utmost importance; too-low concentrations may be ineffective (however, it may be useful for the production of functional males [38]), whereas too high doses often have a so called “paradoxical feminisation” or a sterilising effect [1][41][42].
2.6. Environmental Pollution Issues Related to Sex Reversal under Hatchery Conditions
In recent years, there has been growing concerns about the contamination of the aquatic environment with water pollutants due to anthropogenic activity, which is a serious threat for the reproduction of aquatic animals [43]. Endocrine disrupting chemicals (EDCs) are especially dangerous owing to their interference with endocrine functions. Synthetic androgens used for sex reversal are potential EDCs and their threat is causing increasing awareness in some countries. Therefore, methods to eliminate or decrease their concentrations in water will need to be developed. It seems clear that protocols based on immersion are potentially easy to control because androgens are confined to a restricted volume of vessels for a short time. On the contrary, feeding is more prone to water pollution with hormones, and lasts a long time (usually 60–80 days) so dramatically more hormones are used; moreover, androgens can leak from the diet to the water.
2.7. Species-Specific Characteristics Important for the Efficacy of Sex Reversal
The effective steroid doses for sex inversion were found to vary both between and within salmonid species [31]. Species-specific characteristics important for the efficacy of sex reversal may include the degree of gonadal development at the time of treatment, efficacy and specificity of steroid metabolism, and number and specificity of steroid receptors [13]. For some species, such as rainbow trout, efficient protocols must be developed; however, for others, such as brook trout, the efficacy of the procedure needs to be improved [6][28][32]. An extensive review of earlier protocols for particular species of salmonid fish was provided by other authors [12]. Examples of protocols published later are presented in Table 2.
2.8. Identification of SRF
The identification of SRFs is not necessary when genetic sex XX is clearly established either due to gynogenetic fish or the progeny of SRF and, in such a case, protocols aiming to produce functional (i.e., with normal spermatic ducts and strippable) semen are desired [39][44]. The production of functional males and their use for fertilisation is clearly advantageous because males can be used several times for two to three years. The problem occurs when the normal population is subjected to masculinisation and the sampling of genetic males is probable. The practical strategy in such a case is focused on the production of non-functional SRFs with easily identified phenotypes, such as hermaphrodites or males without spermatic ducts [21][45][46]. This would ensure that SRF population have the XX genotype and are not mixed with individuals presenting the XY genotype. To the best of our knowledge, this strategy seems to be the most popular in the production of salmonid fish SRFs under hatchery conditions. Recently, ultrasonography was found to be potentially useful for the identification of SRF rainbow trout [47].
This entry is adapted from the peer-reviewed paper 10.3390/ijms22020964
References
- Donaldson, E.M. Manipulation of reproduction in farmed fish. Anim. Reprod. Sci. 1996, 42, 381–392.
- Pandian, T.; Sheela, S. Hormonal induction of sex reversal in fish. Aquaculture 1995, 138, 1–22.
- Geffen, A.J.; Evans, J. Sperm traits and fertilization success of male and sex-reversed female rainbow trout (Oncorhynchus mykiss). Aquaculture 2000, 182, 61–72.
- Baroiller, J.-F.; D’Cotta, H. The reversible sex of gonochoristic fish: Insights and consequences. Sex. Dev. 2016, 10, 242–266.
- Chiasson, M.; Benfey, T.J. Gonadal differentiation and hormonal sex reversal in arctic charr (Salvelinus alpinus). J. Exp. Zool. Part A Ecol. Genet. Physiol. 2007, 307, 527–534.
- Haffray, P.; Petit, V.; Guiguen, Y.; Quillet, E.; Rault, P.; Fostier, A. Successful production of monosex female brook trout Salvelinus fontinalis using gynogenetic sex reversed males by a combination of methyltestosterone immersion and oral treatments. Aquaculture 2009, 290, 47–52.
- Fitzpatrick, J.L.; Henry, J.C.; Liley, N.R.; Devlin, R.H. Sperm characteristics and fertilization success of masculinized coho salmon (Oncorhynchus kisutch). Aquaculture 2005, 249, 459–468.
- Budd, A.M.; Banh, Q.Q.T.; Domingos, J.A.; Jerry, D.R. Sex control in fish: Approaches, challenges and opportunities for aquaculture. J. Mar. Sci. Eng. 2015, 3, 329–355.
- Migaud, H.; Bell, G.; Cabrita, E.; McAndrew, B.; Davie, A.; Bobe, J.; Herráez, M.; Carrillo, M. Gamete quality and broodstock management in temperate fish. Rev. Aquac. 2013, 5, S194–S223.
- Weber, G.M.; Lee, C.-S. Current and future assisted reproductive technologies for fish species. In Current and Future Reproductive Technologies and World Food Production; Lamb, G.C., DiLorenzo, N., Eds.; Springer: New York, NY, USA, 2014; pp. 33–76.
- Donaldson, E.M.; Hunter, G.A. Sex control in fish with particular reference to salmonids. Can. J. Fish. Aquat. Sci. 1982, 39, 99–110.
- Hunter, G.A.; Donaldson, E.M. 5 Hormonal sex control and its application to fish culture. Fish Physiol. 1983, 9, 223–303.
- Piferrer, F. Endocrine sex control strategies for the feminization of teleost fish. Aquaculture 2001, 197, 229–281.
- Francis, R.C. Sexual lability in teleosts: Developmental factors. Q. Rev. Biol. 1992, 67, 1–18.
- Padoa, E. Differenziazione e inversione sessuale (femminilizzazione) di avannotti di trota (Salmo irideus) trattati con ormone fol-licolare. Monit. Zool. Ital. 1937, 48, 195–203.
- Ashby, K.R. The effect of steroid hormones on the brown trout (Salmo trutta L.) during the period of gonadal differentiation. J. Embryol. Exp. Morphol. 1957, 5, 225–249.
- Hunter, G.A.; Donaldson, E.M.; Goetz, F.W.; Edgell, P.R. Production of all-female and sterile coho salmon, and experimental evidence for male heterogamety. Trans. Am. Fish. Soc. 1982, 111, 367–372.
- Hunter, G.A.; Donaldson, E.M.; Stoss, J.; Baker, I. Production of monosex female groups of chinook salmon (Oncorhynchus tshawytscha) by the fertilization of normal ova with sperm from sex-reversed females. Aquaculture 1983, 33, 355–364.
- Devlin, R.H.; Nagahama, Y. Sex determination and sex differentiation in fish: An overview of genetic, physiological, and environmental influences. Aquaculture 2002, 208, 191–364.
- Piferrer, F.; Baker, I.J.; Donaldson, E.M. Effects of natural, synthetic, aromatizable, and nonaromatizable androgens in inducing male sex differentiation in genotypic female chinook salmon (Oncorhynchus tshawytscha). Gen. Comp. Endocrinol. 1993, 91, 59–65.
- Guiguen, Y.; Bertho, S.; Herpin, A.; Fostier, A. Sex determination and sex control in salmonidae. In Sex Control in Aquaculture; Wang, H., Piferrer, F., Chen, S., Chen, S.-L., Shen, Z.-G., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 251–280.
- Demska-Zakęś, K.; Hliwa, P.; Matyjewicz, P.; Zakęś, Z. Effect of 17 alpha-methyltestosterone and 11 beta-hydroxyandrostenedione on the development of reproductive system in rainbow trout (Oncorhynchus mykiss Walbaum). Arch. Polish Fish. 1999, 7, 227–235.
- Kuźmiński, H.; Dobosz, S. Effect of sex reversal in rainbow trout (Oncorhynchus mykiss Walbaum) using 17α-methyltestosterone and 11β-hydroxyandrostenedione. Arch. Pol. Fish. 2010, 18, 45–49.
- Feist, G.; Yeoh, C.-G.; Fitzpatrick, M.S.; Schreck, C.B. The production of functional sex-reversed male rainbow trout with 17α-methyltestosterone and 11 β-hydroxyandrostenedione. Aquaculture 1995, 131, 145–152.
- Feist, G.; Schreck, C.B.; Gharrett, A.J. Controlling the Sex of Salmonids; Oregon Sea Grant, Oregon State University: Corvallis, OR, USA, 1996.
- Yossa, R.; Bardon-Albaret, A.; Chiasson, M.A.; Liu, Q.; Duston, J.; Manning, T.; Benfey, T.J. Controlling preharvest maturity in farmed Arctic char: A review from the Canadian perspective. J. World Aquac. Soc. 2019, 50, 894–907.
- Hliwa, P.; Kuźmiński, H.; Dobosz, S.; Nynca, J.; Dietrich, G.J.; Ziomek, E.; Ciereszko, A. Gonad morphology and semen quality of rainbow trout (Oncorhynchus mykiss) neo-males. In New Species in Aquaculture—Reproduction, Breeding, Prophylaxis; Zakęś, Z., Demska-Zakęś, K., Kowalska, A., Eds.; IRŚ: Olsztyn, Poland, 2011; pp. 105–116. (In Polish)
- Fatima, S.; Adams, M.; Wilkinson, R. Sex reversal of brook trout (Salvelinus fontinalis) by 17α-methyltestosterone exposure: A serial experimental approach to determine optimal timing and delivery regimes. Anim. Reprod. Sci. 2016, 175, 39–47.
- Fatima, S.; Adams, M.B.; Wilkinson, R. Histological study of gonadal development and sex differentiation in Salvelinus fontinalis under Tasmanian climate conditions. Aust. J. Zool. 2011, 59, 321–331.
- Valdivia, K.; Jouanno, E.; Volff, J.-N.; Galiana-Arnoux, D.; Guyomard, R.; Helary, L.; Mourot, B.; Fostier, A.; Quillet, E.; Guiguen, Y. High temperature increases the masculinization rate of the all-female (xx) rainbow trout “mal” population. PLoS ONE 2014, 9, e113355.
- Lee, P.; King, H.; Pankhurst, N. Preliminary Assessment of Sex Inversion of Farmed Atlantic Salmon by Dietary and Immersion Androgen Treatments. N. Am. J. Aquac. 2004, 66, 1–7.
- Galbreath, P.F.; Adams, N.D.; Sherrill, L.W., III. Successful sex reversal of brook trout with 17α-methyldihydrotestosterone treatments. N. Am. J. Aquac. 2003, 65, 235–239.
- Chevassus, B.; Krieg, F. Effect of the concentration and duration of methyltestosterone treatment on masculinization rate in the brown trout (Salmo trutta). Aquat. Living Resour. 1992, 5, 325–328.
- Heath, D.D.; Rankin, L.; Bryden, C.A.; Heath, J.W.; Shrimpton, J.M. Heritability and Y-chromosome influence in the jack male life history of chinook salmon (Oncorhynchus tshawytscha). Heredity 2002, 89, 311–317.
- Baron, D.; Montfort, J.; Houlgatte, R.; Fostier, A.; Guiguen, Y. Androgen-induced masculinization in rainbow trout results in a marked dysregulation of early gonadal gene expression profiles. BMC Genom. 2007, 8, 357.
- Baron, D.; Houlgatte, R.; Fostier, A.; Guiguen, Y. Expression profiling of candidate genes during ovary-to-testis trans-differentiation in rainbow trout masculinized by androgens. Gen. Comp. Endocrinol. 2008, 156, 369–378.
- Atar, H.H.; Bekcan, S.; Dogankaya, L. Effects of different hormones on sex reversal of rainbow trout (Oncorhynchus mykissWalbaum) and production of all-female populations. Biotechnol. Biotechnol. Equip. 2009, 23, 1509–1514.
- Ninhaus-Silveira, A.; Foresti, F.; Tabata, Y.A.; Rigolino, M.G.; Veríssimo-Silveira, R. Cryopreservation of semen from functional sex-reversed genotypic females of the rainbow trout, Oncorhynchus mykiss. Braz. Arch. Biol. Technol. 2006, 49, 73–77.
- Weber, G.M.; Leeds, T.D.; Schneider, R.P. Sex reversal of female rainbow trout by immersion in 17α-methyltestosterone. Aquaculture 2020, 528, 735535.
- Barry, T.P.; Marwah, A.; Marwah, P. Stability of 17α-methyltestosterone in fish feed. Aquaculture 2007, 271, 523–529.
- Piferrer, F.; Donaldson, E.M. The comparative effectiveness of the natural and a synthetic estrogen for the direct feminization of chinook salmon (Oncorhynchus tshawytscha). Aquaculture 1992, 106, 183–193.
- Johnstone, R.; MacLachlan, P. Further observations on the sex inversion of Atlantic salmon, Salmo salar L., Using 17α methyl testosterone. Aquac. Res. 1994, 25, 855–859.
- Devaux, A.; Bony, S.; Plenet, S.; Sagnes, P.; Segura, S.; Suaire, R.; Novak, M.; Gilles, A.; Olivier, J.-M. Field evidence of reproduction impairment through sperm DNA damage in the fish nase (Chondrostoma nasus) in anthropized hydrosystems. Aquat. Toxicol. 2015, 169, 113–122.
- Lehnert, S.J.; Heath, D.D.; Pitcher, T.E. Sperm trait differences between wild and farmed Chinook salmon (Oncorhynchus tshawytscha). Aquaculture 2012, 344–349, 242–247.
- Cousin-Gerber, M.; Burger, G.; Boisseau, C.; Chevassus, B. Effect of methyltestosterone on sex differentiation and gonad morphogenesis in rainbow trout Oncorhynchus mykiss. Aquat. Living Resour. 1989, 2, 225–230.
- Baynes, S. Fertilisation procedures for use in all-female brood production. In Trout News; CEFAS: Suffolk, UK, 1999; Volume 28, pp. 23–25.
- Hliwa, P.; Bah, M.M.; Kuźmiński, H.; Dobosz, S.; Ciereszko, A. Ultrasound evaluation of the gonadal structure in sex-reversed rainbow trout females. Aquac. Int. 2013, 22, 89–96.
