Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Electrochemistry
Metalloporphyrins (MP) and metallophtalocyanines (MPc) are innovative materials with catalytic properties that have attracted attention for their application for diverse electrochemical purposes. The presence of metallic centers in their structure offers a redox-active behavior that is being applied in the design of solid electrodes for the quantification of biomolecules, water contaminants, and pharmaceuticals, among others.
- porphyrin complexes
- electro-catalysis
- electrochemical sensors
1. Background
In recent years, the quantification of important molecules by using electrochemical techniques such as chronoamperometry or voltammetry has considerably grown because of their low-cost instruments, excellent reproducibility, high sensitivity, and the low detection limits obtained. Each year, new reports about electrochemical sensors are focused on medical or industrial applications. The technical requirements for the design of electrochemical sensors for these purposes involve the use of modified electrodes that allow the electro-oxidation or reduction of analytes on their surface. In this respect, there is a huge necessity for the synthesis of innovative materials that could work as electrocatalysts in these electrodes. As a result, over the last 10 years, the research in coordination chemistry has played an important role in finding novel materials with redox catalytical activity with wide applicability to electrochemistry. Several metal-coordinated materials are being explored as electrocatalysts with high selectivity and activity toward diverse molecules with biological or environmental interest.
Metalloporphyrin (MP) and metallophthalocyanines (MPc) are a class of organometallic complexes that have attracted attention as active materials on electrochemistry due to their redox-active and enzyme mimic properties [1,2,3]. These electro-catalytic features can generate electrochemical signals with diverse analytes that are useful in sensing applications. As a result, there are a large number of examples where MP and MPc have been applied to chemical and electrochemical sensors.
2. Metalloporphyrins and Metallophthalocyanines Composites as Modifiers
The first challenge on the electrochemical sensor designing is to select an inexpensive and accurate electrode modifier material that could act as an electrocatalyst on the electrode’s surface. In this respect, MP and MPc (Figure 1) are related complexes that contain a N4-macrocyclic coordinated to a metallic ion.
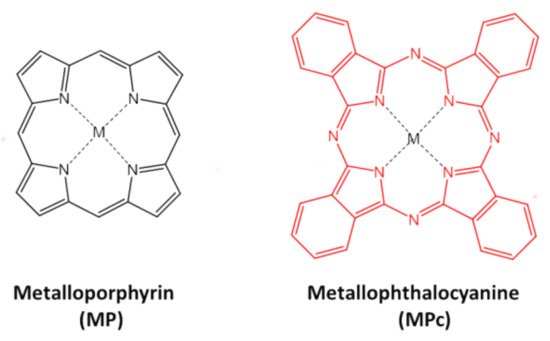
Figure 1. Molecular structure of MP and MPc complexes.
Structurally, porphyrin and phthalocyanine ligands present a highly 18-π conjugated system with a rich electron density perfectly suitable for electron mediator applications [1]. By understanding the complex cytochrome P-450 mechanism, researchers have studied several MP and MPc for bioinspired heterogeneous redox electrocatalysis [2], and at present, the most common MP and MPc used for these purposes contain transition metal ions from Cr3+ to Zn2+. In certain conditions, these metallic ions display highly active redox pairs that catalyze oxidation or reduction reactions of biological molecules. The electronic states of metallic centers of MP and MPc can be tuned by the effect of the ligand and its substituents. As a result, the catalytical properties of the complexes can be enhanced, and the coordination environment allows adsorbing specific analytes that increase the selectivity of the system [3]. This property is highly used on electrochemical sensors design for the electro-catalysis of diverse kinds of molecules on the electrode’s surface; additionally, other applications involve their use in fuel cells [3], electrochemical water splitting [4], and hydrogen evolution reactions [5].
Despite all the interesting properties that MP and MPc could offer as active materials for electrochemical applications, the use of pristine MP and MPc as modifiers materials is scarce, because most of these complexes tend to dissolve in diverse electrolytes, and they are unstable in the presence of highly alkaline or acidic media [3,6]. A smart strategy to overcome this situation is the immobilization of the complexes in a composite. Carbon-based nanomaterials such as graphene, reduced graphene oxide (RGO), graphene oxide (GO), or carbon nanotubes (CNTs) are widely used to immobilize MP or MPc via the π–π non-covalent stacking interactions that occur between porphyrin rings and the nanomaterial’s aromatic rings [7]. This kind of combination enhances the electron-conductive properties of coordination complexes and makes MP and MPc very stable compounds in alkaline or acidic media [8]. As a final result, the obtained composites display highly conductive redox properties and an accurate selectivity.
3. Electrodes Modification with MP and MPc
The second challenge is to load the composite on an electrode’s surface to modify it or use the composite to build electrodes. In literature, the modification of glassy carbon electrodes (GCE) with composites is broadly reported. These kinds of electrodes are mainly preferred due to their chemical inertness and wide electrochemical window; furthermore, GCE can be modified by drop-coating. In this process (Figure 2a), the composite is dispersed in a solvent, and the suspension obtained is cast on the polished electrode to create a thin layer that is adsorbed on the surface and prevents the resolution of the coordination complex during the electrochemical measurements [9].
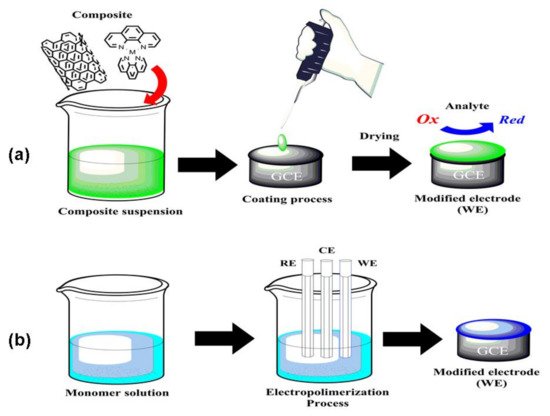
Figure 2. (a) GCE coating modification process; (b) GCE electropolymerization coating process.
Electropolymerization is also used to modify the surface of GCEs. As shown in Figure 2b, the working electrode (WE) is placed in a solution containing a coordination complex monomer and an electrolyte. The process is carried out by using cyclic voltammetry (CV), and during the electropolymerization, the voltammogram displays a progressive increase of the current density initiated by the conductive properties of the polymerized species on the electrode’s surface. In this process, it is important to control the surface coverage concentration (Γ), which may disturb the diffusion of analytes into the electrode and affects the electrocatalytic activity [10].
On the other hand, carbon paste electrodes (CPE) are also broadly used in the elaboration of electrochemical sensors. These electrodes are elaborated with graphite paste that is packed in a plastic tube to fabricate the electrode. The modification of CPEs (Figure 3) involves the ex situ synthesis of a composite made with the graphite powder, a modifier agent (e.g., coordination complexes, nanomaterials, polymers, etc.), and a binding agent (e.g., mineral oil, paraffin oil). In the composite, the ratio of the modifier may vary, and usually, big amounts of modifier in the composite could decrease the catalytic activity and the electron transfer; therefore, researchers evaluate the different proportions to find the best electrochemical response [11,12,13].
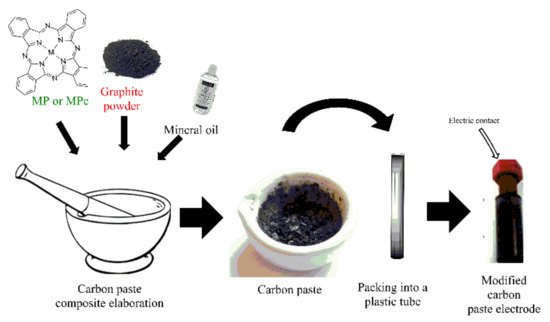
Figure 3. A simple modified CPE’s elaboration process.
4. Characterization of Modified Electrodes
The third challenge is to characterize the electrochemical properties of the modified electrodes. Different tools such as cyclic voltammetry (CV), chronoamperometry (CA), and electrochemical impedance spectroscopy (EIS) are used for this purpose.
For example, cyclic voltammetry (CV) is used to evaluate the electro-catalytic response of metalloporphyrin or metallophthalocyanines in presence of an analyte and helps to identify the presence of characteristic redox peaks, specifically the formation of redox-active pairs (M2+/M3+), which are the key to the electro-catalysis of analytes. During the characterization of the modified electrodes, the pairs are displayed as anodic and cathodic peaks in the voltammograms at specific potentials. Depending on the redox process (oxidation or reduction), the current density of these peaks tends to increase or decrease when the modifier has an electro-catalytic activity toward the analyte. Usually, a linear behavior is observed between the peak current density and the square root of the scan rate, indicating a diffusion-controlled process [10].
Chronoamperometry (CA) is useful to explore the kinetical parameters of redox catalytical processes on the electrode’s surface and is the most used electroanalytical technique for quantitative analysis. This technique involves imposing a specific electric potential, and the current density obtained (proportional to the analyte concentration) is recorded as a function of time [14]. On amperometric sensors, it is desired to get high current densities at low anodic potentials.
Finally, electrochemical impedance spectroscopy (EIS) is broadly used to characterize the electrical resistance of modified electrodes. Researchers often make a comparison between Nyquist plots of several modified electrodes to identify the effect of the proportion of the components on composites over the electrode’s electrical conductivity [15]. On this kind of graph, the semicircle diameter on the Nyquist plot is equal to the electron transfer resistance [16]; in this respect, a bigger semicircle reflects a higher resistance. On EIS evaluations, low resistance values are desired for electrocatalyst applications; however, some coordination complexes and metal–organic frameworks (MOFs) induce an increase of the electrode resistance (Ω), which drastically decreases when these materials are incorporated in a composite with some conductive materials, such as graphene oxide (GO) or reduced graphene (rGO).
A complete example of modified electrodes electrochemical characterization is reported by Chaiyo and coworkers [17]. In their work, diverse screen-printed carbon electrodes were modified with different composites elaborated with cobalt (II) phthalocyanine, graphene, or ionic liquid. The six electrodes were characterized by CV, EIS, and CA. As shown in Figure 4a, the electrodes are firstly evaluated by CV in presence of 0.5 mM [Fe(CN)6]3- in 0.1 M KCl to identify their electrochemical response. The anodic and cathodic peak current densities increased with the composite’s complexity; in this respect, the unmodified electrode presented the lowest electrochemical response, while CoPc/IL/G/SPC electrode presented an elevated peak current density, indicating an excellent response. This behavior matches with the results obtained by using EIS (Figure 4b), where the CoPc/IL/G/SPC displayed a moderate electrical resistance caused by the presence of the coordination complex, which decreases the electron conductivity and enhances the electro-catalytic activity. Figure 4c,d represents the electrochemical response of unmodified and modified electrodes in the absence and presence of an analyte (2.0 mM glucose). The unmodified electrode does not show any response in the absence of the presence of glucose. On the other side, the CoPc/SPC electrode (black line, Figure 4d) showed two redox peaks at −0.33 and 0.26 V assigned to Co3+/Co2+ and Co2+/Co3+ redox pairs. In the presence of glucose, the electrode showed a new anodic peak at 0.65 V (blue line). This effect showed that the CoPc/SPCE has a strong electro-catalytic activity towards glucose, which increases on CoPc/IL/G/SPC as an effect of the graphene electron conductivity properties. Finally, Figure 4e shows the scan rate effect over the electro-oxidation of glucose, presenting a linear tendency that is characteristic of a diffusion-controlled process.
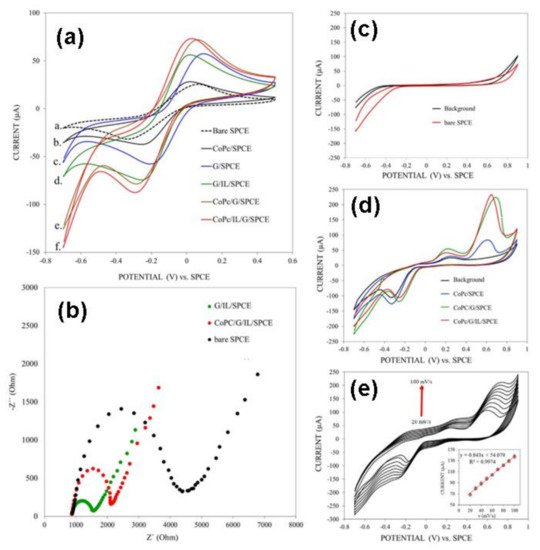
Figure 4. (a) CV characterization of diverse electrodes in presence of [Fe(CN)6]3−. (b) EIS of unmodified SPCE (black), CoPc/IL/SPCE (green), and CoPC/G/IL/SPCE. (c) CV of the unmodified electrode in the absence or presence of glucose. (d) The electrochemical response of the diverse electrodes in presence of 2.0 mM glucose at 0.5 Vs−1. (e) The effect of the scan rate on CoPC/G/IL/SPCE over glucose oxidation. Redrawn with permission of Elsevier from Reference [17].
5. Application of Modified Electrodes Containing MP and MPc for the Quantification of Molecules with Biological Importance
The identification and exact quantification of biomolecules are highly important in biochemistry, clinical chemistry, medical sciences, and environmental sciences. The research on electrochemical sensors for biomolecule sensing purposes is growing constantly, with more than 2500 reports per year according to a Scopus search. Most of the innovative designs involve the use of inorganic materials in conjunction with enzymes to obtain highly selective sensors. In this respect, phthalocyanine complexes have been used to immobilize enzymes and as electrocatalysts. Furthermore, there is a huge interest in the applicability of inorganic redox-active materials for replacing enzymes and designing non-enzymatic sensors with enhanced analytical parameters. In this section, we discuss the applications, key role, and reaction mechanism of MP and MPc on enzymatic and non-enzymatic electrochemical sensors for detecting biomolecules such as ascorbic acid, dopamine, uric acid, glucose, and hydrogen peroxide.
5.1. Electrochemical Sensing of Ascorbic Acid and Uric Acid
Ascorbic acid (H2A) is an important antioxidant that displays a redox couple (ascorbic acid (H2A)/dehydroascorbic (A)) useful in hydroxylation reactions in the body [18], the reduction reaction of several compounds such as reactive oxygen species [19], and protecting cells against oxidative stress caused by infections [20]. Owing to these properties, ascorbic acid plays an important role in the treatment of several respiratory infections such as bronchitis, pneumonia [20], and influenza, and recently, there have been several trials for the treatment of SARS-CoV-2 [21,22,23].
On the other hand, uric acid is a product of purine metabolism present in diverse human fluids such as blood serum or urine, where its concentration range is 0.24–0.50 and 0.214–4.40 mM, respectively. High levels of UA may cause many urinary tract diseases and renal failure.
Ascorbic acid and uric acid sensors have interesting applicability in medical sciences. The examples of these sensors fabricated with coordination compounds are scarce; however, interesting results have been obtained by using diverse composites of porphyrin complexes based on Mn3+, Fe2+, and Zn2+ that display a redox pair M2+ ⇆ M3+, which mimics the active site of several enzymes, and it is useful for oxidation and reduction of diverse kind of biomolecules [24]. The selective oxidation of ascorbic acid or uric acid on the electrode’s surface depends on the metallic center and other components on the electrode. Interesting results were obtained with composites of [Zn2+(Pro)3+R(Cl)] and tetraoctilammonium bromide (TOAB) for the electroanalytical sensing of ascorbic acid at pH 7.0 [25,26]. In the composites, the tetraoctylammonium bromide aids in the oxidation of ascorbic acid through an irreversible oxidation process, while the dye only works as an electron-transfer mediator. An analysis conducted by CV indicated that Zn(II) metalloporphyrins showed four redox process (in an anodic and cathodic sense) between 0.5–1.2 V related to the oxidation and reduction of the porphyrin ligand and the Zn2+ ion. Based on these results, it was proposed that the key role of the dye follows the next mechanism:
2H2A → 2A− + 4H+ 2e−
[Zn2+(Pro)3+R(X)] + 2A− → [Zn2+(Pro)3+R(X)] ···2A−
[Zn2+(Pro)3+R(X)]···2A− → [Zn(Pro)3+R(X)] + 2A
[Zn(Pro)3+R(X)] → [Zn2+(Pro)3+R(X)] + 2e−
The anion (A−) produced in Reaction (1) is coordinated in the complex [Zn2+(Pro)3+R(X)] in Reaction (2) to create a coordination bond with A-; then, in Reaction (3), the electrons are captured by the complex to produce its reduced form [Zn(Pro)3+R(X)] and dehydro-L-ascorbic acid; finally, in Reaction (4), the complex [Zn2+(Pro)3+R(X)] is regenerated again. The oxidation of H2A is carried out by TOAB present in the composite.
For the selective oxidation of uric acid, it is only reported the use of a manganese (III) porphyrin complex (MnNH2TPP) composite [27]. The interesting part of this work is the proposed oxidation mechanism, which suggests that UA is coordinated to the complex, and uric acid is then oxidized in several electron transfer steps as shown in Figure 5, where, after the coordination of UA molecules, these are rearranged to produce carbocations that undergo a dehydrogenation reaction to yield allantoin as the final product.
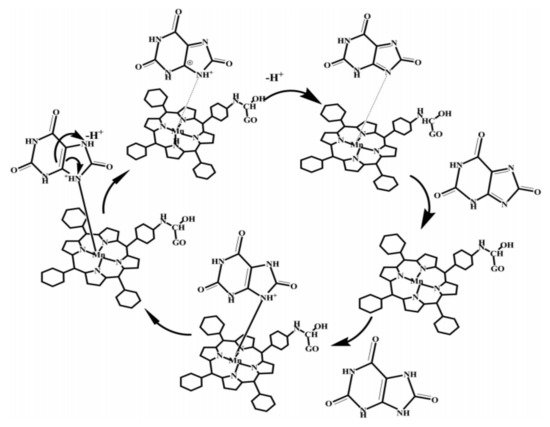
Figure 5. The oxidation process of uric acid oxidation with MnNH2TPP. Redrawn with permission of Elsevier from Reference [27].
On the other hand, in some specific cases, metalloporphyrins catalyze the oxidation of both substrates (ascorbic acid and uric acid). This property was presented on iron (III) porphyrin complexes [24]. The simultaneous oxidation for ascorbic acid and uric acid gives dehydroascorbic acid and allantoin, respectively, as former oxidation products. During the oxidation process, Fe3+ center is reduced to Fe2+ during the oxidation of the analytes at diverse potentials. The final oxidation products obtained from the analytes are represented in Figure 6. The process is highly pH-dependent (pH = 4.0), and the results obtained with these electrodes displayed very low detection limits in the micromolar order and a wide linear range (from micromolar to millimolar). These results demonstrate the potential applicability of MP or MPc as multi-analyte electrochemical sensors; nonetheless, because the application of complexes to electroanalytical chemistry is new, few reports can be found in the literature.
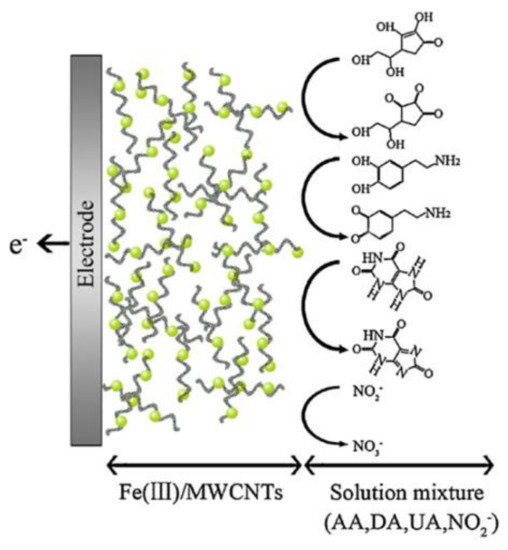
Figure 6. Multiple oxidation process of ascorbic acid (AA), dopamine (DA), uric acid (UA), and nitrite (NO2−). Redrawn with permission of Elsevier from Reference [24].
As part of this work, we summarized the recent examples of electrodes modified with porphyrin complexes for the electroanalytical sensing of ascorbic acid (Table 1) and uric acid (Table 2), including the potentials of work, limits of detection, and other analytic parameters.
Table 1. Examples of porphyrin complexes used in the modification of electrodes for ascorbic acid sensing.
| Electrode | Catalyst | Potential | Technique | LOD | Linear Range | Real Sample | Ref. |
|---|---|---|---|---|---|---|---|
| GCE | Fe(III)P/MWCNTs | 0.20 V | CA | 3.0 µM | 14–2500 µM | Urine/Serum | [24] |
| GCE | Mn(III)TTPCl | 0.30 V | CV | - | 2.6–43.80 µM | - | [28] |
| GCE | Fe(III)TPyP/Clay | 0.40 V | SWV | 0.95 µM | 5–300 µM | Vitamin Syrup | [29] |
| GCE | Zn(II) YD2-o-C8/MWCNTs | 0.40 V | CA | 0.18 µM | 18.72–1850 µM | VC tablets | [26] |
| GCE | Zn(II) YD2-o-C8/GO | 0.54 V | CA | 0.28 µM | 1.33–1460 µM | Soft drinks/Urine | [25] |
Table 2. Examples of porphyrin complexes used in the modification of electrodes for detection of uric acid.
| Electrode | Catalyst | Potential | Technique | LOD | Linear Range | Real Sample | Ref. |
|---|---|---|---|---|---|---|---|
| GCE | Fe(III)P1/MWCNTs | 0.20 V | CA | 0.30 µM | 5.80–1300 µM | Urine/Serum | [24] |
| GCE | Mn(III)NH2TPP | 0.30 V | DPV | 1.74 µM | 20–290 µM | Urine | [27] |
| GCE | Fe(III)TPyP/Clay | 0.40 V | SWV | 0.06 µM | 0.4–25 µM | - | [29] |
P1 = [3,7,12,17-tetramethyl-8,13-divinylporphyrin-2,18-dipropanoato (2-)]iron(III). DPV = differential pulse voltammetry, SWV = square wave voltammetry.
5.2. Electrochemical Sensing of Dopamine
Dopamine (DA) is an important catecholaminergic neurotransmitter that plays an essential role as a chemical messenger in the central nervous system and cardiovascular system. The normal level of dopamine in the brain is around 0.01–1.0 µM [30], and the decrease of this level may cause severe affectations such as Parkinson’s disease or schizophrenia [31]. The development of novel sensors for detecting DA is an important topic in electrochemistry; however, the coexistence of ascorbic acid and uric acid in real samples interferes with the accurate quantification of DA because both molecules are oxidized at the same potential as DA, and electrodes are unable to separate their electroanalytical signals. As a result, there is intensive research on new materials with the capability of selective sensing of DA at low potentials. The biological compatibility of porphyrins and phthalocyanine complexes make them attractive for their use as active materials on electrodes for the electroanalytical sensing of DA. For these purposes, composite-based Co2+, Cu2+, or Zn2+ complexes and carbon nanomaterials have been used in the selective oxidation of dopamine on amperometric sensors, displaying the limits of detection in the micromolar/nanomolar order and low oxidation potentials (under 0.3 V). When these electrodes are in the presence of diverse concentrations of DA, a linear increase of the anodic current is observed, indicating the great sensitivity of the electrodes fabricated with MP and MPc. Additionally, it is reported that the electroanalytical response increases in the presence of diverse scan rates, which suggests that the oxidation of dopamine is a diffusion-controlled mass process [32]. The electro-oxidation of dopamine is carried out by the redox pair M2+/M3+ present in the complexes, where the formation of M3+OOH plays an essential role as a redox catalyst. In an anodic sense, the complex is forced to be oxidized by the potential applied, and as result, unstable and highly oxidant M3+ species are obtained in the electrode surface and reduced to the M2+ due to the electron gain during the oxidation process. As illustrated in Figure 7, the reaction has a loss of two protons and is carried out via two-electron oxidation to yield dopamine-o-quinine as the final oxidation product.
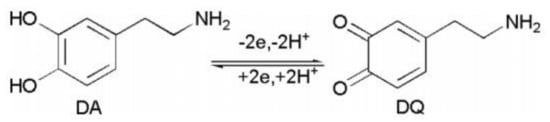
Figure 7. Electro-oxidation process of dopamine. Redrawn from Reference [30].
The obtaining of M3+ species and the electro-oxidation process are dependent on the pH, and better results have been obtained at pH 6–7. At this pH range, the changes in the coordination environment can be clearly seen by cyclic voltammetry. In other cases, the addition of hydrogen peroxide was a fundamental step to obtain the high valence M3+ species [31,32]. In this case, H2O2 activates the complex to obtain the M3+OOH active layer on the electrode’s surface. For now, the results obtained with these electrochemical sensors are promising. These features can be assigned to the conductivity properties of the nanomaterials employed on the composites and the redox properties of complexes. At present, only a few examples of dopamine sensors elaborated with MPc or MP are reported. We expect that in the future, the application of these kinds of complexes will attract the attention of electroanalytical chemistry; nonetheless, it is necessary to evaluate new complexes (such as those based on Ni2+) and enhance their solubility or conductivity to obtain better electrochemical signals. As part of this work, we have summarized in Table 3 the recent electrochemical sensors based on MP or MPc for dopamine quantification.
Table 3. Examples of MP and MPc used in the modification of electrodes for detection of dopamine.
5.3. Electrochemical Sensing of Glucose (Enzyme-Porphyrin Sensors and Non-Enzymatic Sensors)
Today, glucose amperometric sensors are one of the most critical topics in electroanalytical chemistry. The demand for more reliable and exact analytical devices is based on the constant increment of diabetic patients around the world [34]. There are several proposals of innovative and promising wearable devices based on enzymes for detecting glucose in fluids such as sweat [35], saliva [36,37,38], or urine [39,40,41]; however, the use of enzymes is challenging, and several problems are faced during their immobilization on the electrode’s surface. Moreover, the electron transference between the enzyme redox center and the electrode surface is slow, and the glucose oxidation requires elevated positive overpotentials. In this regard, several types of electroactive materials such as metallophthalocyanines and metalloporphyrins are being employed on integrated biosensors as electron-transfer mediators and co-immobilizers of enzymes, helping to enhance the electroanalytical signal.
There is a growing interest in designing bio-amperometric sensors (second generation), based on the use of nanocomposites of cobalt (II) phthalocyanine, and currently, diverse examples can be found in the literature [8,42,43,44]. This particular interest is due to their versatility, biocompatibility, and the redox-active activity of metals in the complex, which present a redox pair (Co2+/Co3+) in presence of alkaline media that is useful in electrocatalytic processes [43] and the electron-transfer capability of the nanomaterials employed to fabricate the nanocomposite.
On nanocomposites of phthalocyanine complexes, graphene is commonly used, because it improves the conductivity problems related to the semiconductor behavior of the complexes. The key role of cobalt (II) phthalocyanine in the nanocomposite is to act as a peroxidase, mediating the electro-oxidation of H2O2 produced during the glucose oxidation with the GOx enzyme. This electro-catalytic process is carried out by the redox pair (Co2+/Co3+) presented on the complex, as illustrated in the reaction in Figure 8, and is directly used to measure the glucose concentration in the function of the amount of hydrogen peroxide produced during the enzymatic reaction. As a result, these electrodes have the capability of sensing glucose or hydrogen peroxide at neutral pH.
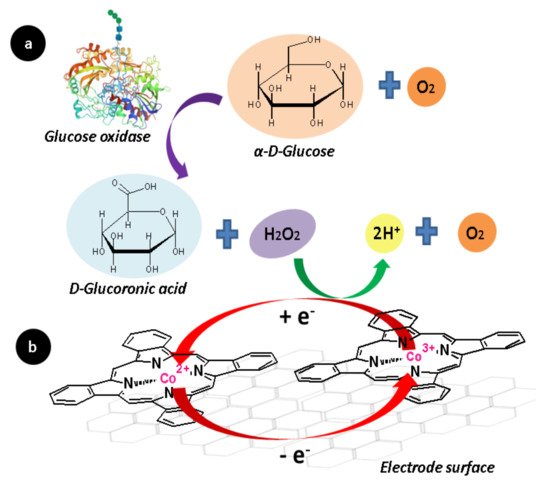
Figure 8. Basic working of an integrated bio-electrode: (a) The GOx enzyme catalyzes the oxidation of glucose and generates hydrogen peroxide as a by-product. (b) Then, the hydrogen peroxide is electro-oxidized by the metal center of the complex, and the electron exchange of the reaction is used in an electroanalytical technique to quantify the amount of glucose in a sample.
As shown in Table 4, the few examples of biosensors elaborated with cobalt phthalocyanine [8,42,43,44] or other kinds of porphyrins derivative complexes [45] have displayed good detection limits (around 1.6–63 µM), although in the presence of real samples of human serum blood or drinks. Despite the low sensitivity and accurate detection limits (in the micromolar order) obtained with these electrodes, the use of expensive enzymes as principal active materials limits the commercial production and applications [46]. As a result, porphyrin and phthalocyanine complexes are being explored as a direct redox-catalyst for glucose oxidation in non-enzymatic electrochemical sensors. It is demonstrated that materials based on specific transition metals such as Co2+, Ni2+, and Cu2+, have redox catalysis activity toward glucose [47]. In this respect, coordination compounds such as MOFs based on these ions are being mainly used as active materials for the selective oxidation of glucose in alkaline media, presenting extraordinary results [13]. Regarding metal porphyrins, films of diverse types of nickel and cobalt porphyrins have been explored on glucose electro-oxidation in alkaline media, and they have displayed high stability in aqueous solutions, low detection limits, low oxidative potentials, and wide linear response. To explain the mechanism of glucose electro-oxidation, the most accepted hypothesis suggests that M3+OOH species obtained in alkaline media from M2+ complexes act as a redox mediator for the electro-oxidation of glucose to obtain gluconolactone as former oxidation product [8,10,13,16,48], according to the following mechanism:
M2+-complex + OH− ⇆ M3+-OOH + e−
M3+-OOH + glucose + e− → M2+-complex + gluconolactone
Table 4. Examples of MP and MPc complexes used in the fabrication of enzyme-based electrodes for electrochemical sensing of glucose.
| Electrode | Catalyst | Potential | Technique | LOD | Linear Range | Sensitivity | Real Sample | Ref. |
|---|---|---|---|---|---|---|---|---|
| GCE | Cu-Hemin MOFs | 0.3 V | LSV | 2.73 µM | 9.10-µM–36.0 mM | 22.77 µAmM−1 | Human serum | [45] |
| paper | CoPc/GO | 0.3 V | CV | 63 µM | 0.1–1 mM | N/R | Orange juice | [43] |
| GCE | Gr-CoPc/GOD | 0.4 V | CV | 1.6 µM | 10 µM–14.8 mM | 5.09 µAmM−1 | Human serum | [44] |
| GCE | (CoPc–(CoTPP)4)/GOD | 0.4 V | CA | 10 µM | N/R | 24.20 nAmM−1 | N/R | [42] |
LSV = linear sweep voltammetry.
In Reaction (5), the M2+ is oxidized to M3+ and produces MOOH, which generates an increase of the anodic peak current in cyclic voltammetry; then, in Reaction (6), MOOH oxidizes glucose (C6H12O6) to gluconolactone (C6H12O7), enhancing the height of the anodic peak. This oxidation process is easily observable by CV for nickel complexes in comparison with the cobalt complex.
For example, Maia and coworkers [49] reported the modification of a GCE with tetra{bis(2,2-bipyridyl)chlororuthenium(II)}{5,10,15,20-tetra(4-pyridyl)porphynatonickel(II)}(Ni-TRP) for electro-oxidation of glucose in alkaline media. In the absence of glucose, the CV experiments displayed the characteristic Ni2+/Ni3+ redox pair between 0.4–0.6 V (Figure 9a). In the presence of glucose (0.95 mM), there was an increment of 20 µA in the anodic peak current at 0.58 V, which increases at diverse glucose concentrations (Figure 9b). The evaluation as an electrochemical sensor was carried out by cyclic voltammetry in a linear range of 0.019–1.8 mM, obtaining a LOD of 6.1 µM.
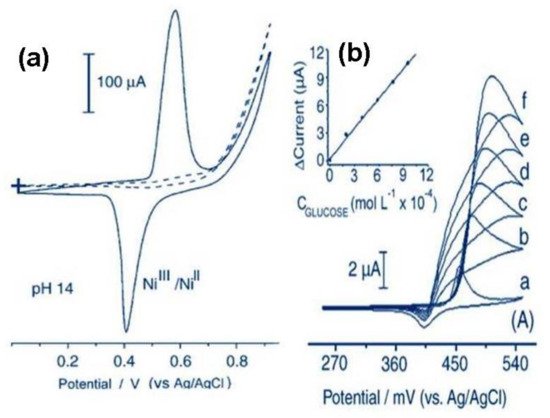
Figure 9. (a) The electrochemical behavior of Ni-TRP in 0.1 M NaOH (solid line) and 1.0 M DMSO. The formations of anodic and cathodic peaks in the alkaline media are assigned to the Ni(II) → Ni(III) and Ni(III) → Ni(II) transitions, respectively; (b) Response of the Ni-TRP electrode in presence of a diverse concentration of glucose (a = 0M, b = 2 × 10−4 M, f = 1 × 10−3 M). Redrawn from Reference [49].
The use of cobalt phthalocyanines complexes is more common due to its high electro-catalytic activity toward biomolecules. As presented before, cobalt phthalocyanines were used in conjunction with enzymes to obtain novel enzymatic electrodes, and diverse examples have demonstrated the capability of cobalt phthalocyanine composites as redox mediators on glucose detection without enzymes, presenting lower detection limits (0.14–0.6 µM) than enzyme-based electrodes. Furthermore, the linear range is over 20 mM, which is very important for glucose detection in real samples. As shown in Table 5, the analytical parameters of non-enzymatic electrodes surpass those reported for enzyme-based electrodes in Table 4. Nonetheless, the research on porphyrins derivative complexes for non-enzymatic glucose sensors is losing ground and is being replaced with other metal-coordinated materials such as MOFs.
Table 5. Examples of non-enzymatic glucose sensors based on MP an MPc.
| Electrode | Catalyst | Potential | Technique | LOD | Linear Range | Sensitivity | Real Sample | Ref. |
|---|---|---|---|---|---|---|---|---|
| GCE | CoPc/MWCNTs | 0.3 V | CA | 0.14 µM | 10–6340 µM | 122.5 µAmM−1 | Human blood | [48] |
| GCE | Ni-TPR | 0.5 V | CV | 0.36 µM | 2.5–1000 µM | N/R | Parentheral solution | [49] |
| GCE | CoPc/Gr | 0.5 V | CA | 14.6 µM | 17.64–1630 µM | - | Human blood | [8] |
| ITO | CoFe-LDH/Mn-TPPS | 0.6 V | CA | 0.79 µM | 0.1–15 mM | 66.3 µA mM−1 | - | [16] |
| paper | CoPc/Gr/IL | 0.7 V | CA | 0.67 µM | 0.01–5.0 mM | - | Honey and wine | [17] |
| GCE | CoPc/DPDE-Ru(III)/MWCNTs | 0.22 V | CV | - | 50–250 mM | - | - | [50] |
5.4. Application of Modified Electrodes Containing MP and MPc for the Quantification of Hydrogen Peroxide
Hydrogen peroxide (H2O2) is a strong oxidizer industrially used for cleaning and bleaching processes. In the body, it plays an important function in the defense of the organism; however, elevated concentrations of this molecule may cause damage to cell proliferation, cancer, or Alzheimer’s disease [51]. On the other hand, H2O2 is a by-product generated during the oxidation of glucose-by-glucose oxidase enzymes, and it can be used for the indirect quantification of glucose by using analytical techniques such as titrimetry and spectrofluorimetry [52], and more recently, hydrogen peroxide sensors are being used for the indirect electrochemical quantification of glucose; nevertheless, this quantification implies the use of enzyme-based electrodes, which are expensive and difficult to build. At present, there is a growing interest in the design of enzyme-free sensors for the accurate detection of H2O2 in neutral and alkaline media [53], and materials based on transition metals are playing an important role as active materials in non-enzymatic electrodes. In this respect, composites of MP and MPc have been demonstrated to be excellent modifiers for the elaboration of innovative electrochemical sensors. Diverse electrodes modified with MP or MPc based on Mn3+, Fe2+, Co2+, and Zn2+ have been successfully applied for the selective electrocatalyst of hydrogen peroxide in neutral or alkaline media.
The electrocatalyst process of hydrogen peroxide is divided into reduction and oxidation. In both cases, the formation of a redox couple (M2+/M3+ or M3+/M4+) on the complex mediates the analyte’s electro-reduction or electro-oxidation. In the reduction process (Figure 10), H2O2 yields H2O in a negative potential, and the metallic ions are oxidized by the effect of H2O2 to temporally yield a high valence state, which is reduced to its stable form. This process is observed as two peaks (anodic and cathodic) in cyclic voltammetry experiments. On the other hand, during the oxidation process, the high valence species produced on anodic potential catalyze the electro-oxidation of hydrogen peroxide to yield oxygen (O2) as the main product. As illustrated in Figure 11, Gao and coworkers [54] proposed that during the oxidation process with Co(II) porphyrin complex, H2O2 is bonded to the high valence metallic center (Co3+) to create an intermediary product, which participates in an H2O2 deprotonation process to give a dioxygen intermediate. The dyoxygen is released and the Co2+ is regenerated again.
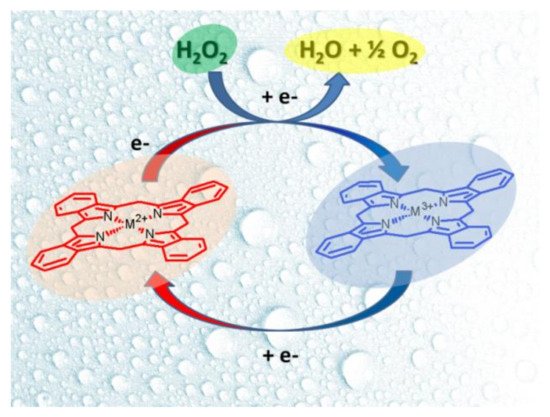
Figure 10. Peroxide electrocatalysis process mediated by MPc or MP in alkaline or neutral media.
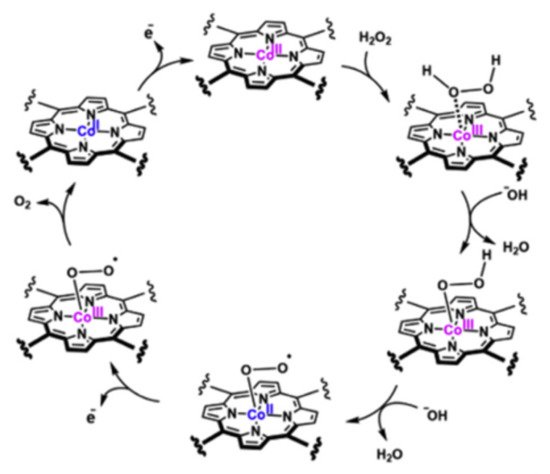
Figure 11. H2O2 electro-oxidation process mediated by a Co(II) porphyrin complex in alkaline media. Redrawn with the permission of Elsevier from Reference [54].
In other papers, the changes in the coordination environment are not mentioned; however, it is possible that metallic ions could be coordinated with hydroxyl ions to form an octahedral structure. This behavior was observed during the evaluation of a Co(II) porphyrin complex by cyclic voltammetry at diverse pH values, where the anodic peak displayed a negative shift when the pH increased, which indicates a strong binding of the hydroxyl ion to the Co(III) [55]. The analytical parameters of electrochemical sensors based on MP or MPc are promising, with limits of detection (LOD) from the nanomolar to micromolar order and a wide linear range. At present, the research on MP and MPc as redox-catalysts on hydrogen peroxide sensors remains active, and diverse materials such MOFs or COFs (covalent–organic frameworks) are being used in combination with MP/MPc to obtain innovative and highly selective composites for electroanalytical application. It is expected that the research on non-enzymatic sensors based on Mp and MPc could increase in the close future and completely replace enzymes. As part of this section, diverse examples of complexes-based electrodes for sensing hydrogen peroxide are summarized in Table 6.
Table 6. Examples of non-enzymatic H2O2 sensors based on MP and MPc complexes.
| Sensors Based on Electro-Reduction of Hydrogen Peroxide | ||||||||
|---|---|---|---|---|---|---|---|---|
| Electrode | Catalyst | Potential | Technique | LOD | Linear Range | Sensitivity | Real Sample | Ref. |
| GCE | Zn(II) FNE57/TOAB | −1.20 V | CA | 0.77 µM | 5.0–295 µM | 3.35 µA mM−1 | - | [56] |
| GCE | Zn(II)P-C60/TOAB | −1.17 V | CA | 0.81 µM | 0.035–3.40 mM | - | - | [57] |
| GCE | Mn(III)TMPyP | −0.45 V | CA | 0.5 µM | - | 0.071 A M−1 | Human serum | [58] |
| GCE | Co(II)TAPP/SWCNTs | −0.4 V | CA | 1.0 µM | - | 194 µA mM−1 | peroxide solution | [58] |
| GCE | Co(II)Fp-Fe(II)P NH2-BTA | −0.20 V | DPV | 0.002 µM | 6.85 nM–7.0 µM | - | - | [59] |
| GCE | Hemoglobine/Carbon blac | −0.20 V | DPV | 4.0 µM | 4.9–390 µM | - | Oxygenated water | [60] |
| Sensors Based on Electro-Oxidation of Hydrogen Peroxide | ||||||||
| Electrode | Catalyst | Potential | Technique | LOD | Linear Range | Sensitivity | Real Sample | Ref. |
| GCE | Co(II)Pc-(CoTPP)4 | 0.45 V | CA | 8.0 µM | >1.50 mM | - | peroxide solution | [55] |
| Carbon cloth | Al-MOF/Co(II)TCCP | 0.40 V | CA | 0.7 µM | 1–1000 µM | 1760 µA mM− | - | [61] |
| GCE | Fe(II)-cytocrome C/Ni foam | 0.45 V | CA | 0.2 µM | - | 1.94 µA mM−1 | - | [54] |
6. Application of Modified Electrodes Containing MP and MPc for the Direct Quantification of Water Contaminants
The direct identification and quantification of organic and inorganic compounds in wastewater by using inexpensive instrumental techniques is an important topic in environmental sciences. Diverse contaminants such as halides, sulphites, nitrates, organohalides, pharmaceuticals, and phenolic compounds are mainly found in wastewater [62]. These compounds are introduced in municipal drain systems by chemical factories, pharmaceutical factories, hospitals, and households. Their presence in aquatic systems represents a severe environmental problem due to their high toxicity and accumulation in living systems [63]. The typical analysis of these compounds requires expensive instrumental methods such as HPLC, gas chromatography, mass spectrometry, and atomic absorption spectroscopy. As a result, new developments on electroanalytical sensors have been focused on the amperometric quantification of organic and inorganic species in aqueous solutions [62,63,64]. In this respect, MP and MPc based on transition metals have played an important role in the redox electrocatalyst of these kinds of compounds in aqueous media. Diverse examples of pristine MP or MPc and their functionalized derivatives have been used on GCE for the elaboration of attractive electrochemical sensors with applicability to environmental sciences. In this section, we summarized and discussed the recent developments in electrochemical sensors for detecting organic and inorganic contaminants in water.
6.1. Electrochemical Sensing of Pharmaceuticals
Few examples of electrochemical sensors for the selective detection of specific analgesics, antibiotics, and phosphodiesterase inhibitors are reported in the literature. The oxidation processes with electrochemical sensors based on complexes do not require high oxidation potentials, and the detection limits obtained with these sensors are very low, similar to those presented with enzymatic sensors [63].
During the design of electrochemical sensors based on MP, functionalized MPs were used as enhanced electrocatalysts with selective properties. For example, tetraruthenated porphyrin complexes have been used as modifiers on GCE for the selective recognition of isoniazid, folic acid, acetaminophen, and tyrosine, presenting low detection limits and good sensitivities. In these cases, the peripheral Ru(II) moieties on the complexes have a strong electron-withdrawing effect on the main metallic center in the porphyrin core, which is oxidizing the catalytic properties of the main metallic center. As mentioned in the other section, the electro-catalytic process is mainly carried out by the effect of the redox couple (M2+/M3+), which is employed for the oxidation or reduction of the pharmaceuticals, while ruthenium is not involved in the catalytical process. Regarding the change in the coordination environment during the electro-catalysis, Ferreira and coworkers [64] have reported changes in the structure of the supramolecular [Ni(II)TPyP(Ru(bipy)2Cl)]4+ complex used for folic acid electro-oxidation. In this case, there is a formation of a µ-peroxo bridge at high alkaline media in the complex structure, which promotes the redox mediation for the electrocatalytic response. On the electro-reduction of metronidazole with a glucosylated Mn(III) porphyrin complex, Gong [65] mentioned that the presence of glucopyranose groups in the complex works as a three-dimensional cage, which acts as a specific recognition site for metronidazole, and induces its coordination to the metallic center. In consequence, similar molecules such as albendazole are not able to be coordinated and recognized by the complex.
Despite the excellent results obtained with MP and MPc, the current research on novel electrocatalysts is focused on porous materials such as MOFs, COFs, and zeolites, which present enhanced electrocatalytic properties. As part of this work, we have summarized in Table 7 diverse examples of electrochemical sensors modified with MP and their electroanalytical parameters.
Table 7. Electrochemical based on MP for sensing pharmaceuticals.
| Electrode | Catalyst | Analyte | Potential | Technique | LOD | Linear Range | Sensitivity | Ref. |
|---|---|---|---|---|---|---|---|---|
| GCE | [Co(II)TRP(Ru(dcbpy)2)4]-Ni/GO | isoniazid | 0.10 V | CA | 3.5 µM | 100–1000 µM | 0.457 mA mM−1 | [66] |
| GCE | [Ni(II)TPyP(Ru(bipy)2Cl)]4+ | Folic acid | 0.50 V | CA | 0.73 µM | 1–200 µM | - | [64] |
| GCE | [Co(II)TPyP(Ru(bipy)2Cl)4]4+ | Tyrosine | 0.72 V | SWV | 0.5 µM | 1–24 µM | 0.47 µA mM−1 | [67] |
| GCE | [Co(II)TPyP(Ru(bipy)2Cl)4]4+ | Acetaminophen | 0.42 V | SWV | 0.1 µM | 1–50 µM | 0.73 µA mM−1 | [67] |
| CPE | Mn(III)T(o-glu)PPCl | Metronidazol | −0.50 V | CV | 0.058 µM | 0.049–2900 µM | - | [65] |
| GCE | Co(II)THPP/MWCNTs | rifampicin | 0.19 V | LSV | 0.008 µM | 0.01–5.0 µM | 217 µA mM−1 | [68] |
| GCE | Zn(II)TSPP | Sildenafil | 0.25 V | CA | 0.0066 µM | 0.1–1000 µM | 19.90 µA mM−1 | [69] |
| GCE | Zn(II)TPP | Sildenafil | 0.25 V | CA | 0.0029 µM | 0.1–10 µM | 312 µA mM−1 | [69] |
| GCE | Zn(II)TNP | Sildenafil | 0.25 V | CA | 0.0026 µM | 0.1–100 µM | 47.9 µA mM−1 | [69] |
6.2. Electrochemical Sensing of Phenolic Pollutants and Other Organic Molecules
The term ‘phenolic pollutants’ includes alkyl, nitro, and halophenols, which are considered important water and soil pollutants due to their slow degradation kinetics and carcinogenic effects [70]. The EPA (Environmental Protection Agency) established a permissible limit of 1 ppb for phenol in water, while toxicity levels for humans are about 9–25 mg/L [71]. As an alternative analytical tool, diverse electrochemical sensors based on coordination compounds have been developed for the identification and quantification of these pollutants in aqueous media. Cobalt (II) and iron (III) phthalocyanine or porphyrin composites are widely preferred as redox-active catalysts on this kind of electrochemical sensor due to their high efficiency in electro-oxidation reactions in acidic media, their high surface area, and the presence of high electron density on their 18 π-electron aromatic ring that make these molecules perfectly suitable for being robust electron mediators [7]. Excellent results have been obtained with these composites, presenting LOD from femtomolar to micromolar order. An interesting example of the use of these complexes is reported by Mazzota and coworkers [72]. In their work, they used a Co-porphyrin (Co(III)tetrakis(o-aminophenyl) porphyrin) as a redox mediator for the electro-mediator of 4-(2,4-dichlorophenoxy)butyric acid (2,4-DB). In this case, the complex was polymerized in the presence of 2,4-DB (used as a template) to create a molecular impressed polymer (MIP). The modified electrode was employed to recognize and quantify 2,4-DB by using chronoamperometry, presenting a LOD of 40 µM and a linear range of 200 µM–2 mM. The electro-reduction mechanism is not reported; nonetheless, it is probable that 2,4-DB is reduced to its corresponding aldehyde or alcohol, while Co(II)/Co(I) species are generated during the catalyst.
On the other hand, there is interest in the electrochemical quantification of hydrazine on wastewater and soils. This short molecule with formula N2H4 has wide applicability as a corrosion inhibitor or jet fuel. Despite its diverse industrial applications, hydrazine is highly toxic to humans and animals, causing carcinogenic effects; therefore, electrochemical sensors based on highly active materials with electro-oxidative properties are being explored in the direct detection of hydrazine. It is demonstrated that cobalt (II) porphyrin complexes have a strong affinity to the electro-catalysis of hydrazine in alkaline and neutral media [73,74]. The d7 electronic configuration and the presence of low-spin fields induct a coordination environment with no repulsive effects, which facilitates the electro-oxidation process of hydrazine.
Canales and coworkers [75] evaluated by CV diverse transition metal octaethylporphyrin modified GC electrodes (M = Co2+, Cu2+, Zn2+, Ru3+, Ni2+) in the presence of hydrazine. In their work (Figure 12), it was observed that only the Co(II)-OEP complex displayed an anodic peak assigned to the N2H4 oxidation, while the other complexes did not show any response.
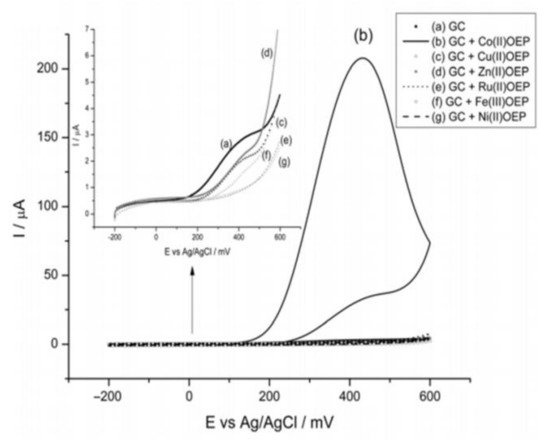
Figure 12. Voltamperometric response of diverse electrodes modified with transition metal OEP complexes in the presence of 5.00 mM hydrazine. pH 7.0, 0.1 Vs−1. Redrawn from Reference [75].
As proposed in the following equations, it is suggested that hydrazine is coordinated to Co(II); this process generates an intermediate cation, which is rapidly oxidized to regenerate Co-OEP, forming N2 as the final oxidation product.
[Co2+-OEP] + N2H4 ⇆ [Co2+-OEP- N2H4]
[Co2+-OEP- N2H4] ⇆ [Co2+-OEP- N2H4]+ + e−
[Co2+-OEP- N2H4] ⇆ [Co2+-OEP] + N2 + 3e− + 4H+
Composites based on Ni2+, Fe3+, or Pt2+ complexes have presented incredible results, with very low detection limits (in the nanomolar order) and excellent reproducibility. Overall, the mechanism suggested for these complexes is similar to that presented by the Co-OEP complex. The decrease in the LOD is an effect caused by the presence of reduced graphene or metallic nanoparticles in the composite, which enhances the electron conductivity and the electro-oxidative properties. As a part of this section, in Table 8, we have collected diverse examples of the recent application of MPc or MP in modified electrodes with applicability in environmental sciences.
Table 8. Examples of electrochemical sensors based on MP and MPc complexes for organic pollutants sensing.
| Electrode | Catalyst | Analyte | Potential | Technique | LOD | Sensitivity | Ref. |
|---|---|---|---|---|---|---|---|
| Gold electrode | (Fe(III)TMPP)/RGO | Bisphenol A | −0.9 V | EIS | 2.1 × 10−7 µM | - | [7] |
| GCE | (Co(III)tetrakis(o-aminophenyl) porphyrin) | 2,4-db | −0.8 V | CV | 40.0 µM | 5.89 µA µM−1 | [72] |
| GCE | Co(II)-OEP | hydrazine | 0.35 V | CV | 50.0 µM | - | [75] |
| GCE | [(CoTPRu4)n8+]/AuNPs | Chatecol | 0.51 V | CV | 1.4 µM | 108 µA µM −1 | [76] |
| GCE | (CoTBTCAPc) | 4-aminophenol | 0.02 V | CA | 0.03 µM | 0.888 µA µM −1 | [77] |
| CPE | 5,10,15,20-tetrakis(pentafluorophenyl)-21H,23H-porphyrin iron (III) chloride | 2,4-db | −0.1 V | CA | 2.1 µM | 1.8 µA µM −1 | [78] |
| GCE | Poly (Ni-TPPS4)/Ni-NPs | hydrazine | 0.45 V | CA | 0.11 µM | 1 mA mM−1 | [79] |
| GCE | Pt-TPP/RGO | hydrazine | −0.25 V | CA | 0.005 µM | - | [80] |
| GCE | Poly (CoOBImPc)/RGO | hydrazine | 0.6 V | CA | 0.033 µM | 56.86 µA µM −1 | [81] |
This entry is adapted from the peer-reviewed paper 10.3390/solids2020014
This entry is offline, you can click here to edit this entry!
