Zinc ions play an essential role in the physiology of brain function. Zinc acts as a potent neuromodulatory agent and signaling ions, regulating healthy brain development and the function of both neurons and glial cells. Therefore, the concentration of zinc within the brain and its cells is tightly controlled. Zinc transporters are key regulators of (extra-)cellular zinc levels, and deregulation of zinc homeostasis and zinc transporters has been associated with neurodegenerative and neuropsychiatric disorders. Here, more information is provided about the presence of specific zinc transporters and their subcellular localization within brain cells (neurons, astrocytes).
1. Introduction
Zinc is one of the most prevalent trace metals in the human brain, where it is found in its free (aqueous) ionic form within cells and inside neurotransmitter vesicles and its protein-bound form. Through its function, acting as a modulator of neurotransmission, signaling ion, and structural or catalytic part of proteins, zinc is vital for several processes, such as neurogenesis, neuronal migration, and differentiation, as well as neurotransmission and synaptic plasticity
[1]. Two different zinc-transporter families regulate cellular zinc homeostasis: the Irt-like protein (ZIP) family (SLC39A) and zinc transporter (ZnT) family (SLC30A). While SLC39As mediate the influx of zinc into the cytosol from the extracellular space and the lumen of intracellular compartments, SLC30A family members facilitate the removal of zinc from the cytosol, either out of the cell or into vesicles and organelles.
The particular function of several of the ten ZnT and fourteen ZIP family members have been described in the brain so far. For example, ZnT3 has been identified as a major transporter for loading synaptic vesicles with zinc
[2], and ZnT1 was shown tightly associated with NMDA receptors at postsynaptic densities
[3]. The expression of ZIP12 is reported in the brain of different species, such as humans, mice, and frogs, where ZIP12 has a role in neurodevelopment
[4].
However, little is known about the function of other zinc transporters in the brain, such as ZIP4, although the expression of
Zip4 has been reported on mRNA level in the choroid plexus, brain capillaries
[5], and gliomas
[6][7]. It has been shown that the expression of zinc transporters is altered in several brain diseases. For example, modified protein and mRNA levels of ZIP1, ZnT1, ZnT4, ZnT6, and ZnT10 have been reported in Alzheimer’s disease (AD)
[8][9], and elevated levels of ZnT6 in Pick’s disease
[10]. Moreover, increased cortical expression of ZIP12 has been found in schizophrenia
[11]. However, a complete expression profile and subcellular distribution of zinc transporters in neural cells have not been described in detail so far.
2. Expression of Zinc Transporters in Adult Rat Brain and Rat Neurons and Astrocytes on mRNA Level
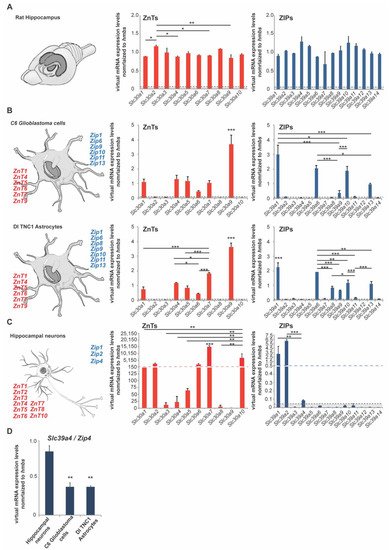
Expression analysis of ZnTs (Slc30a1–Slc30a10) and ZIPs (Slc39a1–Slc39a14) using rat hippocampus lysate from three animals (three female adult rats) reveals that the expression of all zinc transporters can be detected to some extent in the hippocampus. For the zinc transporters of the ZnT family, we noticed the highest expression on mRNA levels for Slc30a2 (ZnT2), Slc30a3 (ZnT3), Slc30a5 (ZnT5), Slc30a8 (ZnT8), and Slc30a10 (ZnT10). Regarding the zinc transporters of the ZIP family, we found the highest expression for the following transporters: Slc39a4 (Zip4), Slc39a5 (Zip5), Slc39a10 (Zip10), Slc39a11 (Zip11), and Slc39a12 (Zip12) (A).
To further investigate to what extent neurons and glial cells (astrocytes) are contributing to the overall expression, we used primary hippocampal neurons from rats as well as two rat astrocyte cell lines (C6 glioblastoma cells and DI TNC1 astrocytes) (B,C). The expression profiles were very consistent between both astrocyte cell lines. In both cell lines, we detected significant expression of Slc30a1 (ZnT1), Slc30a4 (ZnT4), Slc30a5 (ZnT5), Slc30a6 (ZnT6), Slc30a7 (ZnT7), Slc30a9 (ZnT9), and Slc39a1 (Zip1), Slc39a6 (Zip6), Slc39a9 (Zip9), Slc39a10 (Zip10), Slc39a11 (Zip11), Slc39a13 (Zip13) (B). The expression of Slc39a8 (Zip8) was detected in DI TNC1 astrocytes, but significantly less expression was detected in C6 glioblastoma cells, a finding that may have relevance for the pathomechanisms of glioblastomas (B). In astrocytes, the highest expression for zinc transporters was detected for Slc30a9 (ZnT9) and Slc39a1 (Zip1) (B). In neurons, we have detected the prominent expression of Slc30a1 (ZnT1), Slc30a2 (ZnT2), Slc30a3 (ZnT3), Slc30a4 (ZnT4), Slc30a5 (ZnT5), Slc30a6 (ZnT6), Slc30a7 (ZnT7), Slc30a8 (ZnT8), Slc30a10 (ZnT10), and Slc39a1 (Zip1), Slc39a2 (Zip2), and Slc39a4 (Zip4) (C).
Figure 1. mRNA expression of zinc transporters in adult rat brain and rat neurons and astrocytes. mRNA expression was analyzed by qRT-PCR. mRNA levels are shown as average virtual mRNA concentration normalized to hmbs from three biological replicates measured in technical triplicates. (A) Hippocampal brain mRNA lysate was prepared from rats. The expression of all zinc transporters was detected in brain lysate. (B) mRNA lysate was prepared from C6 glioblastoma cells and DI TNC1 astrocytes. Significant differential expression of zinc transporters was detected in both cell lines. The highest expression was detected for ZnT1, ZnT4, ZnT5, ZnT6, ZnT7, ZnT9, and Zip1, Zip6, Zip9, Zip10, Zip11, Zip13. Zip8 expression was significantly higher in C6 glioblastoma cells. (C) mRNA lysate was prepared from primary hippocampal neurons. The highest expression was detected for ZnT1, ZnT2, ZnT6, ZnT7, ZnT10, and Zip1, Zip2 Zip4. (D) Zip4 expression was significantly higher in neurons compared to both astrocyte cell lines. Only expression 5% above of hmbs expression was considered for the analysis (dotted line A–C).
Based on these findings, we detected zinc transporters expressed uniquely in astrocytes, neurons, and zinc transporters expressed in neurons and astrocytes ().
Table 1. List of zinc transporters that are expressed in a cell type-specific manner.
| Cell Type |
ZnT |
Zip |
| Neurons |
ZnT1, ZnT2, ZnT3, ZnT4, ZnT5, ZnT6, ZnT7, ZnT8, ZnT10 |
Zip1, Zip2, Zip4 |
| Astrocytes |
ZnT1, ZnT4, ZnT5, ZnT6, ZnT7, ZnT9 |
Zip1, Zip6, Zip8 1, Zip9, Zip10, Zip11, Zip13 |
3. Zip4 Is Expressed and Localized at Glutamatergic Synapses in the Brain
We used a qRT–PCR-based approach to quantify Zip4 mRNA expression in technical triplicates using RNA lysate from four different brain regions (cortex (ctx), hippocampus (hip), striatum (str), and cerebellum (cer)) from 3 animals. In all brain regions, we detected Zip4 mRNA. The lowest expression was observed in the cortex. Significantly higher levels compared to the cortex were found in the cerebellum and hippocampus. Along with detecting mRNA, the expression of ZIP4 protein was found in all analyzed brain regions. The highest levels were observed in the cerebellum. Given that mRNA and protein levels of ZIP4 were high in the cerebellum and that the detected expression may result from non-neural cells, such as cells of the blood capillary walls or blood cells in the tissue, we further assessed ZIP4 localization in the cerebellum. We detected the high and specific expression of ZIP4 in Purkinje neurons.
We investigated the subcellular localization of ZIP4 in neurons. To that end, we performed protein fractionation of brain lysates to obtain, from homogenate, the S2 fraction containing soluble proteins and the P2 fraction (synaptosomal fraction), a fraction enriched in synaptic proteins. The results show that ZIP4 is enriched in the synaptosomal fraction. Immunocytochemistry using primary neuronal cell cultures confirms the presence of ZIP4 in neurons. ZIP4 immunoreactive signals can be observed in the cell soma and also colocalizing with a synaptic marker protein. High-resolution images reveal a pattern, where the postsynaptic protein Homer1b/c is found closely colocalized with a ZIP4 signal, while ZIP4 is found in close juxtaposition to the presynaptic marker protein BASSOON. These results hint towards a postsynaptic localization of ZIP4. It is possible to co-immunoprecipitate ZIP4 with a major scaffold protein of the postsynaptic density (PSD) of excitatory synapses, SHANK3.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22094511