APOA4, which has been previously known as one of the apolipoproteins, was found to be a soluble PD marker and can be secreted into the blood as a result of HGF-c-Met signaling. Recently, it was reported that APOA4 is not just a constituent of high-density lipoprotein, but it also exerts anti-oxidant and anti-inflammatory effects to protect from liver damage in mice.
- rh-HGF
- APOA4
- ALF
- c-Met
- Fas
1. Introduction
Hepatocyte growth factor (HGF) was originally discovered in the serum of patients with fulminant hepatitis due to its strong capacity to induce hepatocyte proliferation [1,2,3]. Since its discovery, vigorous research has been conducted on HGF world-wide, which revealed its pleiotropic activities [4,5,6], among which its strong and rapid anti-apoptotic effects against murine liver injury led to the dynamic investigation into a fatal and disastrous condition, acute liver failure. Although, to date, liver transplantation is the only curative treatment for the disease, the constant need for a novel drug that can help avoid or at least delay the need of organ transplantation is still unmet [7,8,9]. With the support of the Japan Agency for Medical Research and Development (AMED), we successfully manufactured a recombinant human HGF (rh-HGF) that is currently under evaluation in a phase I study with healthy Japanese volunteers (ClinicalTrials.gov Identifier: NCT03922633). During the process of drug development, it is valuable to evaluate the pharmacodynamic activity of new investigational drugs in healthy volunteers prior to evaluating its clinical efficacy or benefits for diseased conditions. Therefore, we aimed to identify a pharmacodynamic marker for rh-HGF that directly and biologically reflects exogenously administered rh-HGF, in terms of HGF-mediated c-Met receptor activation. DNA microarray analyses of the livers of rh-HGF-administered normal mice showed an induction of various genes as candidate PD markers. Among those, APOA4, which has been previously known as one of the apolipoproteins, was found to be a soluble PD marker and can be secreted into the blood as a result of HGF-c-Met signaling. Recently, it was reported that APOA4 is not just a constituent of high-density lipoprotein, but it also exerts anti-oxidant and anti-inflammatory effects to protect from liver damage in mice [10,11]. Therefore, validation of the utility of APOA4 as a PD marker of rh-HGF in clinical trials would help pharmacodynamic evaluation of rh-HGF, and furthermore, may give clues to elucidate its mechanism of action in humans, not just by direct effect on hepatocytes but also via APOA4 protein.
2. Induction of Hepatic mRNA Expression after rh-HGF Exposure
| Expression Change (versus 0 h) | |||||||
|---|---|---|---|---|---|---|---|
| Gene Symbol | Signal Value (Average) | 8 h | 24 h | ||||
| 0 h | 8 h | 24 h | Ratio | p Value | Ratio | p Value | |
| Apoa4 | 2436 | 15,738 | 13,453 | 6.46 | 0.0002 | 5.52 | 0.0016 |
| Prss8 | 37 | 123 | 198 | 3.32 | 0.0955 | 5.35 | 0.0903 |
| Defa1 | 34 | 37 | 106 | 1.09 | 0.8609 | 3.11 | 0.2846 |
| Tff3 | 56 | 132 | 167 | 2.35 | 0.0623 | 2.97 | 0.1078 |
| Hmgb2 | 701 | 553 | 1679 | 0.79 | 0.0879 | 2.40 | 0.0009 |
| Dnase1 | 46 | 59 | 109 | 1.29 | 0.4958 | 2.39 | 0.2253 |
| Apoc3 | 408 | 1059 | 938 | 2.60 | 0.0002 | 2.30 | 0.0004 |
| Afp | 71 | 349 | 148 | 4.89 | 0.0197 | 2.07 | 0.0170 |
| Spink3 | 83 | 66 | 166 | 0.80 | 0.2707 | 2.01 | 0.2758 |
| Tnfrsf9 | 38,813 | 112,574 | 74,826 | 2.90 | 0.0043 | 1.93 | 0.0192 |
| Il15ra | 44,941 | 118,655 | 83,474 | 2.64 | 0.0092 | 1.86 | 0.0151 |
| Pcsk9 | 567 | 1458 | 1036 | 2.57 | 0.0187 | 1.83 | 0.0431 |
| Gast | 37 | 357 | 63 | 9.54 | 0.0005 | 1.69 | 0.1393 |
| Nrg4 | 75 | 1724 | 112 | 22.85 | 0.0001 | 1.48 | 0.0421 |
| Il6ra | 2912 | 14,047 | 3742 | 4.82 | 0.0005 | 1.29 | 0.1408 |
| Fhad1 | 124 | 1212 | 143 | 9.79 | 0.0003 | 1.16 | 0.0674 |
| Cgref1 | 113 | 1350 | 128 | 11.96 | 0.0013 | 1.13 | 0.6870 |
| Alpl | 411 | 1499 | 456 | 3.64 | 0.0097 | 1.11 | 0.5636 |
| Fam3c | 1807 | 5240 | 1962 | 2.90 | 0.0002 | 1.09 | 0.2547 |
| Serpina7 | 373 | 1636 | 283 | 4.38 | 0.0018 | 0.76 | 0.1721 |
| Creld2 | 11,476 | 34,924 | 8179 | 3.04 | 0.0433 | 0.71 | 0.3436 |
| Cxcl14 | 34 | 161 | 21 | 4.81 | 0.0073 | 0.62 | 0.3495 |
3. Induction of APOA4 in Human Hepatocytes
APOA4 is conserved well in humans. In order to validate whether human APOA4 respond to rh-HGF, primary cultured human hepatocytes were stimulated by rh-HGF. Notably, rh-HGF induced APOA4 mRNA (Figure 1A) and stimulated the production of APOA4 protein (Figure 1B,C) into the supernatants in a dose-dependent manner. A tyrosine receptor kinase, c-Met, is the sole receptor for HGF [6]. In order to evaluate whether APOA4 was indeed induced by c-Met mediated signaling elicited by rh-HGF [6], human hepatocytes were stimulated by rh-HGF in the presence of the c-Met inhibitor, PF-04217903 (100 nM). Inhibition of c-Met signaling significantly suppressed the induction of APOA4 mRNA (Figure 1A) as well as production of APOA4 protein into the supernatants (Figure 1B,C). Considering the inter-species consistency in response of APOA4 to rh-HGF, APOA4 is strongly assumed to represent the pharmacodynamic nature of rh-HGF.
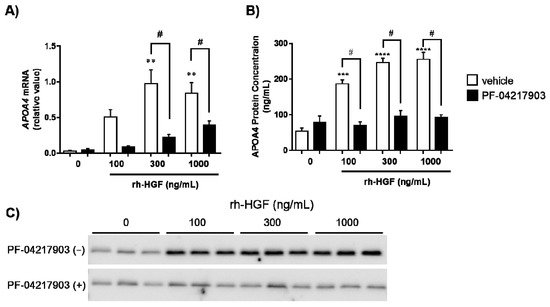
Figure 1. Induction of human APOA4 by rh-HGF, and its c-Met dependency. (A) Relative expression of APOA4 mRNA compared to the expression of 18S rRNA in human hepatocytes, (B) Relative concentration of APOA4 protein in supernatants ** p < 0.01, *** p < 0.001, **** p < 0.0001: comparison between vehicle control (incubation medium) or rh-HGF alone-treated group (Dunnett’s test). # p < 0.05: comparison between rh-HGF alone-treated group and PF-04217903-rh-HGF co-culture group (unpaired t test). The data are presented as mean ± SEM (n = 3). The median value of the triplicate analyses was used for calculation. (C) Analysis of APOA4 protein levels in the supernatants of the culture using Western blot. The picture shows a representative batch of hepatocytes in triplicate.
4. Discussion
There has been substantial progress in medical practice for the care of ALF patients by incorporating an etiology-based approach and artificial liver support. Among these, liver transplantation is the most reliable measure to rescue potentially fatal cases, especially comatose patients with ALF [8,14,15]. However, it is also true that transplantation is not always applicable for the patients who have an indication of liver transplantation, primarily due to the shortage of donors. Therefore, a new treatment option is needed to prevent progressive liver damage, preserve residual mass of functional liver, and promote the repair and regeneration of hepatocytes. HGF, due to its strong anti-apoptotic effect, stimulation of tissue repair, and regeneration activities, is the most promising hope to address this long-standing problem [4,5,16]. We successfully manufactured rh-HGF that pre-clinically showed substantial inhibition of liver damage with the strong preservation of prothrombin time in a Fas-triggering murine ALF model [13]. Prior to conducting a clinical trial with ALF patients, we tried to identify a PD marker of rh-HGF, which could allow us to evaluate the pharmacological outcome of rh-HGF besides the conventional clinical parameters for ALF care, such as AST, ALT, and PT. Moreover, the availability of such a PD marker for healthy subjects may help to determine appropriate dosing regimens of rh-HGF in patients with ALF.
We firstly chose live animals rather than an in vitro cell system to discover PD markers because it might mimic the clinical setting in humans well, considering the bio-distribution of rh-HGF in the body. rh-HGF is known to preferentially distribute to the liver and with much less amount to the kidney and lung [17]. Therefore, we first harvested the murine livers to identify the candidates for PD markers after rh-HGF administration, and, at the same time we pre-filtered candidates by limiting these to the molecules which were present extracellularly or on the plasma membrane. In addition, we selected molecules which showed sustained expression from the early (8 h) to the late (24 h) timepoint to heighten their likelihood of being detected in the serum independent of their serum half-lives. As a result, murine Apoa4 was found to be the highest (Table 1). Besides Apoa4, other genes such as Pcsk9, Tnfrsf9, and Il6ra that were identified using DNA microarray, were remarkably induced by rh-HGF, although the induction was only transient at 8 or 24 h in most cases, or the signal values, which may impact the protein levels, were relatively low. Since rh-HGF impacts cell growth and regeneration, the induction of cell-cycle- or growth-related genes such as Ccnd1, ccne1, and Myc was very obvious and they showed upregulation (data not shown). While these intracellular molecules may be less efficient as soluble PD markers, their upregulation confirmed the in vivo biological impacts of administered rh-HGF [2,18].
Thereafter, the induction of APOA4 was validated by using an in vitro human system utilizing primary cultured human hepatocytes (Figure 1). Consistent with the previous report that human APOA4 is expressed primarily in the small intestine [19], the baseline levels of APOA4 mRNA were quite low in human hepatocytes. However, we found that rh-HGF induced APOA4 mRNA in hepatocytes and stimulated the cells to produce APOA4 protein in a dose-dependent manner, mediated by c-Met (Figure 1). In this culture system, rh-HGF enhanced the APOA4 protein production by four- to five-fold compared to that of the control. Therefore, APOA4 is assumed to have high signal-to-noise ratio when human hepatocytes are treated with rh-HGF. If this observation is proven to be true in clinical settings, APOA4 will be a promising PD marker because of its assay sensitivity.
In order to confirm the adequacy of APOA4 as a PD marker of rh-HGF, the pharmacodynamic profile of APOA4 was evaluated in live animals; initially in normal mice, followed by mice with liver injury. In mice, APOA4 is known to be expressed in the small intestine and the liver [19,20,21]. This was also observed in our study, and the expression of murine Apoa4 was detected in both the organs at baseline with relatively lower values in the liver (Figure 2). However, rh-HGF strongly induced Apoa4 mRNA only in the liver at 8 h in a dose-dependent manner and the induction was sustained throughout the 24 h (Figure 2A) and such an induction of Apoa4 mRNA was not detected in the small intestine (Figure 2B). This discrepancy might be due to preferential bio-distribution of rh-HGF to the liver and not to the small intestine [17]. Even though we cannot deny the possibility that intestinal Apoa4 is a downstream molecule other than c-Met in the intestine, the observed finding at least proves that the liver is the primary organ impacted by administered rh-HGF in vivo. Thereafter, we focused on the induction of hepatic mRNA and serum APOA4 levels, particularly addressing the c-Met dependency. Notably, rh-HGF induced Apoa4 mRNA (Figure 3A) in the liver was associated with a dose-dependent increase in serum APOA4 protein concentration (Figure 3B,C). Importantly, the induction of APOA4 was inhibited by a c-Met inhibitor PF-04217903 both at mRNA and protein levels (Figure 3A–C), therefore, it strongly suggested that APOA4 directly represents the pharmacological effects of rh-HGF as the downstream molecule of c-Met signaling.
Finally, the adequacy of APOA4 as a PD marker was assessed in murine ALF model, which was developed by Fas-mediated cell death using an anti-Fas antibody [5]. Consistent with the observation in normal mice, rh-HGF drastically induced serum APOA4 protein in a dose-dependent manner in mice with ALF (Figure 4). We previously reported that the anti-apoptotic effects of rh-HGF on hepatocytes were significantly associated with the suppression of intrahepatic hemorrhage in which preserved PT was the best parameter that strongly correlated with the decrease in intrahepatic hemorrhages [13]. Considering the PT kinetics after administering rh-HGF [13], the inductive increase in murine APOA4 protein in serum by rh-HGF (Figure 4) reciprocally correlated with the suppression of PT prolongation [13], which might further exhibit the feasibility of APOA4 as a PD marker of rh-HGF.
Here, we primarily identified APOA4, one of the downstream molecules of HGF-c-Met signaling, to be a soluble PD marker of rh-HGF. Interestingly, it has been demonstrated that APOA4 is not just a constituent of high-density lipoprotein but it exerts anti-oxidant and anti-inflammatory effects to protect liver damage in mice [10,11]. Notably, serum APOA4 seemed to increase in murine ALF over normal mice without exogenous rh-HGF (Figure 4A; rh-HGF, 0 mg/kg). Endogenously induced HGF may be responsible for this, although there may be other molecular events regulating APOA4 synthesis. Taken together, it is essential to investigate the clinical utility of serum APOA4 in future clinical trials with healthy subjects and ALF patients at least to evaluate the pharmacodynamic effect of the rh-HGF, E3112. In addition, such human data may help further understanding of how rh-HGF exerts its therapeutic action in clinical settings, not just by direct effect on liver cells but also via APOA4 protein.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22094578
