S-nitrosylation is a selective and reversible post-translational modification of protein thiols by nitric oxide (NO), which is a bioactive signaling molecule, to exert a variety of effects. These effects include the modulation of protein conformation, activity, stability, and protein-protein interactions. S-nitrosylation plays a central role in propagating NO signals within a cell, tissue, and tissue microenvironment, as the nitrosyl moiety can rapidly be transferred from one protein to another upon contact. This modification has also been reported to confer either tumor-suppressing or tumor-promoting effects and is portrayed as a process involved in every stage of cancer progression. In particular, S-nitrosylation has recently been found as an essential regulator of the tumor microenvironment (TME), the environment around a tumor governing the disease pathogenesis.
- NO
- S-nitrosylation
- NOS
- microenvironment
- tumor-associated immune cells
- microbiome
- cancer therapeutics
- ECM
1. Introduction
S-nitrosylation, a protein modification mediated by nitric oxide (NO), exerts a myriad of biological and biochemical functions. In their pioneering study in 1992, Stamler et al. proposed that the formation of NO-adducts at protein thiols would be more stable than the gaseous NO by itself and serve as the primary mechanism for diverse NO bioactivities [1]. Since then, we have gained better understanding of the S-nitrosylation-mediated regulation of cellular signaling. However, this protein modification is less characterized at the molecular level than other modifications, such as phosphorylation. Additionally, the reported consequences of S-nitrosylation in cancer are often conflicting and are largely context-dependent. Thus, there remains a need to clearly define the role of S-nitrosylation of a particular protein in a distinct cell type and microenvironment.
Proteins with nearly all biological functions are subjected to S-nitrosylation. To date, more than 3000 proteins are found to be S-nitrosylated to modulate their conformation, function, stability, and protein-protein interaction [2][3]. Regulation of S-nitrosylation is also essential for maintaining normal or pathological cell signaling [4][5][6][7][8][9]. An imbalance in the regulation of S-nitrosylation could lead to the development of different diseases, including cancer, sepsis, and multi-organ dysfunction [10][11][12][13][14][15]. Especially, S-nitrosylation plays a critical role in the redox regulation of cells and tissues, and its dysregulation is closely linked to pathological conditions (see a comprehensive review by Fernando et al. [16]).
Cancer cells usually succumb to dysregulated levels of NO and S-nitrosylation (either hyper-S-nitrosylation or hypo-S-nitrosylation) owing to the altered expression of nitric oxide synthases (NOS) or denitrosylases as well as oncogenic mutations of target proteins. Such aberrant S-nitrosylation contributes to malignant phenotypes, such as genomic instability, cell proliferation, anti-apoptosis, angiogenesis, and metabolic reprogramming [13][17][18][19][20]. Several anti-cancer strategies are aimed at bringing elevated S-nitrosylation levels down to physiological levels to suppress the pro-tumor effects of S-nitrosylation with NOS inhibitors, NO scavengers, or denitrosylase mimetics [21][22][23][24][25][26][27]. In contrast, several other strategies are aimed to increase S-nitrosylation levels to inhibit cancer cell proliferation and promote cancer cell death and immuno-surveillance [21][28][29][30].
Furthermore, recent studies have attested to the role of S-nitrosylation as a major regulator of the tumor micro-environment (TME) [31][32][33]. TME is a complex collection of diverse populations of stromal cells, including immune cells (myeloid cells and lymphocytes) and fibroblasts as well as the extracellular matrix (ECM) [34]. Fibroblasts in the TME are activated to become cancer-associated fibroblasts (CAFs) under the paracrine signaling from tumor cells [35]. The function of tumor-associated immune cells is also modulated by tumor cells, resulting in an immuno-suppressive milieu to promote tumor progression [36]. The cellular and biochemical composition of the TME play major roles in fostering tumor cell proliferation and metastasis [37] and the refractoriness to cancer therapies. It has been increasingly evident that such tumor-promoting functions of the TME are critically regulated by NO and S-nitrosylation [32][33][38]. For example, in tumor-associated immune cells, the production of chemokines and cytokines as well as cell survival are greatly influenced by S-nitrosylation [27][38].
In this review, we will provide an overview of the role of S-nitrosylation in different cancer types, in different components of the TME, and their effects on cancer progression or suppression. We will also introduce some of the anti-cancer strategies based on modulating the levels of S-nitrosylation.
2. Nitric Oxide (NO) Signaling
NO is a highly reactive molecule with a half-life of 0.09~2 s [39]. NO is produced by nitric oxide synthase (NOS) using amino acid L-arginine as the substrate and a series of redox-active cofactors. In mammalian cells, there are three major NOS isoforms that encompass ~50% homology: neuronal NOS (nNOS/NOS1), inducible NOS (iNOS/NOS2), and endothelial NOS (eNOS/NOS3) [16][40]. nNOS and eNOS are expressed constitutively to produce steady-state NO levels, while their activities are regulated post-translationally, such as by phosphorylation, protein interaction, and cofactor/substrate availability [41][42]. Conversely, the expression of iNOS is regulated inducibly to produce a large amount of NO [41][42]. In addition, mitochondria are reported to possess mitochondrial NOS (mtNOS, nNOS homologue) in the matrix and inner membrane to regulate oxygen consumption and biogenesis of mitochondria [43][44][45][46][47].
The NOS monomer is composed of the carboxyl-terminal reductase and amino-terminal oxygenase domains. The functional NOS is a dimer of two identical monomers tethered by a zinc ion at two CysXXXXCys motifs, at which the substrate L-arginine and cofactor tetrahydrobiopterin (BH4) bind the enzyme [42][48]. In particular, BH4 binding allows “coupling” of the reductase and oxygenase domains for the canonical enzymatic function [40][42]. However, the reduced availability of BH4 or the substrate L-arginine could “uncouple” NOS, impairing the dimerization and NO production. BH4 deficiency could be caused by its excessive degradation under oxidative stress, which contributes to tissue fibrosis and stiffening in chronic disorders, such as obesity, cardiovascular disease, and cancer [48][49][50][51][52][53][54][55].
Most NO studies have focused on its roles in specialized cells, namely, neurons, muscles, endothelia, and immune cells. However, NO, in fact, exerts pleiotropic functions in many different types of cells, including the regulation of epithelial tissue morphogenesis, polarity formation, cellular growth, and movement [56][57][58][59][60][61][62]. NO signaling is classified into the classical and non-classical schemes. In the classical scheme, NO signaling is mediated through its binding and activation of soluble guanylyl cyclase (sGC) to convert guanosine-5′-triphosphate (GTP) to cyclic guanosine monophosphate (cGMP). cGMP, in turn, activates cGMP-dependent protein kinase (PKG) to lower the levels of potassium and calcium ions in the cytosol, leading to membrane hyperpolarization, neurotransmission, and vasodilation [41][42]. In contrast, the non-classical scheme of NO signaling includes covalent post-translational modifications of biomolecules by NO—S-nitrosylation and metal nitrosylation [63].
NO’s bioactivities are largely dependent on its concentration, timing, and context [64][65][66]. In healthy tissues, NO production is tightly regulated to attain the right condition [67]. Conversely, in a diseased state such as cancer, NO production is often dysregulated, leading to too much or too little NO levels that contribute to the disease pathogenesis [65][68][69][70][71][72]. Furthermore, NO’s activities in cancer are complex and contradictory [73]. NO could exert dichotomous effects on diverse cellular activities such as proliferation, apoptosis, angiogenesis, migration, and invasion, depending on its concentration and context [18][65][73][74][75]. For example, at lower concentrations (<300 nM), NO activates pro-tumoral signals (extracellular signal-regulated kinase [ERK] and hypoxia-inducible factor 1α [HIF1-α]), while, at higher concentrations (>300 nM), it activates anti-tumoral signals (p53) [75]. Furthermore, NO could be produced by either tumor cells or tumor-associated macrophages (M1 type), leading to either pro-tumoral or anti-tumoral effects, respectively [73][76]. Such complex NO signaling in cancer have led to conflicting reports and a notion that NO plays a double-edged role as both a cancer-promoter and cancer-inhibitor [67][68][77]. The paradoxical roles of NO in cancer would be partly resolved by clarifying a particular activity of NO in a specific stage and type of cancer under a set context.
3. What Is S-Nitrosylation?
The thiol group of cysteine residue is targeted for various types of covalent/post-translational modifications (PTM). These modifications play key roles in regulating protein function, subcellular localization, and protein-protein interactions [77][78]. One such modification is S-nitrosylation, which is a reversible PTM of a cysteine residue. This modification occurs via the covalent attachment of the nitrosyl (NO-) group to the thiol side chain of a cysteine, forming a S-nitrosothiol (SNO) (Figure 1) [79]. In mammalian cells, S-nitrosylation is a spontaneous reaction mediated by NO produced by NO synthases (NOS). The degrees of S-nitrosylation could range from mono-(single cysteine) to poly-S-nitrosylation (multiple cysteines), depending on the availability of NO, as well as the biochemical properties of the target proteins [3]. There are at least three determinants for the selectivity of S-nitrosylation. The first determinant is the proximity of specific cysteine residues to the NO-donor [80]. In fact, NOS and its interacting proteins are the first to become S-nitrosylated [81][82][83]. The second determinant is the presence of a signature motif (I/L-X-C-X2-D/E) that harbors a cysteine residue flanked by acidic and basic residues [15][84]. The third determinant is the presence of cysteine residues in a hydrophobic pocket that could efficiently bind NO and oxygen [10].
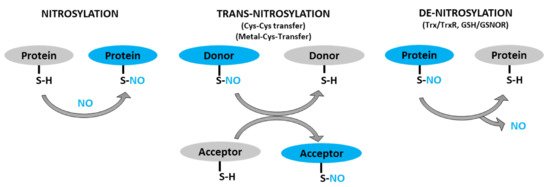
Figure 1. Schematic representation of protein S-nitrosylation, transnitrosylation, and denitrosylation. S-nitrosylation: covalent addition of NO group to the thiol (SH) group of a cysteine residue to form S-nitrosothiol (SNO). Trans-nitrosylation: transfer of NO moiety from donor to acceptor protein. Denitrosylation: removal of NO group from an already S-nitrosylated protein by denitrosylase (Trx/TrxR, GSNO/GSNOR).
S-nitrosothiol-signals could then be transmitted to proteins in distant locales through transnitrosylation [85]. Transnitrosylation is a reaction that involves successive transfer of the NO-group from an already nitrosylated protein (donor) to another protein (acceptor) when the two directly interact and have the appropriate redox potential and signature motifs (Figure 1) [15][86]. During transnitrosylation, the charged amino acids in the signature motifs facilitate electrostatic protein-protein interactions. Then, the donor protein (S-nitrosylase)—with higher redox potential—passes the NO-group to the acceptor protein, while getting denitrosylated. About ten S-nitrosylases have, so far, been identified, such as S-nitrosoglutathione (GSNO), hemoglobin, thioredoxin, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), caspase-3, and cyclin-dependent kinase 5 (CDK5) [86][87]. Each S-nitrosylase targets only a set of proteins involved in particular pathways, allowing for the selectivity of their regulations [85]. There could be hundreds of more S-nitrosylases to be identified, given the total number of S-nitrosylated proteins (>3000) in cells [2][3].
In contrast, denitrosylation is a process that reverts S-nitrosylation through enzymatic reactions (Figure 1). The balance between S-nitrosylation and denitrosylation determines the overall degree of S-nitrosylation. Two denitrosylases have, so far, been well characterized: thioredoxin/thioredoxin reductase (Trx/TrxR) and glutathione/S-nitrosoglutathione reductase (GSH/GSNOR) [88][89]. Nevertheless, recent studies have identified several potential denitrosylases, including glutathione peroxidase, protein disulfide isomerase and ceruloplasmin [87]. Similar to S-nitrosylases, there could be a number of denitrosylases which are yet to be identified.
4. S-Nitrosylation in Diseases
4.1. S-Nitrosylation in Cancer
Cumulative evidence attests to the critical roles of dysregulated NO and S-nitrosylation in the pathogenesis of different types of cancer. Aberrant S-nitrosylation levels are attributed to various factors, including the altered expression of NOSs and denitrosylases, oxidative stress, hypoxia, and oncogenic mutations of target proteins [13][17][18][19][90][91][92].
Numerous s-nitrosylated proteins that contribute to the pathogenesis of different types of cancers have been identified with the help of different bioinformatic analyses (Table 1).
Table 1. S-nitrosylated proteins linked to different diseases.
| Protein | Associated Disease | Status of S-Nitrosylation in Disease | Reference |
|---|---|---|---|
| Cancer | |||
| C-Src | Breast cancer | Increased | [93] |
| H-Ras | Increased | [94] | |
| COX2 | Increased | [95] | |
| HIF1α | Breast cancer | Decreased | [90][96] |
| Galectin-1 | Lung cancer | Increased | [97][98] |
| Ezrin | Lung cancer | Increased | [99] |
| BCL-2 | Increased | [100] | |
| Caveolin-1 | Increased | [101] | |
| Peroxiredoxin-2 | Decreased | [102] | |
| Rac1 | Pancreatic cancer | Increased | [25] |
| Rac2 | Increased | ||
| STAT1 | - | ||
| PGK1 | - | ||
| RB | - | ||
| PFKM | Ovarian cancer | Increased | [103] |
| Caspase-3 | Decreased | [104] | |
| STAT3 | Ovarian cancer Pancreatic cancer Head and neck cancer |
Increased | [25][105] |
| Androgen receptor | Prostate cancer | Increased | [28] |
| Integrin α6 | Increased | [106] | |
| ERK1/2 | Glioma | Decreased | [107] |
| Keap1 | Colon cancer | Increased | [108] |
| LTBP1 | Colorectal cancer | Increased | [109] |
| Neurodegenerative Disease | |||
| PTEN | Alzheimer’s disease | Increased | [110] |
| CDK5 | Increased | ||
| APOE | Increased | ||
| DNM1L | Increased | ||
| Tubulin | Increased | ||
| SOD2 | - | ||
| MMP9 | Cerebral ischemia | Increased | [111] |
| NMDA Receptor | Dementia | Increased | [112] |
| Cardiovascular Disease | |||
| NSF | Pulmonary arterial hypertension | Decreased | [113] |
| NOS3 | Decreased | ||
| CLTC | Decreased | ||
| Thioredoxin 1 (Trx) | Myocardial ischemia | Increased | [114] |
4.1.1. S-Nitrosylation Influenced by Altered Expression of NOSs and Denitrosylases as Well as Oxidative Stress
iNOS is preferentially upregulated in many types of cancers [115][116], leading to elevated NO production and S-nitrosylation. For example, in triple-negative breast cancer cells, increased iNOS level is linked to elevated S-nitrosylation of p21Ras, which promotes oncogenic signaling via mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase 1/2 (ERK)/ETS proto-oncogene 1 (ETS1) [117]. On the other hand, NOS functionality could, instead, be compromised in malignant cells due to the highly oxidizing environment that could degrade the essential cofactor BH4 to “uncouple” the NOS dimer [20][52][118][119][120][121]. In such a condition, NO production and S-nitrosylation would be downmodulated in cancer cells regardless of NOS levels. Alternatively, S-nitrosylation could be elevated in cancer cells because of downmodulation of denitrosylase. For example, the expression of a major denitrosylase GSNOR is downmodulated in ~50% of hepatocellular carcinomas (HCC). GSNOR deficiency was found to elevate S-nitrosylation and proteasomal degradation of the key DNA repair protein, O(6)-alkylguanine-DNA alkyltransferase (AGT), promoting oncogenic mutagenesis [122].
4.1.2. S-Nitrosylation Influenced by Hypoxia
Hypoxia is an inducer of diverse biological events, including angiogenesis and glycolysis, which are two hallmarks of cancer. Hypoxia elevates the expression of eNOS and iNOS in endothelial cells, leading to the increase in S-nitrosylation of hypoxia-inducible factor 1 alpha (HIF1-α) at Cys520 and Cys800. S-nitrosylation of Cys520 in the oxygen-dependent degradation (ODD) domain protects HIF1-α from ubiquitin-mediated degradation and stabilizes the protein. Furthermore, S-nitrosylation of Cys800 promotes HIF1-α interaction with transcription co-factors, such as p300 and CBP, to activate the transcription of a vast array of genes, such as those involved in angiogenesis (e.g., vascular endothelial growth factor [VEGF] and TEK tyrosine kinase [TIE2, angiopoetin-1 receptor]) as well as genes involved in glycolysis (e.g., glucose transporter 1 [GLUT1] and aldolase A) [95][104][105].
4.1.3. S-Nitrosylation Influenced by Oncogenic Mutations
Cancer cells and normal cells often exhibit drastically different S-nitrosylation profiles. Tan et al. performed site-specific proteomic analysis of S-nitrosylated cysteines and identified 397 unique sites in 290 unique proteins for pancreatic ductal adenocarcinoma (PDAC) vs. 25 unique sites in 25 unique proteins for adjacent normal tissues. There were only 66 shared sites in 63 shared proteins for both normal and cancerous tissues [25]. Such differential usage of S-nitrosylation sites and proteins in cancer vs. normal cells could be, in part, attributed to oncogenic mutations in malignant cells. These mutations could have destroyed the reactive cysteines, generated new reaction sites, or altered the local environment of the reaction sites to modulate the accessibility to NO and oxygen [10][15][87]. One such example is p21Ras GTPase. In normal cells, p21Ras is S-nitrosylated at Cys118 within the conserved NKCD motif (Asn116-Lys 117-Cys118-Asp119) involved in nucleotide binding. This S-nitrosylation facilitates guanine nucleotide exchange (from GDP to GTP) of the catalytic site for enzymatic turnover. On the other hand, in cancer cells, p21Ras is often subjected to Gly12Cys and Gly13Cys mutations. This generates the two additional S-nitrosylation sites, increasing the affinity to GTP to further promote guanine nucleotide exchange and exacerbate the pro-tumor activity [123].
4.1.4. Dichotomous Effects of S-Nitrosylation on Cancer
In addition to dysregulated S-nitrosylation levels in cancer, S-nitrosylation itself could elicit dichotomous effects on cancer development, further complicating matters. In some cases, S-nitrosylation inhibits tumor progression, whereas, in other cases, it promotes tumor growth [25][107][108]. Such bifurcated consequences of S-nitrosylation likely depend on the tumor types, target proteins, and specific TME.
For example, in non-small cell lung carcinoma (NSCLC) cells, S-nitrosylation of the antioxidant enzyme peroxiredoxin-2 (PRDX2) inhibits its enzymatic activity, resulting in H2O2 accumulation. This causes the activation of 5′ adenosine monophosphate-activated protein kinase (AMPK), which, in turn, phosphorylates Sirtuin 1 (SIRT1) to inhibit its deacetylase activity toward p53 and forkhead Box O1 (FOXO1) for their repression. This liberates the pro-apoptotic functions of the two tumor suppressor proteins, leading to cancer cell death [102]. If such pro-apoptotic signaling triggered by S-nitrosylation of PRDX2 takes place in neurons, however, this could lead to neurodegenerative disorders, such as Parkinson’s disease [124]. Moreover, even in the same NSCLC cell, if S-nitrosylation is targeted to ezrin, which is a linker between the plasma membrane and actin cytoskeleton, this could lead to tumor promotion. S-nitrosylation of ezrin promotes its interactions with actin and, thus, elevates cytoskeletal tension at the plasma membrane, increasing the invasiveness of cancer cells [99]. In addition, S-nitrosylation of an anti-apoptotic protein, B-cell lymphoma 2 (BCL-2), in lung cancer cells also exerts a pro-tumor function. S-nitrosylated BCL-2 binds Beclin-1, which is a critical autophagy regulator [125]. The BCL-2-Beclin-1 complex, in turn, inhibits the formation of autophagosomes and, ultimately, autophagy-mediated cell death, promoting lung cancer progression.
Likewise, in glioma cells, S-nitrosylation of ERK1/2 mitogen-activated protein kinases (MAPK) suppresses their phosphorylation and activation, promoting apoptosis [107]. In normal neurons, however, ERK1/2 plays essential roles in cell survival and should not be inhibited by S-nitrosylation [126]. Thus, S-nitrosylation instead takes place at the upstream protein p21Ras, which conversely activates ERK1/2 pathway for neurogenesis [127]. Moreover, even in glioma cells, if S-nitrosylation is targeted to caspase-3, this inhibits its pro-apoptotic activity and promotes tumor cell growth [128]. These examples clearly demonstrate that S-nitrosylation of different proteins in different cell types could result in contrasting effects on cell growth and survival.
Most of these studies attribute the source of the NO-group for S-nitrosylation to GSNO, which is the most abundant nitrosothiol in cells. However, in some cases, NO produced by NOS could rapidly S-nitrosylate proteins in close proximity. For example, in colorectal cancer cells, iNOS forms a complex with latent TGF-β binding protein (LTBP1) along with 6-pyruvoyl-tetrahydropterin synthase (PTPS), a critical enzyme for the biosynthesis of BH4—the essential cofactor of NOS. In response to hypoxia, AMPK phosphorylates PTPS, which induces PTPS to bind LTBP1, leading to the formation of the PTPS/iNOS/LTBP1 complex. Both BH4 and NO are produced within the same complex, which facilitates the efficient S-nitrosylation of LTBP1 to target the protein for proteasome-mediated degradation. Loss of LTBP1 then inhibits TGF-β secretion, allowing for continuous tumor cell growth under hypoxia [109]. It should be noted that TGF-β plays dichotomous roles in cancer depending on cancer type, stage, and context, which complicates the consequence of its inhibition. For example, TGF-β suppresses tumor growth in the early-stage colon, gastric, bladder, and ovarian cancer in a cell autonomous manner. In contrast, TGF-β promotes tumor growth in the advanced stage breast, esophageal, lung, and pancreatic cancer in a non-cell-autonomous manner (via interactions with the microenvironment) [129].
4.2. S-Nitrosylation in Other Diseases
In addition to its role in cancer pathogenesis, S-nitrosylation could also play key roles in the development of other types of diseases (Table 1). For example, as discussed above, increased levels of S-nitrosylated proteins are implicated in the progression of neurodegenerative diseases [116][119][120]. In the brain tissues of patients with Alzheimer’s disease, 45 differentially S-nitrosylated proteins have been identified [130]. In contrast, S-nitrosylation plays a beneficial role in cardio-protection. For example, upon the incidence of ischemia that lowers the oxygen supply to the cardiac muscle, a number of proteins become S-nitrosylated. This helps the heart become preconditioned for the low oxygen level and also for the upcoming reperfusion that elicits oxidative tissue injury. S-nitrosylation of these proteins not only lowers the cells’ need for oxygen and prevents their necrosis and apoptosis, but also protects the proteins from oxidation during reperfusion [131]. Moreover, in renal proximal tubules, inhibitory S-nitrosylation of enzymes involved in intermediary metabolism could protect the kidney against acute injury [132].
The above examples clearly depict dichotomous effects of S-nitrosylation on the pathogenesis of different diseases. The overall consequences of this protein modification depend on the context, cell/tissue type, target proteins, and the resulting molecular events. However, these intricate roles of S-nitrosylation remain the subject matter of further investigations. Such a complexity becomes amplified when S-nitrosylation takes place in the tissue/tumor microenvironment composed of a collection of cells and secreted proteins as we discuss in the following section.
5. S-Nitrosylation in the Tumor Micro-Environment
The tumor micro-environment (TME) is composed of a heterogeneous group of cells, including cancer cells, fibroblasts, vasculature, immune cells, adipocytes, and other types of cells, as well as the ECM and other secreted proteins (Figure 2) [133][134]. Crosstalk between different resident cells in the TME promotes tumor progression and contributes to the acquisition of therapeutic resistance. In particular, tumor cells could reprogram the functions of different components of TME for their own growth advantage [135][136].
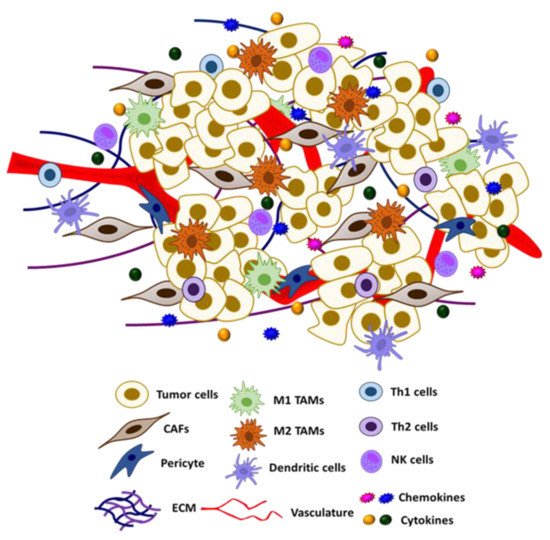
Figure 2. Schematic representation of tumor microenvironment and its constituents.
In the TME, a broad variety of cell types, including immune cells such as macrophages and natural killer (NK) cells, can produce NO and exert autocrine and paracrine effects. Macrophages represent a significant source of NO in the TME. Recent studies have revealed novel roles of S-nitrosylation/denitrosylation in modifying the phenotype of tumor-associated immune cells and other types of stromal cells, such as fibroblasts and endothelial cells. Dysregulated S-nitrosylation in these cells could reshape the TME from an immuno-active to an immuno-suppressive environment. In the following section, we will discuss the effects of S-nitrosylation on different resident cells of the TME.
5.1. S-Nitrosylation in Tumor-Associated Immune Cells
It has been established that NO plays crucial roles in both innate and adaptive immunity. Aside from its well-known roles in anti-microbial responses, NO acts as a key regulator of tumor-associated immune cells, which can switch their immunogenicity (i.e., immuno-active vs. immuno-suppressive) in response to dynamic signal interactions in the TME. Recent studies have unveiled that such NO’s influences on immune cell functions are partly mediated through protein S-nitrosylation [38][137]. Numerous immune cell-specific S-nitrosylated proteins have been identified [138][139] (Table 2). Despite the fact that the number of these proteins continues to grow, little is known about how the S-nitrosylated proteins impact immune cell functions. The following section discusses some known examples of NO-mediated immune cell regulation that prominently affect tumor immunity.
Table 2. Different S-nitrosylated proteins in the resident cells of the TME and their impact on cancer.
| Protein | Signaling Pathway | Impact on Protein | Physiological Impact of S–Nitrosylation during Cancer | S-Nitrosylation Site (* Potential Site) | Ref |
|---|---|---|---|---|---|
| Endothelial cells | |||||
| VE–Cadherin | Disassembled adherens junction between endothelial cells | Induced phosphorylation and internalization | Increased cell migration; hyperpermeability | – | [140] |
| p120 | Disassembled adherens junction between endothelial cells | Inhibited binding with β–Catenin | Increased cell migration | Cys579 | [141] |
| β–Catenin | Disassembled adherens junction between endothelial cells | Inhibited binding with p120 | Increased cell migration | Csy619 | [142] |
| HIF1–α | Activated HIF1 signaling pathway | Increased activation and stability | Increased angiogenesis and cancer metastasis | Cys533 | [143] |
| Dynamin | Promoted clathrin–dependent endocytosis of β–Adrenergic receptor | Increased self–assembly and GTPase activity | Increased angiogenesis | Cys86, Cys607 | [144][145] |
| MKP7 | Activated JNK3 signaling pathway | Inhibited phosphatase activity | Increased angiogenesis and migration | Cys244 * | [13][146] |
| Immune cells | |||||
| T cell receptor | – | – | Decreased T cell proliferation and migration; increased T cell apoptosis | – | [147] |
| CCL–2 | Reduced activity of CCR2/CCL2 signaling pathway | Decreased protein expression. | Decreased T cell infiltration | – | [148] |
| NF–kB | Inactivated NF–kB signaling pathway | Inhibited DNA binding activity | Decreased inflammation | Cys179 | [149] |
| STAT3 | Inactivated STAT3 signaling pathway | Inhibited activation | Decreased immune inflammatory response | Cys259 | [150] |
| Caspase–1 | Inhibited activation of NLRP3–Caspase–1 inflammasome | Inhibited activation | Decreased immune inflammatory response | Cys285 | [32] |
| Caspase–3 | Inhibited downstream activation of Caspase–3 signaling | Inhibited activation | Decreased cancer cell apoptosis | Cys163 | [151] |
| JNK1 | Inhibited activation of JNK signaling pathway | Inhibited activation | Decreased inflammation | – | [32] |
| NLRP3 | Inhibited activation of NLRP3–Caspase–1 inflammasome | Inhibited activity | Decreased immune inflammatory response | – | [152][153][154] |
| NOS2 | – | Suppressed activity | Decreased immune inflammatory response | – | [32] |
| ARG1 | – | Increased protein stability | Increased immunosuppressive response | Cys303 | [155] |
| Others (e.g., tumor cells) | |||||
| p21Ras | Promoted Guanine Nucleotide Exchange and activate downstream signaling pathways | Promoted protein activity | Increased Ras induced tumor growth | Cys118 | [94][156] |
| p21Ras (oncogenic) | – | – | Increased tumorigenic growth | Gly12Cy, Gly13Cys | [123] |
| COX2 | – | Stimulated protein activity | Increased inflammation | Cys526 | [95][157] |
| EGFR | Inhibited activation of EGF/EGFR signaling pathway | Impaired tyrosine kinase activity | Decreased tumorigenic growth | – | [158] |
| OGG1 | Reduced activity of BER (Base excision repair) pathway | Inhibited activity | Impaired DNA damage repair response | – | [159] |
| AGT1 | Suppressed activity of direct DNA repair pathway | Promoted protein degradation | Impaired DNA damage repair response | Cys145 | [160] |
| Apo2L/TRAIL receptor DR4 | Inhibited activation of death receptor signaling pathway | Inhibited activity | Decreased cancer cell apoptosis | Cys336 | [161] |
| Bcl–2 | – | Promoted protein stability | Decreased cancer cell apoptosis | Cys158, Cys229 | [100][162][163] |
| ERK | Suppressed activity of ERK/MAPK pathway | Suppressed kinase activity | Increased cancer cell apoptosis | Cys183 | [164] |
| HDAC2 | Induced protein release from chromatin. | Increased acetylation activity. | Increased histone acetylation | Cys262, Cys274 | [165][166] |
| PTEN | Activated downstream Akt signaling pathway | Inhibited enzymatic activity | Increased tumor progression | – | [167] |
| Src | Activated oncogenic signaling pathways (Akt, c–MYC) | Increased kinase activity | Increased tumor growth and proliferation | Cys498 | [93] |
| Androgen receptor | Suppression of androgen receptor signaling | Suppressed DNA binding activity | Increased tumor growth | Cys601 | [28] |
| Integrin α6 | – | Suppressed binding to ECM | Increased cell migration | Cys86 | [106] |
| Caveolin–1 | – | Prevented proteasomal degradation | Increased tumor progression | Cys156 | [33] |
| p53 | – | Induced activation | Increased transactivation of antioxidant genes | – | [168] |
| MDM2 | – | Inhibited activity | Decreased p53 binding and inhibition | Cys77 | [169] |
| Fas | Activated Fas/FasL signaling pathway | Increased sensitivity to Fas ligand | Increased cancer cell apoptosis | Cys304 | [170] |
| MKP1 | – | Increased phosphatase activity | Decreased radiation induced apoptosis | Cys258 | [145] |
| TRAP1 | Increased mitochondrial ROS production & permeability transition pore opening | Promoted proteasomal degradation | Increased cell death in GSNOR deficient cells (HCC) | Cys501 | [33][171][172] |
5.1.1. Tumor Associated Macrophages (TAMs)
Tumor associated macrophages (TAMs) are a heterogeneous group of macrophages that occupy more than 50% of the TME [173]. Immunogenicity of the TME is predominantly regulated by the ratio between two distinct subtypes of TAMs: M1 TAMs, which exert immuno-stimulatory responses, and M2 TAMs, which exert immuno-suppressive responses. Importantly, M1 TAMs produce high levels of NO compared to M2 TAMs [174][175]. M1 macrophages are activated by pro-inflammatory stimuli (e.g., IFN-γ, LPS) that upregulate iNOS expression to generate a burst of NO. Apart from its inherent cytotoxic effects, the anti-tumorigenic and immunogenic responses of NO produced by M1 macrophages are largely carried out by protein S-nitrosylation. Such S-nitrosylation-regulated immunological pathways include CC chemokine receptor 5 (CCR5) signaling (protein Kinase C delta [PRKCD], Receptor For Activated C Kinase 1 [RACK1] and G Protein Subunit Beta 1 [GNB1]); interleukin 12 (IL-12) production and signaling (lysozyme [LYZ], CCAAT enhancer binding protein beta [CEBP-β]); and phagocytosis (Rac Family Small GTPase 1 [RAC1], RAC2 and beta-actin [ACTB]) [138]. Despite large amounts of NO production, activated M1 macrophages are able to protect themselves against toxic levels of NO and reactive nitrogen species (RNS) by compartmentalizing nitrosative stress in the phagosomes with the help of denitrosylases, such as GSH/GSNOR and Trx/TrxR [176].
5.1.2. T Cells
T cells are a major component of the adaptive immune system induced to attack target cells carrying specific antigens presented by antigen-presenting cells (APC). Tumor infiltrating T cells fall into different subtypes and exert distinct immune responses. T helper 1 (Th1) and cytotoxic (CD8+) T cells induce immunogenic responses, while Th2, Th17, and Treg cells induce immuno-suppressive mechanisms. Upon engagement of APC, the microtubule-organizing center (MTOC) and the associated Golgi apparatus in T helper cells translocate to the APC contact site. This induces the recruitment of the Golgi-localized eNOS to the immune synapse, a specialized intercellular site where the T cell receptor (TCR) accumulates. This activates NO production by eNOS and potentiates TCR signaling in response to the antigen binding [177]. In T cell-mediated immunity, NO signals are propagated via S-nitrosylation/denitrosylation that regulates the differentiation and activation of T cells [178]. For instance, effector T cells, including Th1 and CD8+ T cells are activated by NO produced from M1 TAMs. There has been increasing evidence that suggests the role of denitrosylation in T-cell activation [179]. Nitrosothiols could be denitrosylated through the conversion of GSH to GSNO, which is further reduced by GSNOR. Denitrosylation is particularly important for T cell function because the development of T cells is impaired in GSNOR-deficient mice [180]. A recent study has also shown that GSNOR-dependent denitrosylation of protein kinase B (AKT) is involved in the T cell activation during hyper-homocysteinemia (HHcy)-induced atherosclerosis [181].
In the TME, high levels of NO induce S-nitrosylation of the chemokine CCL2, which prevents the recruitment of CD8+ T cells to tumor tissues and renders the TME immuno-suppressive [148]. In addition, upregulation of iNOS along with arginase 1 (ARG1) in myeloid-derived suppressor cells (MDSCs) leads to arginine depletion in the TME, inducing S-nitrosylation of the T cell receptor (TCR) and T-cell apoptosis [147].
5.1.3. Natural Killer (NK) Cells
NK cells are granular lymphocytes of the innate immune system that induce cytotoxicity against virally infected or tumorigenic cells. Activation of NK cells is a complex process, which requires stimulation by various cytokines (i.e., IL-2, IL-12, and IL-15) and a shift of the signaling cascade from inhibitory receptors (human leukocyte antigen [HLA]-A, -B, CD48) to activating receptors (NKp46, NKG2D, and NKp30) [182]. Upon activation, NK cells produce high levels of NO that facilitates their cytolytic function [183][184]. Furthermore, activated NK cells regulate the activation and maturation of T cells, dendritic cells, and macrophages, which subsequently leads to an improved anti-tumor response [185]. Apart from being an effector molecule, NO also regulates the activation and survival of NK cells. Nevertheless, the exact impact of NO on NK cells and the underlying mechanisms remain to be determined [183]. The impact of S-nitrosylation on some of the crucial immune regulatory proteins is discussed below.
5.1.4. Nuclear Factor Kappa B (NF-κB)
NF-κB is an evolutionarily conserved family of transcription factors that form a heterodimer to regulate inflammatory and immune responses, cell proliferation, and apoptosis. Therefore, the NF-κB signaling pathway is considered to be a crucial target for cancer therapeutics [14]. Different cell surface receptors such as the tumor necrosis factor (TNF) receptor, Toll-like receptor (TLR), and T cell/B cell receptors activate NF-κB signaling in response to diverse external stimuli. In the cytoplasm, a NF-κB heterodimer is in a complex with the inhibitor of NF-κB (IκB) and remains inactive. In response to an activation signal, IκB becomes phosphorylated and is targeted for proteasomal degradation, liberating NF-κB which, then, translocates to the nucleus [186][187]. In the nucleus, NF-κB transactivates a multitude of target genes including cytokines (interferon gamma [IFNγ], IL1α and IL12); immunoreceptors (CD48, major histocompatibility complex I [MHC I] and MHCII); apoptotic regulators (Fas, FasL and BCL-xL); and growth factors (granulocyte-colony-stimulating factor [G-CSF] and macrophage-CSF [M-CSF]) that are essential for regulating immune responses and tumor growth [188].
S-nitrosylation has been shown to regulate NF-κB activity by targeting multiple components [149]. In macrophages and T cells, S-nitrosylation of IκB kinase (IKKβ) at Cys179 residue represses its activity to phosphorylate IκB for proteasomal degradation (Figure 3A) [14][162][163]. As a result, the NF-κB-IκB complex remains inactive in the cytoplasm. Furthermore, p50 and p65 subunits of the NF-κB heterodimer are also inactivated via S-nitrosylation at Cys62 and Cys38, respectively (Figure 3A) [149][189]. Inactivation of these subunits inhibits their DNA binding for gene transcription. In contrast, S-nitrosylation has also been reported to activate the downstream molecules of NF-κB signaling, such as p21Ras, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and thioredoxin [149]. Such conflicting results could be attributed to dichotomous effects of NO and S-nitrosylation in a cell type-dependent and concentration-dependent manner.
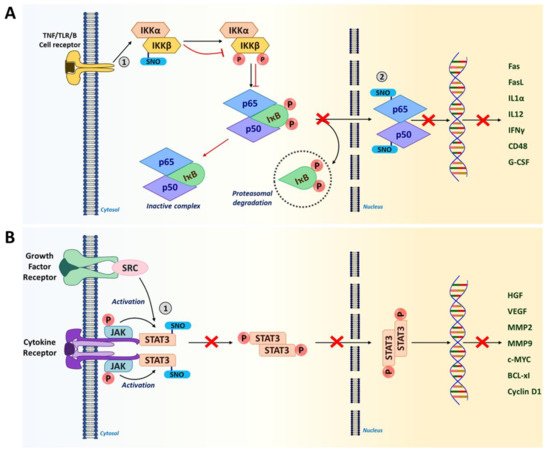
Figure 3. S-nitrosylation in NF-κB and STAT3 signaling pathways. (A) IKKβ and NF-κB subunits (p65 and p50) are S-nitrosylated in the NF-κB signaling pathway. S-nitrosylation of IKKβ at Cys179 prevents the phosphorylation of IκB and subsequent proteasomal degradation. This results in the inactive NF-κB-IκB complex sequestered in the cytosol. Furthermore, S-nitrosylation of NF-κB subunits p65 (Cys38) and p50 (Cys62) inhibits their DNA binding, in turn, preventing the transcription of NF-κB target genes including a number of pro-inflammatory cytokines. (Red arrows show the impact of S-nitrosylation.) (B) In tumor cells, when STAT3 is phosphorylated, it leads to the expression of genes related to proliferation and angiogenesis that promote tumor progression. However, S-nitrosylation of STAT3 at Cys259 leads to its inactivation by preventing phosphorylation. This could lead to improved immunogenicity in the TME. (1 and 2 represent the site of S-nitrosylation in the pathway).
5.1.5. Signal Transducer and Activator of Transcription 3 (STAT3)
STAT3 is a transcription factor which acts at the intersection of many signaling pathways induced by different cytokines (e.g., IL-6, IL-11, IFNα) and growth factors (human epidermal growth factor receptor 2 [HER2], epidermal growth factor [EGFR] and VEGF) [190][191]. STAT3 is constitutively active in many types of cancer with high frequency [192]. While being highly activated in both tumor-associated immune cells and tumor cells, STAT3 plays crucial roles in downregulating anti-tumor responses to promote tumorigenesis [193][194]. In immune cells, STAT3 elevates the expression of anti-inflammatory factors (IL-10, transforming growth factor beta [TGFβ] and cyclooxygenase 2 [COX2]) and inhibits the expression of pro-inflammatory factors (tumor necrosis factor alpha [TNFα] and IFNγ) (32). Thus, STAT3 activation leads to the downmodulation of dendritic cell maturation, M1 TAM polarization, and Th1-type immune responses, while promoting the expansion of M2 TAMs, Th2 cells, Tregs and MDSCs [194][195]. In addition, STAT3 activation in tumor cells elevates the expression of genes linked to proliferation (cellular myelocytomatosis oncogene [c-MYC], Cyclin D1, BCL-xl) and angiogenesis (VEGF, hepatocyte growth factor [HGF]); and metastasis (matrix metalloproteinase-2 [MMP2], MMP9, and twist-related protein 1 [TWIST1]), promoting malignant progression [190][191]. Therefore, STAT3 is a newly emerging target of immunotherapies for many types of cancer [192][196]. Recent studies unveiled that STAT3 activity could be inhibited by S-nitrosylation at Cys259 in tumor-associated immune cells (Figure 3B) [32][172][182]. This finding suggests that S-nitrosylation-mediated suppression of STAT3 could improve the immunogenicity of the TME.
5.1.6. Caspases
Caspases are a family of proteases consisting of 11 members that cleave target proteins at the C-terminus of aspartate residues. These proteolytic enzymes are categorized into three functional groups, including inflammatory caspases (Caspase-1, Caspase-4 and Caspase-5); initiator caspases (Caspase-8, Caspase-9 and Caspase-10); and executioner caspases (Caspase-3, Caspase-6 and Caspase-7) [197]. Apart from their well-known function in regulating programmed cell death and inflammation, caspases are also known to regulate cell proliferation, differentiation and aging [198][199]. More importantly, they are implicated in many hallmarks of cancer, such as evasion of cell death, sustained proliferation, immune evasion and tumor-promoting inflammation, making caspases an ideal target for anti-cancer therapies [200]. NO was found to regulate the activity of many of these caspases primarily through S-nitrosylation/denitrosylation of the cysteine groups in their active sites [14]. The impact of S-nitrosylation on two important caspases that regulate the TME are discussed below.
Caspase-1 is an inflammatory caspase and is predominantly involved in the differentiation, activation and polarization of phagocytic cells, such as macrophages [198][201]. This enzyme is activated within inflammasomes, which are the cytosolic complexes of multi-protein immune receptors (i.e., pattern recognition receptors). Caspase-1 mediates immune responses by promoting the maturation and secretion of pro-inflammatory cytokines, such as IL-1β and IL-18, and by regulating NF-κB signaling [32][198]. S-nitrosylation of caspase-1 at the catalytic site cysteine (Cys285) inhibits its activation, lowering the production of IL-1β and IL-18 by inflammasomes (Figure 4A). In particular, S-nitrosylation of caspase-1 inhibits the functions of NLR family pyrin domain containing 3 [NLRP3] inflammasome, which is an activation platform of caspase-1 [14][154]. It was shown that inhibiting NLRP3 inflammasome through caspase-1 S-nitrosylation was able to suppress angiogenesis, invasion and metastasis of melanoma and breast cancer cells [186][189][190].
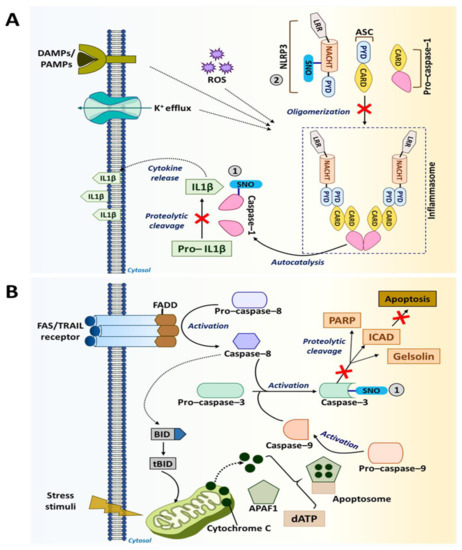
Figure 4. S-nitrosylation in caspase pathways. (A) Caspase-1 promotes the maturation and secretion of pro-inflammatory cytokines, such as IL-1β mediating immune response. However, S-nitrosylation of caspase-1 at Cys285 inhibits the maturation and secretion of IL-1β. NLRP3 inflammasome helps in the activation of caspase-1. Additionally, NLRP3 also undergoes S-nitrosylation and becomes inactive. (B) Cell death receptors, such as Fas/TNF-related apoptosis-inducing ligand [TRAIL], activate caspase-3-mediated apoptotic signaling in the presence of caspase-8 and caspase-9. However, S-nitrosylation of caspase-3 at the catalytic site cysteine (Cys163) causes the inhibition of its apoptotic activity.
Caspase-3 is an executioner caspase with a well-known function in regulating apoptosis. Located within the cytoplasm and the mitochondrial intermembrane space in mammalian cells, caspase-3 becomes activated by extrinsic death signals transduced via death receptors, such as TRAIL and Fas [14][151]. Activation of this executioner caspase requires cleavage by initiator caspases, such as caspase-8 and caspase-9. Upon activation, caspase-3 undergoes a conformational change. The active enzyme then targets key structural and regulatory proteins associated with cell survival (e.g., poly [adenosine diphosphate (ADP-ribose)] polymerase [PARP], EGFR and gelsolin), leading cells to apoptosis [202][203]. However, S-nitrosylation of caspase-3 at the catalytic site cysteine (Cys163) inhibits its activity (Figure 4B) [14]. Such anti-apoptotic effects of caspase-3 S-nitrosylation are utilized by tumor-associated immune cells to improve their anti-tumor responses. On the other hand, suppression of apoptosis in tumor cells is a key driving force for tumorigenesis [204]. Nevertheless, recent studies revealed that activation of caspase-3-mediated apoptosis in cancer cells could also induce the “Phoenix Rising” pathway that produces biochemical signals to regenerate tumor tissues. As such, elevated caspase-3 levels in breast cancer are associated with a worse clinical outcome [205][206]. Therefore, caspase-3 inhibition in certain conditions could have anti-tumor effects rather than pro-tumor effects.
5.2. S-Nitrosylation in Endothelial Cells
The first known biological role of NO was the regulation of endothelial cell functions, such as vascular tone, migration and permeability [207]. In endothelial cells, NO is produced by eNOS, which mediates S-nitrosylation of multiple proteins regulating protein trafficking, cell migration, redox state and cell cycle. Decreased levels of S-nitrosylated proteins in endothelial cells have been linked to a variety of disease conditions, including congestive heart failure and hypertension. Conversely, increased levels of S-nitrosylated proteins in endothelial cells could promote tumor pathogenesis by triggering angiogenesis and tumor cell attachment to endothelium, allowing for tumor growth and metastasis [208][209]. Below are some examples of endothelial proteins that act to promote tumor progression upon S-nitrosylation.
Vascular endothelium-cadherin (VE-cadherin), expressed specifically in endothelial cells, is located at adherens junctions between endothelial cells. It associates with several cytoplasmic proteins, including α-catenin, β-catenin, γ-catenin and δ-catenin (p120), to maintain the endothelial barrier. In glioblastoma, however, IL-8 secreted by tumor cells triggers eNOS-mediated NO production and then S-nitrosylation of VE-cadherin and p120, which impairs their associations and induces hyperpermeability of blood vessels [140].
VEGF plays a major role in inducing vascular permeability with the help of eNOS. VEGF promotes eNOS-dependent S-nitrosylation of β-catenin at Cys619, leading to its dissociation from VE cadherin. This, in turn, triggers the disassembly of the adherens junction complex, elevating the vascular permeability [142]. In fact, eNOS-deficient mice were found to be defective in vascular permeabilization even in the presence of VEGF, suggesting the critical role of eNOS in executing the VEGF signal [142].
HIF-1α is a subunit of a heterodimeric transcription factor HIF1, which induces angiogenesis in response to hypoxia to maintain tissue metabolism [210][211][212]. Hyperactivation of HIF-1α plays an important role in cancer metastasis [90][213][214][215][216]. For example, S-nitrosylation of HIF-1α at Cys533 elevates the activity, resulting in the increased expression of various angiogenic factors that stimulate angiogenesis and promote cancer metastasis [143].
Given the adversary effects of excessive S-nitrosylation of endothelial proteins, eNOS inhibitors have been tested for their efficacies of minimizing tumor metastasis. A study by Gao and colleagues showed that knocking-down eNOS expression or treatment with eNOS inhibitors, 1400 W and L-NIO, suppressed angiogenesis and compromised colorectal cancer progression [217]. The same group also showed that Celastrol, a phytochemical which inhibits NOS activity, impaired angiogenesis in colorectal cancer [218].
5.3. S-Nitrosylation in the Extracellular Matrix (ECM)
ECM is the acellular component of the TME, which provides not only the structural support, but also a variety of signals to regulate tissue homeostasis [219]. The ECM constituents, also known as the “core matrisome”, are about 300 proteins including fibrous proteins, growth factors, ECM-modifying enzymes and other ECM-associated proteins [220].The ECM components dynamically remodel in response to varying environmental cues. The ECM then regulates a variety of cellular functions, including cell survival, differentiation, maintenance of tissue architecture and migration [220].
Normal epithelial cells require attachments to the ECM for their growth, differentiation and survival. If epithelial cells detach from the ECM, they undergo programmed cell death through a phenomenon termed anoikis [221]. During cancer progression, however, malignant cells acquire the ability to survive and metastasize without the need of attachment to the ECM—anoikis-resistance [221][222][223]. Such anoikis-resistance of cancer cells is partly attributed to S-nitrosylation of various proteins and is, otherwise, associated with the ECM, as discussed below [131][217][218].
Integrin αβ-heterodimers are essential cell surface receptors that not only mediate cell adhesion to the ECM, but also are involved in transduction of various biochemical and mechanical signals [224][225][226]. During the formation of cell-ECM adhesions, integrin-ECM interactions trigger their clustering and induction of Src kinase to phosphorylate focal adhesion kinase 1 (FAK1). This causes the assembly of focal adhesion complexes and their linkages to cytoskeletal networks [227]. These adhesion complexes, in fact, play essential roles in the induction of anoikis. However, in prostate cancer cells with iNOS overexpression, the α6-integrin subunit becomes S-nitrosylated at Cys86, which causes a shift of its dimerization partner from the canonical β4-integrin to β1-integrin. This lowers the number of integrin α6β4 heterodimers (laminin receptors) on the cell surface and the cells’ ability to bind the ECM, inducing cell migration and anoikis-resistance [106]. In addition to integrins, Src kinase could also be S-nitrosylated at Cys498, which induces autophosphorylation of the protein and promotes cells’ invasiveness and anoikis-resistance [93][228]. This mode of anoikis-resistance plays a role in estrogen-driven tumor progression.
Caveolin-1 (CAV-1) is a major structural component of caveolae, a subset of lipid rafts in the plasma membrane involved in endocytosis, ECM organization, mechano-sensing, and biochemical signaling. CAV-1 physically associates with eNOS for mutual suppression. CAV-1 binding keeps eNOS in the inactive state [229][230]. Once eNOS becomes activated in response to a stress, such as a mechanical stress, NO S-nitrosylates CAV-1 at Cys156 to target the protein for proteasomal degradation [231][232]. CAV-1 is elevated in several metastatic cancers. However, cancer-associated caveolae are often dysfunctional [233][234]. It is yet to be determined whether this is, in part, attributed to aberrant S-nitrosylation of CAV-1.
Transglutaminase 2 (TG2) is a multi-functional enzyme present in the cytosol and ECM. TG2 is highly expressed in stromal cells, such as endothelial cells, fibroblasts and monocytes/macrophages. Cytosolic TG2 primarily acts as a GTPase, while extracellular TG2 catalyzes deamidation and cross-linking of ECM proteins to regulate the tensile properties of tissues [235]. TG2 becomes S-nitrosylated to suppress the activity when the NO levels are elevated [235]. However, in aged vasculatures where the NO levels are low, TG2 S-nitrosylation is compromised, leading to excessive crosslinking of matrix proteins and tissue stiffening [236]. Importantly, TG2 is highly elevated in various types of cancer and plays major roles in establishing stiff ECM that exacerbates tumor progression. Thus, TG2 is an emerging therapeutic and diagnostic target for cancer and could be inhibited by S-nitrosylation [237].
5.4. S-Nitrosylation in Tumor Microbiome
The TME, as described earlier, consists of innate and adaptive immune cells, which is a network of blood and lymphatic vessels and other types of stromal cells. Furthermore, recent findings unveiled that the microbiome serves as an additional core component of the TME and impacts tumor progression [238][239][240][241]. A comprehensive analysis of the tumor microbiome by Nejmen et al. found that metabolically active bacteria live intracellularly in both cancer and immune cells and could affect the TME. They reported that the microbial composition varied according to tumor type and tissue origin (lung, breast, ovary, pancreatic, melanoma, brain and bone). In addition, there were close similarities of metabolic profiles between bacteria and host tumor cells, suggesting that these bacteria play critical roles in the tumor phenotype [238]. Microbiome compositions could also affect the TME by modulating the host immune response. Riquelme et al. transplanted human fecal microbes from PDAC patients into mice by oral gavage, and later xenografted these mice with cancer cells. They found that PDAC-derived microbes modulated the host immune system and exacerbated tumor development [239].
Better understanding of microbial biology and its influence on the TME would significantly impact the development of new cancer treatments. For example, a recent study reported that modified bacterial strains engineered to migrate to tumor hypoxic sites could be utilized to deliver anti-cancer agents to necrotic sites of tumors to overcome the difficulty in drug delivery owing to aberrant tumor vasculature [242]. Recently, Seth et al., using C. elegans as in vivo models, unraveled that NO-producing bacteria (i.e., Bacillus. Subtilis) in the gut microbiome largely influence the host physiology by S-nitrosylating host proteins [243]. This is the first-time demonstration that NO, by means of S-nitrosylation, could serve as a common language for interspecies communications between gut bacteria and host cells. A total of 924 host proteins were found to be S-nitrosylated by bacterial NO. About 200 of them were involved in metabolism while other proteins were involved in the maintenance of immunity, suggesting the role of bacterial NO in modulating the host metabolism and immunity [243]. It is, however, yet to be examined whether such NO-mediated interspecies communications take place in mammalian guts, where the host cells are separated from the gut microbes by the mucosal barrier.
Furthermore, recent studies have also unveiled the essential functions of the microbiota of various tissues/organs other than the gut. For example, the microbiota of the breast is composed of seven phyla, including Firmicutes (e.g., Bacillus spp.) and Actinobacteria (e.g., Adlercreutzia spp.), which produce NO and NOS cofactor BH4, respectively [244]. These bacteria are also enriched in breast milk, indicating their roles not only in breast functions, but also in neonatal development [245]. Furthermore, breast milk is the major source of maternally transferred microbes, accounting for about 40% of the gut bacteria of newborns in the first month of life [246]. However, it is yet to be determined whether these microbial contributions are attributed to their NO production. As expected, the microbiota of the breast is dramatically altered in both benign and malignant tissues compared to the normal breast [247]. As such, the discovery of microbial NO that could S-nitrosylate host proteins has added another factor of complexity in the regulation of the TME. Too much or too little NO production by the microbiome may dysregulate host protein functions and contribute to cancer pathogenesis (Figure 5). Such information could be utilized to develop novel strategies for cancer treatment.
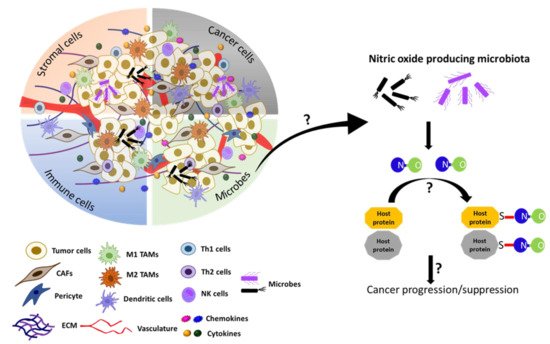
Figure 5. Model speculating the role of microbial NO in tumor progression or suppression in the tumor microenvironment. Recently, the gut microbiota in C. elegans. has been shown to produce NO and regulate the S-nitrosylation of host proteins and affect the host development [243]. The TME also contains microbes in addition to cancer cells, stromal cells, immune cells, and acellular components [238][239][240][241]. We speculate here that, if the microbiome is present in the TME, it could produce NO and regulate the tumor progression or suppression by affecting S-nitrosylation of cellular proteins in the TME.
6. S-Nitrosylation in Anti-Cancer Therapy
It is now evident that aberrant S-nitrosylation plays a key role in cancer development [248][249][250][251][252]. The altered S-nitrosylation levels are likely attributed to the dysregulated expression or function of NOSs (specifically iNOS) and denitrosylases (such as Trx and GSNOR) as well as oxidative stress, hypoxia and oncogenic mutations of target proteins. However, given that most S-nitrosylation inactivates the target, the biological consequence of this modification largely depends on the primary function of the protein. For example, S-nitrosylation of proteins with tumor-suppressive functions (e.g., caspases and PTEN) could exacerbate tumor development. In contrast, S-nitrosylation of proteins with tumor-promoting functions (e.g., NF-ĸB and AKT) could suppress tumor progression [209][253][254][255]. Such dichotomous effects of S-nitrosylation become apparent when different cancer types are compared. Accordingly, a therapeutic strategy to either reduce or increase S-nitrosylation could be decided based on the type and nature of cancer as discussed below [21] (Table 3).
Table 3. S-Nitrosylation in anti-cancer therapy.
| Drug | Molecular Signaling Changes | Biological Outcome | Model and Cell Type | Reference |
|---|---|---|---|---|
| Reducing S-Nitrosylation | ||||
| 1400W, L-NAME, L-NMMA | iNOS inhibition, HIF-1α, and IRE1α/XBP1 impairment | Decreased cell growth and motility | TNBC, MDA-MB-231 and SUM159 | [251] |
| L-NMMA+ Docetaxel | iNOS inhibition, ASK1 activation | Increased cytotoxicity in docetaxel-resistant cells | TNBC, SUM-159PT, MDA-MB-436, and MDA-MB-468 | [256] |
| 1400W | Rescues AGT depletion | Reduced DNA mutagenesis | HCC, Diethylnitrosamine (DEN) induced HCC in murine model | [257] |
| MDA-7/IL-24 | Increased BCL-2 denitrosylation | Increased apoptosis | Pan cancer, melanoma A375, and renal carcinoma 7860 | [258] |
| 1400W | Increased OGG1 activity | Increased DNA-repair activity | Cholangiocarcinoma, KMBC | [24] |
| 1400W, L-NIO | Inhibition of angiogenesis related genes | Decreased cell growth, migration, and angiogenesis | CRC; HT 29, and HCT 116 | [217] |
| L-NAME | Inhibition of MAPK signaling | Decreased cell growth and survival | Breast cancer, LM-2, LM-3, LMM3, MDA-MB-231 | [22] |
| Increasing S-nitrosylation | ||||
| SNP, GSNO | Increased ERK1/2 S-nitrosylation | Decreased cell growth | Glioma, U251 cells | |
| GSNO | Increased STAT3 S-nitrosylation | Decreased cell growth of chemo-resistant cells | Ovarian cancer. Ovarian cancer cell lines and HNSCC | [105][259] |
| GTN | cIAP S-nitrosylation | Increased apoptosis and cell death | Colon and breast cancer. SW480, CT26, MDA-MB-231, and EMT6, macrophages | [260] |
| JSK | Inhibition of ubiquitination | Decreased cell growth | Prostate cancer, LNCaP, and C4-2 | [261] |
| NO-ASA and NO-naproxen | Increased NF-κB S-nitrosylation | Decreased cell growth | Colon cancer, HT-29 cells | [262] |
| NO-NSAID | Increased NF-κB and caspase-3 S-nitrosylation | Decreased cell growth | Pan-cancer | [263] |
| SNOC, GSNO, and DETA-NO | Increased Androgen receptor | Decreased cell growth | Prostate cancer, LNCaP, PC3, and 22Rv1 cells | [28] |
| SNP | Increased ERK1/2 S-nitrosylation | Increased apoptosis | Breast cancer, MCF-7 cells | [164] |
6.1. Reducing S-Nitrosylation
Pharmacological inhibition of NOS is the most commonly used approach for reducing S-nitrosylation. Especially, 1400W (iNOS inhibitor), L-NAME and L-NMMA (pan NOS inhibitors) have shown preclinical feasibility for the treatment of triple-negative breast cancer (TNBC), resulting in the reduction of cell proliferation and cell motility [264]. In particular, the combinatory use of a NOS inhibitor with a chemotherapeutic agent, docetaxel, was found to improve the cytotoxicity of the drug in docetaxel-resistant TNBC cells by activating activation of apoptosis signal regulating kinase (ASK1) [256]. iNOS inhibitors have also been utilized for the treatment of liver tumors that have increased iNOS expression and reduced expression of a denitrosylase, GSNOR. These tumors were found to have hyper-S-nitrosylation of AGT, a DNA damage repair protein, which targets the protein for degradation. In these tumors, the iNOS inhibitor was able to rescue the AGT activity and block mutagenesis [257]. Another example is MDA-7/IL-24, which is a tumor suppressive cytokine currently in the early stages of FDA pre-IND drug trials [265]. This cytokine induces apoptosis of various types of tumors through denitrosylation of an anti-apoptotic protein BCL-2, targeting the protein for ubiquitination and degradation. (S-nitrosylation of BCL-2 at Cys158 and Cys229 was found to be a major mechanism to suppress apoptosis of tumor cells under stress.) This pro-apoptotic activity of MDA-7/IL-24 was shown to be mediated through both the decrease of iNOS expression and increase of a denitrosylase TRXR1 [162][258].
6.2. Increasing S-Nitrosylation
NO donors and NOS-inducing drugs, such as SNP, GSNO, NO-ASA (NO-releasing Aspirin) and JSK (NO pro-drug), are utilized to increase S-nitrosylation levels for cancer treatment [30][259][263][266]. This approach is based on the well-established notion that increased nitrosative stress could trigger growth inhibition and cytotoxicity in tumor cells. One of the key mechanisms by which NO donors exert such anti-tumor effects is elevated S-nitrosylation. For example, S-nitrosylation of ERK1/2 at Cys183 impaired its phosphorylation and mitogenic activity, leading to the growth inhibition of glioma cells and apoptosis of breast cancer cells [107]. Moreover, GSNO treatment resulted in increased STAT3 S-nitrosylation and inhibited the growth of ovarian cancer [105] as well as head and neck squamous cell carcinoma [259]. In addition, an NO donor, glyceryl trinitrate (GTN), was shown to induce S-nitrosylation of inhibitor of apoptosis (cIAP) at Cys571 and Cys574, which led to the assembly of a death complex in colon and breast cancer cells [260]. Among these NO donors, NO-nonsteroidal anti-inflammatory drugs (NO-NSAIDs), including NO-ASA, NONO-ASA and NO-naproxen, are the most widely used for cancer treatment [266]. These NO-releasing compounds are utilized by themselves or in combination with other chemotherapeutic agents to induce apoptosis of drug-resistant cancer cells [267]. The anti-cancer effects of NO-NSAID involve the S-nitrosylation and subsequent inactivation of various pro-tumor proteins, including NF-κB and β-catenin [263].
Dysregulated S-nitrosylation levels have been linked to metabolic reprogramming, in part owing to altered mitochondrial functions, in cancer cells and stroma cells [268][269]. In mitochondria, six out of a total of eight enzymes in the tricarboxylic acid (TCA) cycle are targeted for S-nitrosylation. These six enzymes are aconitase, citrate, succinyl-CoA synthase, isocitrate, α-ketoglutarate dehydrogenase and succinate dehydrogenase [270][271][272]. S-nitrosylation of these TCA enzymes lowers the production of metabolic intermediates and energy necessary for most cellular activities. In addition, S-nitrosylation of the complex I (subunit ND3), IV, and V (ATP synthase) in the mitochondrial electron transport chain (ETC) lowers the electron fluxes and respiratory capacity of mitochondria [273][274][275]. In contrast, succinate dehydrogenase (SDH, i.e., complex II) is not targeted for S-nitrosylation. SDH could instead be subjected to cancer-associated mutations and be utilized as a target for cancer therapy. It was found that the efficacy of an SDH targeting drug (mitocans) for liver cancer treatment could be improved by increasing S-nitrosylation of mitochondrial chaperone TNF receptor associated protein 1 (TRAP1) at Cys50 by inhibiting a denitrosylase GSNOR. TRAP1 is highly expressed in different types of cancer and regulates metabolic rewiring [171][276][277]. S-nitrosylation of TRAP1 causes its degradation, destabilizing SDH and inducing apoptosis of cancer cells [172][278][279].
6.3. Challenges in S-Nitrosylation-Based Anti-Cancer Therapy
Despite the emerging cancer therapeutics based on S-nitrosylation, the efficacy of this approach is challenged by the multifaceted roles of S-nitrosylation in cancer. As discussed above, S-nitrosylation could exert either pro-tumor or anti-tumor effects depending on different parameters, including the context, tumor type, target cell, and target protein. Since pharmaceutical agents that modulate S-nitrosylation levels could affect all different cells in the TME, as well as in the body, they could produce adverse off-target effects that offset the advantage. For example, many types of NO-donors are utilized in S-nitrosylation-based anti-cancer treatment. These NO donors could be conjugated to different drugs, such as NSAIDs and doxorubicin, to improve the cytotoxicity [280][281][282]. However, these NO donors rapidly release NO by simply reacting with water. Such uncontrolled NO release would affect not only tumor cells but also normal cells throughout the body. Thus, the development of a tumor cell-targeted delivery system for these drugs is essential for moving this field forward [281]. In fact, there have been several studies demonstrating the efficacy of utilizing nanoparticles and liposomes to specifically deliver NO donors to tumors [281]. There have been, in fact, concerted efforts to improve the precision targeting of these cancer drugs that modulate S-nitrosylation levels. For example, in recent decades, isoform-specific NOS inhibitors have been engineered to replace the previously developed pan-NOS inhibitors, aiming to mitigate the side effects. Additional promising approaches would be advances in biomaterials and the combination of S-nitrosylation-based cancer drugs with radiotherapy, immunotherapy, and chemotherapy.
7. Conclusions
Endogenous NO is unstable and has a rather short half-life [39]. NO, which is synthesized in different tissues, may diffuse across cell membranes, and exert its biological function. This NO combines with the heme group of soluble guanylate cyclase (sGC)—the first intracellular NO receptor—and activates sGC to produce cyclic guanosine monophosphate (cGMP), which is a unique second messenger molecule in cells [283]. However, increasing evidence indicates that NO performs a variety of biological functions through cGMP-independent S-nitrosylation of proteins. Various physiological functions are determined by the degree of S-nitrosylation in different tissues.
In the past two decades, S-nitrosylation has garnered considerable attention for its multifaceted roles in regulating diverse signaling events in the TME to regulate cancer development [248][249][250][251][252]. A recent study reported nitrosoproteome in PDAC patient samples and revealed that many of these S-nitrosylated proteins are involved in the regulation of the cell cycle, focal adhesions, adherent junctions, and cytoskeletal functions [24]. As we described in this review, S-nitrosylation modulates the activities of different resident cells in the tissue/TME, including immune cells (macrophages, T cells, and NK cells) and other types of stromal cells (endothelial cells). It is well accepted that the levels of S-nitrosylation are aberrant in most components of the TME. However, strategies to normalize S-nitrosylation levels for cancer treatment depend on cancer types/origins and cancer-causing proteins, which are either hyper-S-nitrosylated or hypo-S-nitrosylated. Considering such a complexity, the development of new precision cancer medicine could be aimed at restoring the physiological S-nitrosylation level of a particular protein for each cell type of the TME.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22094600
References
- Stamler, J.S.; Simon, D.I.; Osborne, J.A.; Mullins, M.E.; Jaraki, O.; Michel, T.; Singel, D.J.; Loscalzo, J. S-nitrosylation of proteins with nitric oxide: Synthesis and characterization of biologically active compounds. Proc. Natl. Acad. Sci. USA 1992, 89, 444–448.
- Mnatsakanyan, R.; Markoutsa, S.; Walbrunn, K.; Roos, A.; Verhelst, S.H.L.; Zahedi, R.P. Proteome-wide detection of S-nitrosylation targets and motifs using bioorthogonal cleavable-linker-based enrichment and switch technique. Nat. Commun. 2019, 10, 2195.
- Furuta, S. Basal S-Nitrosylation Is the Guardian of Tissue Homeostasis. Trends Cancer 2017, 3, 744–748.
- Chouchani, E.T.; Methner, C.; Nadtochiy, S.M.; Logan, A.; Pell, V.R.; Ding, S.; James, A.M.; Cocheme, H.M.; Reinhold, J.; Lilley, K.S.; et al. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat. Med. 2013, 19, 753–759.
- Yasinska, I.M.; Sumbayev, V.V. S-nitrosation of Cys-800 of HIF-1alpha protein activates its interaction with p300 and stimulates its transcriptional activity. FEBS Lett. 2003, 549, 105–109.
- Okamoto, S.; Nakamura, T.; Cieplak, P.; Chan, S.F.; Kalashnikova, E.; Liao, L.; Saleem, S.; Han, X.; Clemente, A.; Nutter, A.; et al. S-nitrosylation-mediated redox transcriptional switch modulates neurogenesis and neuronal cell death. Cell Rep. 2014, 8, 217–228.
- Chouchani, E.T.; James, A.M.; Methner, C.; Pell, V.R.; Prime, T.A.; Erickson, B.K.; Forkink, M.; Lau, G.Y.; Bright, T.P.; Menger, K.E.; et al. Identification and quantification of protein S-nitrosation by nitrite in the mouse heart during ischemia. J. Biol. Chem. 2017, 292, 14486–14495.
- Raju, K.; Doulias, P.T.; Evans, P.; Krizman, E.N.; Jackson, J.G.; Horyn, O.; Daikhin, Y.; Nissim, I.; Yudkoff, M.; Nissim, I.; et al. Regulation of brain glutamate metabolism by nitric oxide and S-nitrosylation. Sci. Signal 2015, 8, ra68.
- Okamoto, S.; Lipton, S.A. S-Nitrosylation in neurogenesis and neuronal development. Biochim. Biophys. Acta 2015, 1850, 1588–1593.
- Hess, D.T.; Matsumoto, A.; Kim, S.O.; Marshall, H.E.; Stamler, J.S. Protein S-nitrosylation: Purview and parameters. Nat. Rev. Mol. Cell Biol. 2005, 6, 150–166.
- Sun, J.; Picht, E.; Ginsburg, K.S.; Bers, D.M.; Steenbergen, C.; Murphy, E. Hypercontractile female hearts exhibit increased S-nitrosylation of the L-type Ca2+ channel alpha1 subunit and reduced ischemia/reperfusion injury. Circ. Res. 2006, 98, 403–411.
- Kovacs, I.; Lindermayr, C. Nitric oxide-based protein modification: Formation and site-specificity of protein S-nitrosylation. Front. Plant. Sci. 2013, 4, 137.
- Wang, Z. Protein S-nitrosylation and cancer. Cancer Lett. 2012, 320, 123–129.
- Plenchette, S.; Romagny, S.; Laurens, V.; Bettaieb, A. S-Nitrosylation in TNF superfamily signaling pathway: Implication in cancer. Redox Biol. 2015, 6, 507–515.
- Stomberski, C.T.; Hess, D.T.; Stamler, J.S. Protein S-Nitrosylation: Determinants of Specificity and Enzymatic Regulation of S-Nitrosothiol-Based Signaling. Antioxid. Redox Signal. 2019, 30, 1331–1351.
- Fernando, V.; Zheng, X.; Walia, Y.; Sharma, V.; Letson, J.; Furuta, S. S-Nitrosylation: An Emerging Paradigm of Redox Signaling. Antioxidants 2019, 8, 404.
- Rizza, S.; Filomeni, G. Role, Targets and Regulation of (de)nitrosylation in Malignancy. Front. Oncol. 2018, 8, 334.
- Vannini, F.; Kashfi, K.; Nath, N. The dual role of iNOS in cancer. Redox Biol. 2015, 6, 334–343.
- Karlenius, T.C.; Tonissen, K.F. Thioredoxin and Cancer: A Role for Thioredoxin in all States of Tumor Oxygenation. Cancers 2010, 2, 209–232.
- Ren, G.; Zheng, X.; Bommarito, M.; Metzger, S.; Walia, Y.; Letson, J.; Schroering, A.; Kalinoski, A.; Weaver, D.; Figy, C.; et al. Reduced Basal Nitric Oxide Production Induces Precancerous Mammary Lesions via ERBB2 and TGFβ. Sci. Rep. 2019, 9, 6688.
- Rizza, S.; Filomeni, G. Exploiting S-nitrosylation for cancer therapy: Facts and perspectives. Biochem. J. 2020, 477, 3649–3672.
- Sciacca, M.; Belgorosky, D.; Zambrano, M.; Gomez Escalante, J.I.; Roca, F.; Langle, Y.V.; Sandes, E.O.; Lodillinsky, C.; Eijan, A.M. Inhibition of breast tumor growth by N(G)-nitro-l-arginine methyl ester (l-NAME) is accompanied by activation of fibroblasts. Nitric Oxide 2019, 93, 34–43.
- Christmann, M.; Verbeek, B.; Roos, W.P.; Kaina, B. O(6)-Methylguanine-DNA methyltransferase (MGMT) in normal tissues and tumors: Enzyme activity, promoter methylation and immunohistochemistry. Biochim. Biophys. Acta 2011, 1816, 179–190.
- Jaiswal, M.; LaRusso, N.F.; Nishioka, N.; Nakabeppu, Y.; Gores, G.J. Human Ogg1, a protein involved in the repair of 8-oxoguanine, is inhibited by nitric oxide. Cancer Res. 2001, 61, 6388–6393.
- Tan, C.; Li, Y.; Huang, X.; Wei, M.; Huang, Y.; Tang, Z.; Huang, H.; Zhou, W.; Wang, Y.; Hu, J. Extensive protein S-nitrosylation associated with human pancreatic ductal adenocarcinoma pathogenesis. Cell Death Dis. 2019, 10, 914.
- Flaherty, R.L.; Intabli, H.; Falcinelli, M.; Bucca, G.; Hesketh, A.; Patel, B.A.; Allen, M.C.; Smith, C.P.; Flint, M.S. Stress hormone-mediated acceleration of breast cancer metastasis is halted by inhibition of nitric oxide synthase. Cancer Lett. 2019, 459, 59–71.
- Yarlagadda, K.; Hassani, J.; Foote, I.P.; Markowitz, J. The role of nitric oxide in melanoma. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 500–509.
- Qin, Y.; Dey, A.; Purayil, H.T.; Daaka, Y. Maintenance of androgen receptor inactivation by S-nitrosylation. Cancer Res. 2013, 73, 6690–6699.
- Williams, J.L.; Ji, P.; Ouyang, N.; Kopelovich, L.; Rigas, B. Protein nitration and nitrosylation by NO-donating aspirin in colon cancer cells: Relevance to its mechanism of action. Exp. Cell Res. 2011, 317, 1359–1367.
- Song, J.M.; Upadhyaya, P.; Kassie, F. Nitric oxide-donating aspirin (NO-Aspirin) suppresses lung tumorigenesis in vitro and in vivo and these effects are associated with modulation of the EGFR signaling pathway. Carcinogenesis 2018, 39, 911–920.
- Aranda, E.; Lopez-Pedrera, C.; De La Haba-Rodriguez, J.R.; Rodriguez-Ariza, A. Nitric Oxide and Cancer: The Emerging Role of S-Nitrosylation. Curr. Mol. Med. 2012, 12, 50–67.
- Benhar, M. Emerging Roles of Protein S-Nitrosylation in Macrophages and Cancer Cells. Curr. Med. Chem. 2016, 23, 2602–2617.
- Bignon, E.; Allega, M.F.; Lucchetta, M.; Tiberti, M.; Papaleo, E. Computational Structural Biology of S-nitrosylation of Cancer Targets. Front. Oncol. 2018, 8, 272.
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125, 5591–5596.
- Prajapati, P.; Lambert, D.W. Cancer-associated fibroblasts—Not-so-innocent bystanders in metastasis to bone? J. Bone Oncol. 2016, 5, 128–131.
- Munn, D.H.; Bronte, V. Immune suppressive mechanisms in the tumor microenvironment. Curr. Opin. Immunol. 2016, 39, 1–6.
- McAllister, S.S.; Weinberg, R.A. Tumor-host interactions: A far-reaching relationship. J. Clin. Oncol. 2010, 28, 4022–4028.
- Hernansanz-Agustin, P.; Izquierdo-Alvarez, A.; Garcia-Ortiz, A.; Ibiza, S.; Serrador, J.M.; Martinez-Ruiz, A. Nitrosothiols in the immune system: Signaling and protection. Antioxid. Redox Signal. 2013, 18, 288–308.
- Thomas, D.D.; Liu, X.; Kantrow, S.P.; Lancaster, J.R.J. The biological lifetime of nitric oxide: Implications for the perivascular dynamics of NO and O2. Proc. Natl. Acad. Sci. USA 2001, 98, 355–360.
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357, 593–615.
- Francis, S.H.; Busch, J.L.; Corbin, J.D.; Sibley, D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharm. Rev. 2010, 62, 525–563.
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837.
- Lacza, Z.; Puskar, M.; Figueroa, J.P.; Zhang, J.; Rajapakse, N.; Busija, D.W. Mitochondrial nitric oxide synthase is constitutively active and is functionally upregulated in hypoxia. Free Radic. Biol. Med. 2001, 31, 1609–1615.
- Ghafourifar, P.; Cadenas, E. Mitochondrial nitric oxide synthase. Trends Pharm. Sci. 2005, 26, 190–195.
- Nisoli, E.; Tonello, C.; Cardile, A.; Cozzi, V.; Bracale, R.; Tedesco, L.; Falcone, S.; Valerio, A.; Cantoni, O.; Clementi, E.; et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 2005, 310, 314–317.
- Zhu, L.; Wang, Q.; Zhang, L.; Fang, Z.; Zhao, F.; Lv, Z.; Gu, Z.; Zhang, J.; Wang, J.; Zen, K.; et al. Hypoxia induces PGC-1α expression and mitochondrial biogenesis in the myocardium of TOF patients. Cell Res. 2010, 20, 676–687.
- Victor, V.M.; Nun˜ez, C.; D’Oco´n, P.; Taylor, C.T.; Esplugues, J.V.; Moncada, S. Regulation of oxygen distribution in tissues by endothelial nitric oxide. Circ. Res. 2009, 104, 1178–1183.
- Moens, A.L.; Kass, D.A. Tetrahydrobiopterin and cardiovascular disease. Arter. Thromb. Vasc. Biol. 2006, 26, 2439–2444.
- Edgar, K.S.; Matesanz, N.; Gardiner, T.A.; Katusic, Z.S.; McDonald, D.M. Hyperoxia depletes (6R)-5,6,7,8-tetrahydrobiopterin levels in the neonatal retina: Implications for nitric oxide synthase function in retinopathy. Am. J. Pathol. 2015, 185, 1769–1782.
- Kuzkaya, N.; Weissmann, N.; Harrison, D.G.; Dikalov, S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: Implications for uncoupling endothelial nitric-oxide synthase. J. Biol. Chem. 2003, 278, 22546–22554.
- Veron, D.; Aggarwal, P.K.; Velazquez, H.; Kashgarian, M.; Moeckel, G.; Tufro, A. Podocyte-specific VEGF-a gain of function induces nodular glomerulosclerosis in eNOS null mice. J. Am. Soc. Nephrol. 2014, 25, 1814–1824.
- Rabender, C.S.; Alam, A.; Sundaresan, G.; Cardnell, R.J.; Yakovlev, V.A.; Mukhopadhyay, N.D.; Graves, P.; Zweit, J.; Mikkelsen, R.B. The Role of Nitric Oxide Synthase Uncoupling in Tumor Progression. Mol. Cancer Res. 2015, 13, 1034–1043.
- Hoang, H.H.; Padgham, S.V.; Meininger, C.J. L-arginine, tetrahydrobiopterin, nitric oxide and diabetes. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 76–82.
- Gámez-Méndez, A.M.; Vargas-Robles, H.; Arellano-Mendoza, M.; Cruz-Laguna, E.; Rios, A.; Escalante, B. Early stage of obesity potentiates nitric oxide reduction during the development of renal failure. J. Nephrol. 2014, 27, 281–287.
- Landmesser, U.; Dikalov, S.; Price, S.R.; McCann, L.; Fukai, T.; Holland, S.M.; Mitch, W.E.; Harrison, D.G. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Investig. 2003, 111, 1201–1209.
- Antosova, M.; Plevkova, J.; Strapkova, A.; Buday, T. Nitric oxide—Important messenger in human body. OJMIP 2012, 2, 98–106.
- Peunova, N.; Scheinker, V.; Ravi, K.; Enikolopov, G. Nitric oxide coordinates cell proliferation and cell movements during early development of Xenopus. Cell Cycle 2007, 6, 3132–3144.
- Young, S.L.; Evans, K.; Eu, J.P. Nitric oxide modulates branching morphogenesis in fetal rat lung explants. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 282, L379–L385.
- Cole, A.G.; Mashkournia, A.; Parries, S.C.; Goldberg, J.I. Regulation of early embryonic behavior by nitric oxide in the pond snail Helisoma trivolvis. J. Exp. Biol. 2002, 205, 3143–3152.
- Slezinger, M.S.; Kuzin, B.A. Nitric oxide synthase mediates regulation of cell polarity and movement during Drosophila melanogaster morphogenesis. Ontogenez 2009, 40, 40–47.
- Bradley, S.; Tossell, K.; Lockley, R.; McDearmid, J.R. Nitric oxide synthase regulates morphogenesis of zebrafish spinal cord motoneurons. J. Neurosci. 2010, 30, 16816–16831.
- Lee, N.P.; Cheng, C.Y. Nitric oxide/nitric oxide synthase, spermatogenesis, and tight junction dynamics. Biol. Reprod. 2004, 70, 267–276.
- Martínez-Ruiz, A.; Araújo, I.M.; Izquierdo-Álvarez, A.; Hernansanz-Agustín, P.; Lamas, S.; Serrador, J. Specificity in S-nitrosylation: A short-range mechanism for NO signaling? Antioxid. Redox Signal. 2013, 19, 1220–1235.
- Scicinski, J.; Oronsky, B.; Ning, S.; Knox, S.; Peehl, D.; Kim, M.M.; Langecker, P.; Fanger, G. NO to cancer: The complex and multifaceted role of nitric oxide and the epigenetic nitric oxide donor, RRx-001. Redox Biol. 2015, 6, 1–8.
- Choudhari, S.K.; Chaudhary, M.; Sachin, B.; Gadbail, A.R.; Joshi, V. Nitric oxide and cancer: A review. World J. Surg. Oncol. 2013, 11, 118.
- Thomas, D.D.; Espey, M.G.; Ridnour, L.A.; Hofseth, L.J.; Mancardi, D.; Harris, C.C.; Wink, D.A. Hypoxic inducible factor 1alpha, extracellular signal-regulated kinase, and p53 are regulated by distinct threshold concentrations of nitric oxide. Proc. Natl. Acad. Sci. USA 2004, 101, 8894–8899.
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharm. Rev. 1991, 43, 109–142.
- Thomsen, L.L.; Miles, D.W.; Happerfield, L.; Bobrow, L.G.; Knowles, R.G.; Moncada, S. Nitric oxide synthase activity in human breast cancer. Br. J. Cancer 2020, 72, 41–44.
- Sangle, V.A.; Chaware, S.J.; Kulkarni, M.A.; Ingle, Y.C.; Singh, P.; Pooja, V.K. Elevated tissue nitric oxide in oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2018, 22, 35–39.
- Abdel-Salam, O.; Youness, E.; Hafez, H. The antioxidant status of the plasma in patients with breast cancer undergoing chemotherapy. Open J. Mol. Integr. Physiol. 2011, 1, 29–35.
- Simeone, A.M.; Broemeling, L.D.; Rosenblum, J.; Tari, A.M. HER2/neu reduces the apoptotic effects of N-(4-hydroxyphenyl)retinamide (4-HPR) in breast cancer cells by decreasing nitric oxide production. Oncogenet 2003, 22, 6739–6747.
- Furuta, S.; Ren, G.; Mao, J.; Bissell, M.J. Laminin signals initiate the reciprocal loop that informs breast-specific gene expression and homeostasis by activating NO, p53 and microRNA. eLife 2018, 7, e26148.
- Hickok, J.R.; Thomas, D.D. Nitric oxide and cancer therapy: The emperor has NO clothes. Curr. Pharm. Des. 2010, 16, 381–391.
- Burke, A.J.; Sullivan, F.J.; Giles, F.J.; Glynn, S.A. The yin and yang of nitric oxide in cancer progression. Carcinogenesis 2013, 34, 503–512.
- Ridnour, L.A.; Thomas, D.D.; Switzer, C.; Flores-Santana, W.; Isenberg, J.S.; Ambs, S.; Roberts, D.D.; Wink, D.A. Molecular mechanisms for discrete nitric oxide levels in cancer. Nitric Oxide 2000, 19, 73–76.
- Zheng, X.; Turkowski, K.; Mora, J.; Brüne, B.; Seeger, W.; Weigert, A.; Savai, R. Redirecting tumor-associated macrophages to become tumoricidal effectors as a novel strategy for cancer therapy. Oncotarget 2017, 8, 48436–48452.
- Gould, N.; Doulias, P.T.; Tenopoulou, M.; Raju, K.; Ischiropoulos, H. Regulation of protein function and signaling by reversible cysteine S-nitrosylation. J. Biol. Chem. 2013, 288, 26473–26479.
- Chung, H.S.; Wang, S.B.; Venkatraman, V.; Murray, C.I.; Van Eyk, J.E. Cysteine oxidative posttranslational modifications: Emerging regulation in the cardiovascular system. Circ. Res. 2013, 112, 382–392.
- Aguilar, G.; Koning, T.; Ehrenfeld, P.; Sanchez, F.A. Role of NO and S-nitrosylation in the Expression of Endothelial Adhesion Proteins That Regulate Leukocyte and Tumor Cell Adhesion. Front. Physiol. 2020, 11, 595526.
- Nakamura, T.; Lipton, S.A. Protein S-Nitrosylation as a Therapeutic Target for Neurodegenerative Diseases. Trends. Pharm. Sci. 2016, 37, 73–84.
- Bartberger, M.D.; Liu, W.; Ford, E.; Miranda, K.M.; Switzer, C.; Fukuto, J.M.; Farmer, P.J.; Wink, D.A.; Houk, K.N. The reduction potential of nitric oxide (NO) and its importance to NO biochemistry. Proc. Natl. Acad. Sci. USA 2002, 99, 10958–10963.
- Moller, M.N.; Li, Q.; Vitturi, D.A.; Robinson, J.M.; Lancaster, J.R., Jr.; Denicola, A. Membrane “lens” effect: Focusing the formation of reactive nitrogen oxides from the *NO/O2 reaction. Chem. Res. Toxicol. 2007, 20, 709–714.
- Broniowska, K.A.; Hogg, N. The chemical biology of S-nitrosothiols. Antioxid. Redox Signal. 2012, 17, 969–980.
- Jia, J.; Arif, A.; Terenzi, F.; Willard, B.; Plow, E.F.; Hazen, S.L.; Fox, P.L. Target-selective protein S-nitrosylation by sequence motif recognition. Cell 2014, 159, 623–634.
- Nakamura, T.; Lipton, S.A. Emerging role of protein-protein transnitrosylation in cell signaling pathways. Antioxid. Redox Signal. 2013, 18, 239–249.
- Hess, D.T.; Stamler, J.S. Regulation by S-nitrosylation of protein post-translational modification. J. Biol. Chem. 2012, 287, 4411–4418.
- Anand, P.; Stamler, J.S. Enzymatic mechanisms regulating protein S-nitrosylation: Implications in health and disease. J. Mol. Med. 2012, 90, 233–244.
- Anand, P.; Hausladen, A.; Wang, Y.J.; Zhang, G.F.; Stomberski, C.; Brunengraber, H.; Hess, D.T.; Stamler, J.S. Identification of S-nitroso-CoA reductases that regulate protein S-nitrosylation. Proc. Natl. Acad. Sci. USA 2014, 111, 18572–18577.
- Zhang, Y.; Deng, Y.; Yang, X.; Xue, H.; Lang, Y. The Relationship Between Protein S-Nitrosylation and Human Diseases: A Review. Neurochem. Res. 2020, 45, 2815–2827.
- Ehrenfeld, P.; Cordova, F.; Duran, W.N.; Sanchez, F.A. S-nitrosylation and its role in breast cancer angiogenesis and metastasis. Nitric Oxide 2019, 87, 52–59.
- Basudhar, D.; Somasundaram, V.; de Oliveira, G.A.; Kesarwala, A.; Heinecke, J.L.; Cheng, R.Y.; Glynn, S.A.; Ambs, S.; Wink, D.A.; Ridnour, L.A. Nitric Oxide Synthase-2-Derived Nitric Oxide Drives Multiple Pathways of Breast Cancer Progression. Antioxid. Redox Signal. 2017, 26, 1044–1058.
- Thompson, L.; Dong, Y.; Evans, L. Chronic hypoxia increases inducible NOS-derived nitric oxide in fetal guinea pig hearts. Pediatr. Res. 2009, 65, 188–192.
- Rahman, M.A.; Senga, T.; Ito, S.; Hyodo, T.; Hasegawa, H.; Hamaguchi, M. S-nitrosylation at cysteine 498 of c-Src tyrosine kinase regulates nitric oxide-mediated cell invasion. J. Biol. Chem. 2010, 285, 3806–3814.
- Marshall, H.E.; Foster, M.W. S-nitrosylation of Ras in breast cancer. Breast Cancer Res. 2012, 14, 113.
- Jindal, S.; Pennock, N.D.; Klug, A.; Narasimhan, J.; Calhoun, A.; Roberts, M.R.; Tamimi, R.M.; Eliassen, A.H.; Weinmann, S.; Borges, V.F.; et al. S-nitrosylated and non-nitrosylated COX2 have differential expression and distinct subcellular localization in normal and breast cancer tissue. NPJ Breast Cancer 2020, 6, 62.
- Thomas, D.D.; Espey, M.G.; Pociask, D.A.; Ridnour, L.A.; Donzelli, S.; Wink, D.A. Asbestos redirects nitric oxide signaling through rapid catalytic conversion to nitrite. Cancer Res. 2006, 66, 11600–11604.
- Liu, M.; Hou, J.; Huang, L.; Huang, X.; Heibeck, T.H.; Zhao, R.; Pasa-Tolic, L.; Smith, R.D.; Li, Y.; Fu, K.; et al. Site-specific proteomics approach for study protein S-nitrosylation. Anal. Chem. 2010, 82, 7160–7168.
- Ben-Lulu, S.; Ziv, T.; Weisman-Shomer, P.; Benhar, M. Nitrosothiol-Trapping-Based Proteomic Analysis of S-Nitrosylation in Human Lung Carcinoma Cells. PLoS ONE 2017, 12, e0169862.
- Zhang, X.; Li, G.; Guo, Y.; Song, Y.; Chen, L.; Ruan, Q.; Wang, Y.; Sun, L.; Hu, Y.; Zhou, J.; et al. Regulation of ezrin tension by S-nitrosylation mediates non-small cell lung cancer invasion and metastasis. Theranostics 2019, 9, 2555–2571.
- Chanvorachote, P.; Nimmannit, U.; Stehlik, C.; Wang, L.; Jiang, B.H.; Ongpipatanakul, B.; Rojanasakul, Y. Nitric oxide regulates cell sensitivity to cisplatin-induced apoptosis through S-nitrosylation and inhibition of Bcl-2 ubiquitination. Cancer Res. 2006, 66, 6353–6360.
- Chanvorachote, P.; Nimmannit, U.; Lu, Y.; Talbott, S.; Jiang, B.H.; Rojanasakul, Y. Nitric oxide regulates lung carcinoma cell anoikis through inhibition of ubiquitin-proteasomal degradation of caveolin-1. J. Biol. Chem. 2009, 284, 28476–28484.
- Zhang, Y.; Sun, C.; Xiao, G.; Shan, H.; Tang, L.; Yi, Y.; Yu, W.; Gu, Y. S-nitrosylation of the Peroxiredoxin-2 promotes S-nitrosoglutathione-mediated lung cancer cells apoptosis via AMPK-SIRT1 pathway. Cell Death Dis. 2019, 10, 1–15.
- Gao, W.; Huang, M.; Chen, X.; Chen, J.; Zou, Z.; Li, L.; Ji, K.; Nie, Z.; Yang, B.; Wei, Z.; et al. The role of S-nitrosylation of PFKM in regulation of glycolysis in ovarian cancer cells. Cell Death Dis. 2021, 12, 408.
- Saed, G.M.; Ali-Fehmi, R.; Jiang, Z.L.; Fletcher, N.M.; Diamond, M.P.; Abu-Soud, H.M.; Munkarah, A.R. Myeloperoxidase serves as a redox switch that regulates apoptosis in epithelial ovarian cancer. Gynecol. Oncol. 2010, 116, 276–281.
- Giri, S.; Rattan, R.; Deshpande, M.; Maguire, J.L.; Johnson, Z.; Graham, R.P.; Shridhar, V. Preclinical Therapeutic Potential of a Nitrosylating Agent in the Treatment of Ovarian Cancer. PLoS ONE 2014, 9, e97897.
- Isaac, J.; Tarapore, P.; Zhang, X.; Lam, Y.W.; Ho, S.M. Site-specific S-nitrosylation of integrin alpha6 increases the extent of prostate cancer cell migration by enhancing integrin beta1 association and weakening adherence to laminin-1. Biochemistry 2012, 51, 9689–9697.
- Jin, L.; Cao, Y.; Zhang, T.; Wang, P.; Ji, D.; Liu, X.; Shi, H.; Hua, L.; Yu, R.; Gao, S. Effects of ERK1/2 S-nitrosylation on ERK1/2 phosphorylation and cell survival in glioma cells. Int. J. Mol. Med. 2018, 41, 1339–1348.
- Li, C.Q.; Kim, M.Y.; Godoy, L.C.; Thiantanawat, A.; Trudel, L.J.; Wogan, G.N. Nitric oxide activation of Keap1/Nrf2 signaling in human colon carcinoma cells. Proc. Natl. Acad. Sci. USA 2009, 106, 14547–14551.
- Zhao, Q.; Zheng, K.; Ma, C.; Li, J.; Zhuo, L.; Huang, W.; Chen, T.; Jiang, Y. PTPS facilitates compartmentalized LTBP1 S-nitrosylation and promotes tumor growth under hypoxia. Mol. Cell 2020, 77, 95–107.
- Zhao, Q.-F.; Yu, J.-T.; Tan, L. S-Nitrosylation in Alzheimer’s disease. Mol. Neurobiol. 2015, 51, 268–280.
- Gu, Z.; Kaul, M.; Yan, B.; Kridel, S.J.; Cui, J.; Strongin, A.; Smith, J.W.; Liddington, R.C.; Lipton, S.A. S-nitrosylation of matrix metalloproteinases: Signaling pathway to neuronal cell death. Science 2002, 297, 1186–1190.
- Lipton, S.A.; Stamler, J.S. Actions of redox-related congeners of nitric oxide at the NMDA receptor. Neuropharmacology 1994, 33, 1229–1233.
- Mukhopadhyay, S.; Lee, J.; Sehgal, P.B. Depletion of the ATPase NSF from Golgi membranes with hypo-S-nitrosylation of vasorelevant proteins in endothelial cells exposed to monocrotaline pyrrole. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1943–H1955.
- Tao, L.; Gao, E.; Bryan, N.S.; Qu, Y.; Liu, H.R.; Hu, A.; Christopher, T.A.; Lopez, B.L.; Yodoi, J.; Koch, W.J.; et al. Cardioprotective effects of thioredoxin in myocardial ischemia and reperfusion: Role of S-nitrosation [corrected]. Proc. Natl. Acad. Sci. USA 2004, 101, 11471–11476.
- Ambs, S.; Ogunfusika, M.O.; Merriam, W.G.; Bennett, W.P.; Billiar, T.R.; Harris, C.C. Up-regulation of inducible nitric oxide synthase expression in cancer-prone p53 knockout mice. Proc. Natl. Acad. Sci. USA 1998, 95, 8823–8828.
- Vakkala, M.; Kahlos, K.; Lakari, E.; Pääkkö, P.; Kinnula, V.; Soini, Y. Inducible Nitric Oxide Synthase Expression, Apoptosis, and Angiogenesis in in Situ and Invasive Breast Carcinomas. Clin. Cancer Res. 2000, 6, 2408–2416.
- Switzer, C.H.; Cheng, R.Y.S.; Ridnour, L.A.; Glynn, S.A.; Ambs, S.; Wink, D.A. Ets-1 is a transcriptional mediator of oncogenic nitric oxide signaling in estrogen receptor-negative breast cancer. Breast Cancer Res. 2012, 14, R125.
- Cai, S.; Khoo, J.; Channon, K.M. Augmented BH4 by gene transfer restores nitric oxide synthase function in hyperglycemic human endothelial cells. Cardiovasc. Res. 2005, 65, 823–831.
- Santhanam, A.V.; d’Uscio, L.V.; Smith, L.A.; Katusic, Z.S. Uncoupling of eNOS causes superoxide anion production and impairs NO signaling in the cerebral microvessels of hph-1 mice. J. Neurochem. 2012, 122, v.
- Melo, F.H.; Molognoni, F.; Morais, A.S.; Toricelli, M.; Mouro, M.G.; Higa, E.M.; Lopes, J.D.; Jasiulionis, M.G. Endothelial nitric oxide synthase uncoupling as a key mediator of melanocyte malignant transformation associated with sustained stress conditions. Free Radic. Biol. Med. 2011, 50, 1263–1273.
- Jo, H.; Otani, H.; Jo, F.; Shimazu, T.; Okazaki, T.; Yoshioka, K.; Fujita, M.; Kosaki, A.; Iwasaka, T. Inhibition of nitric oxide synthase uncoupling by sepiapterin improves left ventricular function in streptozotocin-induced diabetic mice. Clin. Exp. Pharm. Phys. 2011, 38, 485–493.
- Wei, W.; Li, B.; Hanes, M.A.; Kakar, S.; Chen, X.; Liu, L. S-nitrosylation from GSNOR deficiency impairs DNA repair and promotes hepatocarcinogenesis. Sci. Transl. Med. 2010, 2, 19ra13.
- Messina, S.; De Simone, G.; Ascenzi, P. Cysteine-based regulation of redox-sensitive Ras small GTPases. Redox Biol. 2019, 26, 101282.
- Fang, J.; Nakamura, T.; Cho, D.H.; Gu, Z.; Lipton, S.A. S-nitrosylation of peroxiredoxin 2 promotes oxidative stress-induced neuronal cell death in Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2007, 104, 18742–18747.
- Wright, C.; Iyer, A.K.V.; Kulkarni, Y.; Azad, N. S-Nitrosylation of Bcl-2 Negatively Affects Autophagy in Lung Epithelial Cells. J. Cell Biochem. 2016, 117, 521–532.
- Hetman, M.; Gozdz, A. Role of extracellular signal regulated kinases 1 and 2 in neuronal survival. Eur. J. Biochem. 2004, 271, 2050–2055.
- Santos, A.I.; Carreira, B.P.; Izquierdo-Álvarez, A.; Ramos, E.; Lourenço, A.S.; Filipa Santos, D.; Morte, M.I.; Ribeiro, L.F.; Marreiros, A.; Sánchez-López, N.; et al. S-Nitrosylation of Ras Mediates Nitric Oxide-Dependent Post-Injury Neurogenesis in a Seizure Model. Antioxid. Redox Signal. 2018, 28, 15–30.
- Shen, X.; Burguillos, M.A.; Osman, A.M.; Frijhoff, J.; Carrillo-Jiménez, A.; Kanatani, S.; Augsten, M.; Saidi, D.; Rodhe, J.; Kavanagh, E. Glioma-induced inhibition of caspase-3 in microglia promotes a tumor-supportive phenotype. Nat. Immunol. 2016, 17, 1282–1290.
- Huang, J.J.; Blobe, G.C. Dichotomous roles of TGF-β in human cancer. Biochem. Soc. Trans. 2016, 44, 1441–1454.
- Zahid, S.; Khan, R.; Oellerich, M.; Ahmed, N.; Asif, A. Differential S-nitrosylation of proteins in Alzheimer’s disease. Neuroscience 2014, 256, 126–136.
- Sun, J.; Murphy, E. Protein S-nitrosylation and cardioprotection. Circ. Res. 2010, 106, 285–296.
- Zhou, H.L.; Zhang, R.; Anand, P.; Stomberski, C.T.; Qian, Z.; Hausladen, A.; Wang, L.; Rhee, E.P.; Parikh, S.M.; Karumanchi, S.A.; et al. Metabolic reprogramming by the S-nitroso-CoA reductase system protects against kidney injury. Nature 2019, 565, 96–100.
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogenet 2008, 27, 5904–5912.
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal 2020, 18, 59.
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322.
- Frisch, J.; Angenendt, A.; Hoth, M.; Prates Roma, L.; Lis, A. STIM-Orai Channels and Reactive Oxygen Species in the Tumor Microenvironment. Cancers 2019, 11, 457.
- Duan, S.; Chen, C. S-nitrosylation Denitrosylation and Apoptosis of Immune Cells. Cell Mol. Immunol. 2007, 4, 353–358.
- Ibanez-Vea, M.; Huang, H.; Martinez de Morentin, X.; Perez, E.; Gato, M.; Zuazo, M.; Arasanz, H.; Fernandez-Irigoyen, J.; Santamaria, E.; Fernandez-Hinojal, G.; et al. Characterization of Macrophage Endogenous S-Nitrosoproteome Using a Cysteine-Specific Phosphonate Adaptable Tag in Combination with TiO2 Chromatography. J. Proteome Res. 2018, 17, 1172–1182.
- Jaffrey, S.R.; Snyder, S.H. The biotin switch method for the detection of S-nitrosylated proteins. Sci. STKE 2001, 2001, pl1.
- Guequen, A.; Zamorano, P.; Cordova, F.; Koning, T.; Torres, A.; Ehrenfeld, P.; Boric, M.P.; Salazar-Onfray, F.; Gavard, J.; Duran, W.N.; et al. Interleukin-8 Secreted by Glioblastoma Cells Induces Microvascular Hyperpermeability Through NO Signaling Involving S-Nitrosylation of VE-Cadherin and p120 in Endothelial Cells. Front. Physiol. 2019, 10, 988.
- Marin, N.; Zamorano, P.; Carrasco, R.; Mujica, P.; Gonzalez, F.G.; Quezada, C.; Meininger, C.J.; Boric, M.P.; Duran, W.N.; Sanchez, F.A. S-Nitrosation of beta-Catenin and p120 Catenin A Novel Regulatory Mechanism in Endothelial Hyperpermeability. Circ. Res. 2012, 111, U248–U553.
- Thibeault, S.; Rautureau, Y.; Oubaha, M.; Faubert, D.; Wilkes, B.C.; Delisle, C.; Gratton, J.P. S-Nitrosylation of beta-Catenin by eNOS-Derived NO Promotes VEGF-Induced Endothelial Cell Permeability. Mol. Cell 2010, 39, 468–476.
- Li, F.; Sonveaux, P.; Rabbani, Z.N.; Liu, S.; Yan, B.; Huang, Q.; Vujaskovic, Z.; Dewhirst, M.W.; Li, C.Y. Regulation of HIF-1alpha stability through S-nitrosylation. Mol. Cell 2007, 26, 63–74.
- Kang-Decker, N.; Cao, S.; Chatterjee, S.; Yao, J.; Egan, L.J.; Semela, D.; Mukhopadhyay, D.; Shah, V. Nitric oxide promotes endothelial cell survival signaling through S-nitrosylation and activation of dynamin-2. J. Cell Sci. 2007, 120, 492–501.
- Wang, G.; Moniri, N.H.; Ozawa, K.; Stamler, J.S.; Daaka, Y. Nitric oxide regulates endocytosis by S-nitrosylation of dynamin. Proc. Natl. Acad. Sci. USA 2006, 103, 1295–1300.
- Pi, X.; Wu, Y.; Ferguson, J.E., 3rd; Portbury, A.L.; Patterson, C. SDF-1alpha stimulates JNK3 activity via eNOS-dependent nitrosylation of MKP7 to enhance endothelial migration. Proc. Natl. Acad. Sci. USA 2009, 106, 5675–5680.
- Parker, K.H.; Beury, D.W.; Ostrand-Rosenberg, S. Myeloid-Derived Suppressor Cells: Critical Cells Driving Immune Suppression in the Tumor Microenvironment. Adv. Cancer Res. 2015, 128, 95–139.
- Molon, B.; Ugel, S.; Del Pozzo, F.; Soldani, C.; Zilio, S.; Avella, D.; De Palma, A.; Mauri, P.; Monegal, A.; Rescigno, M.; et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J. Exp. Med. 2011, 208, 1949–1962.
- Marshall, H.E.; Hess, D.T.; Stamler, J.S. S-nitrosylation—Physiological regulation of NF-κB. Proc. Natl. Acad. Sci. USA 2004, 101, 8841–8842.
- Kim, J.; Won, J.S.; Singh, A.K.; Sharma, A.K.; Singh, I. STAT3 regulation by S-nitrosylation: Implication for inflammatory disease. Antioxid. Redox Signal. 2014, 20, 2514–2527.
- Mannick, J.B.; Schonhoff, C.; Papeta, N.; Ghafourifar, P.; Szibor, M.; Fang, K.; Gaston, B. S-Nitrosylation of mitochondrial caspases. J. Biol. Chem. 2001, 154, 1111–1116.
- Mao, K.; Chen, S.; Chen, M.; Ma, Y.; Wang, Y.; Huang, B.; He, Z.; Zeng, Y.; Hu, Y.; Sun, S.; et al. Nitric oxide suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock. Cell Res. 2013, 23, 201–212.
- Mishra, B.B.; Rathinam, V.A.; Martens, G.W.; Martinot, A.J.; Kornfeld, H.; Fitzgerald, K.A.; Sassetti, C.M. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1beta. Nat. Immunol. 2013, 14, 52–60.
- Hernandez-Cuellar, E.; Tsuchiya, K.; Hara, H.; Fang, R.; Sakai, S.; Kawamura, I.; Akira, S.; Mitsuyama, M. Cutting Edge: Nitric Oxide Inhibits the NLRP3 Inflammasome. J. Immunol. 2012, 189, 5113.
- Santhanam, L.; Lim, H.K.; Lim, H.K.; Miriel, V.; Brown, T.; Patel, M.; Balanson, S.; Ryoo, S.; Anderson, M.; Irani, K.; et al. Inducible NO synthase dependent S-nitrosylation and activation of arginase1 contribute to age-related endothelial dysfunction. Circ. Res. 2007, 101, 692–702.
- Batista, W.L.; Ogata, F.T.; Curcio, M.F.; Miguel, R.B.; Arai, R.J.; Matsuo, A.L.; Moraes, M.S.; Stern, A.; Monteiro, H.P. S-nitrosoglutathione and endothelial nitric oxide synthase-derived nitric oxide regulate compartmentalized ras S-nitrosylation and stimulate cell proliferation. Antioxid. Redox Signal. 2013, 18, 221–238.
- Kim, S.F.; Huri, D.A.; Snyder, S.H. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science 2005, 310, 1966–1970.
- Switzer, C.H.; Glynn, S.A.; Cheng, R.Y.; Ridnour, L.A.; Green, J.E.; Ambs, S.; Wink, D.A. S-nitrosylation of EGFR and Src activates an oncogenic signaling network in human basal-like breast cancer. Mol. Cancer Res. 2012, 10, 1203–1215.
- Tang, C.H.; Wei, W.; Liu, L. Regulation of DNA repair by S-nitrosylation. Biochim. Biophys. Acta 2012, 1820, 730–735.
- Liu, L.; Xu-Welliver, M.; Kanugula, S.; Pegg, A.E. Inactivation and degradation of O(6)-alkylguanine-DNA alkyltransferase after reaction with nitric oxide. Cancer Res. 2002, 62, 3037–3043.
- Tang, Z.; Bauer, J.A.; Morrison, B.; Lindner, D.J. Nitrosylcobalamin promotes cell death via S nitrosylation of Apo2L/TRAIL receptor DR4. Mol. Cell Biol. 2006, 26, 5588–5594.
- Azad, N.; Vallyathan, V.; Wang, L.; Tantishaiyakul, V.; Stehlik, C.; Leonard, S.S.; Rojanasakul, Y. S-nitrosylation of Bcl-2 inhibits its ubiquitin-proteasomal degradation. A novel antiapoptotic mechanism that suppresses apoptosis. J. Biol. Chem. 2006, 281, 34124–34134.
- Iyer, A.K.; Azad, N.; Wang, L.; Rojanasakul, Y. Role of S-nitrosylation in apoptosis resistance and carcinogenesis. Nitric Oxide 2008, 19, 146–151.
- Feng, X.; Sun, T.; Bei, Y.; Ding, S.; Zheng, W.; Lu, Y.; Shen, P. S-nitrosylation of ERK inhibits ERK phosphorylation and induces apoptosis. Sci. Rep. 2013, 3, 1814.
- Nott, A.; Nitarska, J.; Veenvliet, J.V.; Schacke, S.; Derijck, A.A.; Sirko, P.; Muchardt, C.; Pasterkamp, R.J.; Smidt, M.P.; Riccio, A. S-nitrosylation of HDAC2 regulates the expression of the chromatin-remodeling factor Brm during radial neuron migration. Proc. Natl. Acad. Sci. USA 2013, 110, 3113–3118.
- Nott, A.; Watson, P.M.; Robinson, J.D.; Crepaldi, L.; Riccio, A. S-Nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature 2008, 455, 411–415.
- Yu, C.X.; Li, S.; Whorton, A.R. Redox regulation of PTEN by S-nitrosothiols. Mol. Pharm. 2005, 68, 847–854.
- Calmels, S.; Hainaut, P.; Ohshima, H. Nitric oxide induces conformational and functional modifications of wild-type p53 tumor suppressor protein. Cancer Res. 1997, 57, 3365–3369.
- Schonhoff, C.M.; Daou, M.C.; Jones, S.N.; Schiffer, C.A.; Ross, A.H. Nitric oxide-mediated inhibition of Hdm2-p53 binding. Biochemistry 2002, 41, 13570–13574.
- Leon-Bollotte, L.; Subramaniam, S.; Cauvard, O.; Plenchette-Colas, S.; Paul, C.; Godard, C.; Martinez-Ruiz, A.; Legembre, P.; Jeannin, J.F.; Bettaieb, A. S-nitrosylation of the death receptor fas promotes fas ligand-mediated apoptosis in cancer cells. Gastroenterology 2011, 140, 2009–2018.e2.
- Sciacovelli, M.; Guzzo, G.; Morello, V.; Frezza, C.; Zheng, L.; Nannini, N.; Calabrese, F.; Laudiero, G.; Esposito, F.; Landriscina, M.; et al. The mitochondrial chaperone TRAP1 promotes neoplastic growth by inhibiting succinate dehydrogenase. Cell Metab. 2013, 17, 988–999.
- Rizza, S.; Montagna, C.; Cardaci, S.; Maiani, E.; Di Giacomo, G.; Sanchez-Quiles, V.; Blagoev, B.; Rasola, A.; De Zio, D.; Stamler, J.S.; et al. S-nitrosylation of the Mitochondrial Chaperone TRAP1 Sensitizes Hepatocellular Carcinoma Cells to Inhibitors of Succinate Dehydrogenase. Cancer Res. 2016, 76, 4170–4182.
- Poh, A.R.; Ernst, M. Targeting Macrophages in Cancer: From Bench to Bedside. Front. Oncol. 2018, 8, 49.
- Rath, M.; Muller, I.; Kropf, P.; Closs, E.I.; Munder, M. Metabolism via Arginase or Nitric Oxide Synthase: Two Competing Arginine Pathways in Macrophages. Front. Immunol. 2014, 5, 532.
- Zheng, X.; Fernando, V.; Sharma, V.; Walia, Y.; Letson, J.; Furuta, S. Correction of arginine metabolism with sepiapterin-the precursor of nitric oxide synthase cofactor BH4-induces immunostimulatory-shift of breast cancer. Biochem. Pharm. 2020, 176, 113887.
- Virág, L.; Jaén, R.I.; Regdon, Z.; Boscá, L.; Prieto, P. Self-defense of macrophages against oxidative injury: Fighting for their own survival. Redox Biol. 2019, 26, 101261.
- Ibiza, S.; Víctor, V.M.; Boscá, I.; Ortega, A.; Urzainqui, A.; O’Connor, J.E.; Sánchez-Madrid, F.; Esplugues, J.V.; Serrador, J.M. Endothelial Nitric Oxide Synthase Regulates T Cell Receptor Signaling at the Immunological Synapse. Immunity. 2006, 24, 753–765.
- Garcia-Ortiz, A.; Serrador, J.M. Nitric Oxide Signaling in T Cell-Mediated Immunity. Trends Mol. Med. 2018, 24, 412–427.
- Iwakiri, Y.; Satoh, A.; Chatterjee, S.; Toomre, D.K.; Chalouni, C.M.; Fulton, D.; Groszmann, R.J.; Shah, V.H.; Sessa, W.C. Nitric oxide synthase generates nitric oxide locally to regulate compartmentalized protein S-nitrosylation and protein trafficking. Proc. Natl. Acad. Sci. USA 2006, 103, 19777–19782.
- Yang, Z.; Wang, Z.E.; Doulias, P.T.; Wei, W.; Ischiropoulos, H.; Locksley, R.M.; Liu, L. Lymphocyte development requires S-nitrosoglutathione reductase. J. Immunol. 2010, 185, 6664–6669.
- Li, J.; Zhang, Y.; Zhang, Y.; Lü, S.; Miao, Y.; Yang, J.; Huang, S.; Ma, X.; Han, L.; Deng, J.; et al. GSNOR modulates hyperhomocysteinemia-induced T cell activation and atherosclerosis by switching Akt S-nitrosylation to phosphorylation. Redox Biol. 2018, 17, 386–399.
- Paul, S.; Lal, G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1124.
- Cifone, M.G.; Ulisse, S.; Santoni, A. Natural killer cells and nitric oxide. Int. Immunopharmacol. 2001, 1, 1513–1524.
- Jyothi, M.D.; Khar, A. Induction of nitric oxide production by natural killer cells: Its role in tumor cell death. Nitric Oxide 1999, 3, 409–418.
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510.
- Huxford, T.; Ghosh, G. A structural guide to proteins of the NF-kappaB signaling module. Cold Spring Harb. Perspect. Biol. 2009, 1, a000075.
- Zheng, C.; Yin, Q.; Wu, H. Structural studies of NF-kappaB signaling. Cell Res. 2011, 21, 183–195.
- Pahl, H.L. Activators and target genes of Rel/NF-κB transcription factors. Oncogenet 1999, 18, 6853–6866.
- Caviedes, A.; Maturana, B.; Corvalán, K.; Engler, A.; Gordillo, F.; Varas-Godoy, M.; Smalla, K.-H.; Batiz, L.F.; Lafourcade, C.; Kaehne, T.; et al. eNOS-dependent S-nitrosylation of the NF-κB subunit p65 has neuroprotective effects. bioRxiv 2020.
- Carpenter, R.L.; Lo, H.-W. STAT3 Target Genes Relevant to Human Cancers. Cancers 2014, 6, 897–925.
- Wang, Y.; Shen, Y.; Wang, S.; Shen, Q.; Zhou, X. The role of STAT3 in leading the crosstalk between human cancers and the immune system. Cancer Lett. 2018.
- Burdelya, L.; Kujawski, M.; Niu, G.; Zhong, B.; Wang, T.; Zhang, S.; Kortylewski, M.; Shain, K.; Kay, H.; Djeu, J.; et al. Stat3 Activity in Melanoma Cells Affects Migration of Immune Effector Cells and Nitric Oxide-Mediated Antitumor Effects. J. Immunol. 2005, 174, 3925.
- Butturini, E.; Carcereri de Prati, A.; Mariotto, S. Redox Regulation of STAT1 and STAT3 Signaling. Int. J. Mol. Sci. 2020, 21, 7034.
- Hillmer, E.J.; Zhang, H.; Li, H.S.; Watowich, S.S. STAT3 signaling in immunity. Cytokine Growth Factor Rev. 2016, 31, 1–15.
- Lee, H.; Pal, S.K.; Reckamp, K.; Figlin, R.A.; Yu, H. STAT3: A target to enhance antitumor immune response. Curr. Top. Microbiol. Immunol. 2011, 344, 41–59.
- Zou, S.; Tong, Q.; Liu, B.; Huang, W.; Tian, Y.; Fu, X. Targeting STAT3 in Cancer Immunotherapy. Mol. Cancer 2020, 19, 145.
- Julien, O.; Wells, J.A. Caspases and their substrates. Cell Death Differ. 2017, 24, 1380–1389.
- Niu, Z.; Tang, J.; Zhang, W.; Chen, Y.; Huang, Y.; Chen, B.; Li, J.; Shen, P. Caspase-1 promotes monocyte–macrophage differentiation by repressing PPARγ. FEBS J. 2017, 284, 568–585.
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, new and emerging functions of caspases. Cell Death. Differ. 2015, 22, 526–539.
- Xu, D.C.; Arthurton, L.; Baena-Lopez, L.A. Learning on the Fly: The Interplay between Caspases and Cancer. BioMed Res. Int. 2018, 2018, 5473180.
- Vande Walle, L.; Lamkanfi, M. Inflammasomes: Caspase-1-Activating Platforms with Critical Roles in Host Defense. Front. Microbiol. 2011, 2.
- Devarajan, E.; Sahin, A.A.; Chen, J.S.; Krishnamurthy, R.R.; Aggarwal, N.; Brun, A.M.; Sapino, A.; Zhang, F.; Sharma, D.; Yang, X.H.; et al. Down-regulation of caspase 3 in breast cancer: A possible mechanism for chemoresistance. Oncogenet 2002, 21, 8843–8851.
- Ponder, K.G.; Boise, L.H. The prodomain of caspase-3 regulates its own removal and caspase activation. Cell Death Discov. 2019, 5, 56.
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674.
- Huang, Q.; Li, F.; Liu, X.; Li, W.; Shi, W.; Liu, F.-F.; O’Sullivan, B.; He, Z.; Peng, Y.; Tan, A.-C.; et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat. Med. 2011, 17, 860–866.
- Li, F.; Huang, Q.; Chen, J.; Peng, Y.; Roop, D.R.; Bedford, J.S.; Li, C.-Y. Apoptotic cells activate the “phoenix rising” pathway to promote wound healing and tissue regeneration. Sci. Signal 2010, 3, ra13-ra13.
- Sessa, W.C. eNOS at a glance. J. Cell Sci. 2004, 117, 2427–2429.
- Tonini, T.; Rossi, F.; Claudio, P.P. Molecular basis of angiogenesis and cancer. OncoGene 2003, 22, 6549–6556.
- Kostourou, V.; Cartwright, J.E.; Johnstone, A.P.; Boult, J.K.; Cullis, E.R.; Whitley, G.; Robinson, S.P. The role of tumour-derived iNOS in tumour progression and angiogenesis. Br. J. Cancer 2011, 104, 83–90.
- Lee, J.W.; Bae, S.H.; Jeong, J.W.; Kim, S.H.; Kim, K.W. Hypoxia-inducible factor (HIF-1)alpha: Its protein stability and biological functions. Exp. Mol. Med. 2004, 36, 1–12.
- Semenza, G.L. HIF-1: Upstream and downstream of cancer metabolism. Curr. Opin. Genet. Dev. 2010, 20, 51–56.
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-Inducible Factor-1 Is a Basic-Helix-Loop-Helix-Pas Heterodimer Regulated by Cellular O-2 Tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514.
- Gilkes, D.M.; Semenza, G.L. Role of hypoxia-inducible factors in breast cancer metastasis. Future Oncol. 2013, 9, 1623–1636.
- Li, Z.; Bao, S.; Wu, Q.; Wang, H.; Eyler, C.; Sathornsumetee, S.; Shi, Q.; Cao, Y.; Lathia, J.; McLendon, R.E.; et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell 2009, 15, 501–513.
- Mimeault, M.; Batra, S.K. Hypoxia-inducing factors as master regulators of stemness properties and altered metabolism of cancer- and metastasis-initiating cells. J. Cell Mol. Med. 2013, 17, 30–54.
- Rankin, E.B.; Giaccia, A.J. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ. 2008, 15, 678–685.
- Gao, Y.; Zhou, S.; Xu, Y.; Sheng, S.; Qian, S.Y.; Huo, X. Nitric oxide synthase inhibitors 1400W and L-NIO inhibit angiogenesis pathway of colorectal cancer. Nitric Oxide 2019, 83, 33–39.
- Gao, Y.; Zhou, S.; Pang, L.; Yang, J.; Li, H.J.; Huo, X.; Qian, S.Y. Celastrol suppresses nitric oxide synthases and the angiogenesis pathway in colorectal cancer. Free Radic. Res. 2019, 53, 324–334.
- Herrmann, H.; Aebi, U. Intermediate filaments: Molecular structure, assembly mechanism, and integration into functionally distinct intracellular Scaffolds. Annu. Rev. Biochem. 2004, 73, 749–789.
- Hynes, R.O.; Naba, A. Overview of the matrisome–an inventory of extracellular matrix constituents and functions. Cold Spring Harb. Perspect. Biol. 2012, 4, a004903.
- Buchheit, C.L.; Weigel, K.J.; Schafer, Z.T. Cancer cell survival during detachment from the ECM: Multiple barriers to tumour progression. Nat. Rev. Cancer 2014, 14, 632–641.
- Chiarugi, P.; Giannoni, E. Anoikis: A necessary death program for anchorage-dependent cells. Biochem. Pharmacol. 2008, 76, 1352–1364.
- Paoli, P.; Giannoni, E.; Chiarugi, P. Anoikis molecular pathways and its role in cancer progression. BBA Mol. Cell Res. 2013, 1833, 3481–3498.
- Berrier, A.L.; Yamada, K.M. Cell-matrix adhesion. J. Cell Physiol. 2007, 213, 565–573.
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687.
- Miranti, C.K.; Brugge, J.S. Sensing the environment: A historical perspective on integrin signal transduction. Nat. Cell Biol. 2002, 4, E83–E90.
- Parsons, J.T.; Martin, K.H.; Slack, J.K.; Taylor, J.M.; Weed, S.A. Focal adhesion kinase: A regulator of focal adhesion dynamics and cell movement. Oncogenet 2000, 19, 5606–5613.
- da Costa, P.E.; Batista, W.L.; Moraes, M.S.; Stern, A.; Monteiro, H.P. Src kinase activation by nitric oxide promotes resistance to anoikis in tumour cell lines. Free Radic. Res. 2018, 52, 592–604.
- Bernatchez, P.N.; Bauer, P.M.; Yu, J.; Prendergast, J.S.; He, P.; Sessa, W.C. Dissecting the molecular control of endothelial NO synthase by caveolin-1 using cell-permeable peptides. Proc. Natl. Acad. Sci. USA 2005, 102, 761–766.
- Zhao, Y.-Y.; Zhao, Y.D.; Mirza, M.K.; Huang, J.H.; Potula, H.-H.S.K.; Vogel, S.M.; Brovkovych, V.; Yuan, J.X.J.; Wharton, J.; Malik, A.B. Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. J. Clin. Investig. 2009, 119, 2009–2018.
- Bakhshi, F.R.; Bonini, M.; Chen, Z.; NieuwAmerongen, G.; Comhair, S.; Erzurum, S.; Minshall, R. Role of Caveolin-1 S-Nitrosylation, Ubiquitination, and Degradation in Idiopathic Pulmonary Arterial Hypertension. FASEB J. 2013, 27, 878.877–878.877.
- Chen, Z.; D S Oliveira, S.; Zimnicka, A.M.; Jiang, Y.; Sharma, T.; Chen, S.; Lazarov, O.; Bonini, M.G.; Haus, J.M.; Minshall, R.D. Reciprocal regulation of eNOS and caveolin-1 functions in endothelial cells. Mol. Biol. Cell 2018, 29, 1190–1202.
- Bailey, K.M.; Liu, J. Caveolin-1 up-regulation during epithelial to mesenchymal transition is mediated by focal adhesion kinase. J. Biol. Chem. 2008, 283, 13714–13724.
- Lamaze, C.; Torrino, S. Caveolae and cancer: A new mechanical perspective. Biomed. J. 2015, 38, 367–379.
- Bakker, E.N.; Pistea, A.; VanBavel, E. Transglutaminases in vascular biology: Relevance for vascular remodeling and atherosclerosis. J. Vasc. Res. 2008, 45, 271–278.
- Santhanam, A.V.; Smith, L.A.; Katusic, Z.S. Brain-derived neurotrophic factor stimulates production of prostacyclin in cerebral arteries. Stroke 2010, 41, 350–356.
- Tabolacci, C.; De Martino, A.; Mischiati, C.; Feriotto, G.; Beninati, S. The Role of Tissue Transglutaminase in Cancer Cell Initiation, Survival and Progression. Med. Sci. 2019, 7, 19.
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 2020, 368, 973–980.
- Riquelme, E.; Zhang, Y.; Zhang, L.; Montiel, M.; Zoltan, M.; Dong, W.; Quesada, P.; Sahin, I.; Chandra, V.; San Lucas, A.; et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 2019, 178, 795–806.
- Gopalakrishnan, V.; Helmink, B.A.; Spencer, C.N.; Reuben, A.; Wargo, J.A. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell 2018, 33, 570–580.
- Laniewski, P.; Cui, H.; Roe, D.J.; Chase, D.M.; Herbst-Kralovetz, M.M. Vaginal microbiota, genital inflammation, and neoplasia impact immune checkpoint protein profiles in the cervicovaginal microenvironment. NPJ Precis. Oncol. 2020, 4, 22.
- Bashiardes, S.; Tuganbaev, T.; Federici, S.; Elinav, E. The microbiome in anti-cancer therapy. Semin. Immunol. 2017, 32, 74–81.
- Seth, P.; Hsieh, P.N.; Jamal, S.; Wang, L.; Gygi, S.P.; Jain, M.K.; Coller, J.; Stamler, J.S. Regulation of MicroRNA Machinery and Development by Interspecies S-Nitrosylation. Cell 2019, 176, 1014–1025.e1012.
- Urbaniak, C.; Cummins, J.; Brackstone, M.; Macklaim, J.M.; Gloor, G.B.; Baban, C.K.; Scott, L.; O’Hanlon, D.M.; Burton, J.P.; Francis, K.P.; et al. Microbiota of human breast tissue. Appl. Environ. Microbiol. 2014, 80, 3007–3014.
- Urbaniak, C.; Burton, J.P.; Reid, G. Breast, milk and microbes: A complex relationship that does not end with lactation. Women Health 2012, 8, 385–398.
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K.; et al. Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr. 2017, 171, 647–654.
- Urbaniak, C.; Gloor, G.B.; Brackstone, M.; Scott, L.; Tangney, M.; Reid, G. The Microbiota of Breast Tissue and Its Association with Breast Cancer. Appl. Environ. Microbiol. 2016, 82, 5039–5048.
- Fukumura, D.; Kashiwagi, S.; Jain, R.K. The role of nitric oxide in tumour progression. Nat. Rev. Cancer 2006, 6, 521–534.
- Jadeski, L.C.; Chakraborty, C.; Lala, P.K. Role of nitric oxide in tumour progression with special reference to a murine breast cancer model. Can. J. Physiol. Pharm. 2002, 80, 125–135.
- Lala, P.K.; Chakraborty, C. Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol. 2001, 2, 149–156.
- Thomsen, L.L.; Miles, D.W. Role of nitric oxide in tumour progression: Lessons from human tumours. Cancer Metastasis Rev. 1998, 17, 107–118.
- Lim, S.Y.; Raftery, M.; Cai, H.; Hsu, K.; Yan, W.X.; Hseih, H.L.; Watts, R.N.; Richardson, D.; Thomas, S.; Perry, M.; et al. S-nitrosylated S100A8: Novel anti-inflammatory properties. J. Immunol. 2008, 181, 5627–5636.
- Cianchi, F.; Cortesini, C.; Fantappie, O.; Messerini, L.; Schiavone, N.; Vannacci, A.; Nistri, S.; Sardi, I.; Baroni, G.; Marzocca, C.; et al. Inducible nitric oxide synthase expression in human colorectal cancer: Correlation with tumor angiogenesis. Am. J. Pathol. 2003, 162, 793–801.
- Thomas, D.D.; Wink, D.A. NOS2 as an Emergent Player in Progression of Cancer. Antioxid. Redox Signal. 2017, 26, 963–965.
- Lopez-Sanchez, L.M.; Corrales, F.J.; Lopez-Pedrera, C.; Aranda, E.; Rodriguez-Ariza, A. Pharmacological impairment of s-nitrosoglutathione or thioredoxin reductases augments protein S-Nitrosation in human hepatocarcinoma cells. AntiCancer Res. 2010, 30, 415–421.
- Davila-Gonzalez, D.; Choi, D.S.; Rosato, R.R.; Granados-Principal, S.M.; Kuhn, J.G.; Li, W.F.; Qian, W.; Chen, W.; Kozielski, A.J.; Wong, H.; et al. Pharmacological Inhibition of NOS Activates ASK1/JNK Pathway Augmenting Docetaxel-Mediated Apoptosis in Triple-Negative Breast Cancer. Clin. Cancer Res. 2018, 24, 1152–1162.
- Tang, C.H.; Wei, W.; Hanes, M.A.; Liu, L. Hepatocarcinogenesis driven by GSNOR deficiency is prevented by iNOS inhibition. Cancer Res. 2013, 73, 2897–2904.
- Tian, H.; Wang, J.; Zhang, B.; Di, J.; Chen, F.; Li, H.; Li, L.; Pei, D.; Zheng, J. MDA-7/IL-24 induces Bcl-2 denitrosylation and ubiquitin-degradation involved in cancer cell apoptosis. PLoS ONE 2012, 7, e37200.
- Kaliyaperumal, K.; Sharma, A.K.; McDonald, D.G.; Dhindsa, J.S.; Yount, C.; Singh, A.K.; Won, J.S.; Singh, I. S-Nitrosoglutathione-mediated STAT3 regulation in efficacy of radiotherapy and cisplatin therapy in head and neck squamous cell carcinoma. Redox Biol. 2015, 6, 41–50.
- Romagny, S.; Bouaouiche, S.; Lucchi, G.; Ducoroy, P.; Bertoldo, J.B.; Terenzi, H.; Bettaieb, A.; Plenchette, S. S-Nitrosylation of cIAP1 Switches Cancer Cell Fate from TNFalpha/TNFR1-Mediated Cell Survival to Cell Death. Cancer Res. 2018, 78, 1948–1957.
- Tan, G.; Qiu, M.; Chen, L.; Zhang, S.; Ke, L.; Liu, J. JS-K, a nitric oxide pro-drug, regulates growth and apoptosis through the ubiquitin-proteasome pathway in prostate cancer cells. BMC Cancer 2017, 17, 376.
- Chattopadhyay, M.; Goswami, S.; Rodes, D.B.; Kodela, R.; Velazquez, C.A.; Boring, D.; Crowell, J.A.; Kashfi, K. NO-releasing NSAIDs suppress NF-κB signaling in vitro and in vivo through S-nitrosylation. Cancer Lett. 2010, 298, 204–211.
- Kashfi, K. Nitric Oxide-Releasing Hybrid Drugs Target Cellular Processes Through S-Nitrosylation. Immunopathol. Dis. Ther. 2012, 3, 97–108.
- Granados-Principal, S.; Liu, Y.; Guevara, M.L.; Blanco, E.; Choi, D.S.; Qian, W.; Patel, T.; Rodriguez, A.A.; Cusimano, J.; Weiss, H.L.; et al. Inhibition of iNOS as a novel effective targeted therapy against triple-negative breast cancer. Breast Cancer Res. 2015, 17, 25.
- Tong, A.W.; Nemunaitis, J.; Su, D.; Zhang, Y.; Cunningham, C.; Senzer, N.; Netto, G.; Rich, D.; Mhashilkar, A.; Parker, K.; et al. Intratumoral injection of INGN 241, a nonreplicating adenovector expressing the melanoma-differentiation associated gene-7 (mda-7/IL24): Biologic outcome in advanced cancer patients. Mol. Ther. J. Am. Soc. Gene Ther. 2005, 11, 160–172.
- Rigas, B.; Williams, J.L. NO-donating NSAIDs and cancer: An overview with a note on whether NO is required for their action. Nitric Oxide Biol. Chem. 2008, 19, 199–204.
- Bonavida, B.; Garban, H. Nitric oxide-mediated sensitization of resistant tumor cells to apoptosis by chemo-immunotherapeutics. Redox Biol. 2015, 6, 486–494.
- Caneba, C.A.; Yang, L.; Baddour, J.; Curtis, R.; Win, J.; Hartig, S.; Marini, J.; Nagrath, D. Nitric oxide is a positive regulator of the Warburg effect in ovarian cancer cells. Cell Death Dis. 2014, 5, e1302.
- Lopez-Sanchez, L.M.; Aranda, E.; Rodriguez-Ariza, A. Nitric oxide and tumor metabolic reprogramming. Biochem. Pharm. 2020, 176, 113769.
- Drapier, J.C.; Hibbs, J.B., Jr. Aconitases: A class of metalloproteins highly sensitive to nitric oxide synthesis. Methods Enzymol. 1996, 269, 26–36.
- Piantadosi, C.A. Regulation of mitochondrial processes by protein S-nitrosylation. Biochim. Biophys. Acta 2012, 1820, 712–721.
- Yang, E.S.; Richter, C.; Chun, J.S.; Huh, T.L.; Kang, S.S.; Park, J.W. Inactivation of NADP(+)-dependent isocitrate dehydrogenase by nitric oxide. Free Radic. Biol. Med. 2002, 33, 927–937.
- Boveris, A.; Costa, L.E.; Poderoso, J.J.; Carreras, M.C.; Cadenas, E. Regulation of mitochondrial respiration by oxygen and nitric oxide. Ann. N. Y. Acad. Sci. 2000, 899, 121–135.
- Dahm, C.C.; Moore, K.; Murphy, M.P. Persistent S-nitrosation of complex I and other mitochondrial membrane proteins by S-nitrosothiols but not nitric oxide or peroxynitrite: Implications for the interaction of nitric oxide with mitochondria. J. Biol. Chem. 2006, 281, 10056–10065.
- Montagna, C.; Cirotti, C.; Rizza, S.; Filomeni, G. When S-Nitrosylation Gets to Mitochondria: From Signaling to Age-Related Diseases. Antioxid. Redox Signal. 2020, 32, 884–905.
- Rasola, A.; Neckers, L.; Picard, D. Mitochondrial oxidative phosphorylation TRAP(1)ped in tumor cells. Trends Cell Biol. 2014, 24, 455–463.
- Yoshida, S.; Tsutsumi, S.; Muhlebach, G.; Sourbier, C.; Lee, M.J.; Lee, S.; Vartholomaiou, E.; Tatokoro, M.; Beebe, K.; Miyajima, N.; et al. Molecular chaperone TRAP1 regulates a metabolic switch between mitochondrial respiration and aerobic glycolysis. Proc. Natl. Acad. Sci. USA 2013, 110, E1604–E1612.
- Chen, G.; Wang, F.; Trachootham, D.; Huang, P. Preferential killing of cancer cells with mitochondrial dysfunction by natural compounds. Mitochondrion 2010, 10, 614–625.
- Neuzil, J.; Dong, L.F.; Rohlena, J.; Truksa, J.; Ralph, S.J. Classification of mitocans, anti-cancer drugs acting on mitochondria. Mitochondrion 2013, 13, 199–208.
- Kashfi, K.; Rigas, B. Molecular targets of nitric-oxide-donating aspirin in cancer. Biochem. Soc. Trans. 2005, 33, 701–704.
- Alimoradi, H.; Greish, K.; Gamble, A.B.; Giles, G.I. Controlled Delivery of Nitric Oxide for Cancer Therapy. Pharm. Nanotechnol. 2019, 7, 279–303.
- Chegaev, K.; Riganti, C.; Lazzarato, L.; Rolando, B.; Guglielmo, S.; Campia, I.; Fruttero, R.; Bosia, A.; Gasco, A. Nitric Oxide Donor Doxorubicins Accumulate into Doxorubicin-Resistant Human Colon Cancer Cells Inducing Cytotoxicity. ACS Med. Chem. Lett. 2011, 2, 494–497.
- Murad, F. Cyclic guanosine monophosphate as a mediator of vasodilation. J. Clin. Investig. 1986, 78, 1–5.
