The application of plant extracts for therapeutic purposes has been used in traditional medicine because plants contain bioactive compounds with beneficial properties for health. Currently, the use of these compounds that are rich in polyphenols for the treatment and prevention of diseases such as cancer, diabetes, and cardiovascular diseases, many of them related to oxidative stress, is gaining certain relevance. Polyphenols have been shown to have antimutagenic, antioxidant, and anti-inflammatory properties.
- cancer cells
- polyphenols
- grape stem
- proteasome
- ROS
- TrxR1
1. Introduction
Grape stems are by-products generated in great quantity in the winemaking process, and their elimination causes environmental problems. Therefore, it is important to find strategies that allow the reuse of these products. This residue is a rich source of phenolic compounds, celluloses, hemicelluloses, and lignins [1,2,3,4,5,6]. Among them, phenolic compounds confer antioxidant properties to the extracts obtained from grape stems. For this reason, different studies have been conducted in order to determine the polyphenolic composition of grape stems, and several proanthocyanidins, anthocyanidins, flavonols, hydroxycinnamic acids, and stilbenes have been found. Among them, the most characteristic polyphenolic substances referred to in most of the studies are trans-resveratrol, ε-viniferin, caftaric acid, gallic acid, catechin (one of the most abundant polyphenols), epicatechin, malvidin derivatives, quercetin, and glycosylated derivatives of quercetin in position 3 [2,3,5,7].
According to current research results, grape stem extracts possess important biological activities with multiple benefits for human health due to antioxidant and anti-inflammatory properties [5,7,8,9,10,11]. Veskoukis et al. [10] found that this by-product is particularly rich in flavonoids and stilbenes, such as trans-resveratrol and viniferin, which are found in considerably high concentrations. These authors also found that such extracts exhibited significant antioxidant properties, and, even at low concentrations, they showed a strong ability to prevent the oxidation of low-density lipoprotein (LDL) and to reduce intracellular levels of reactive oxygen species (ROS). In this way, Gonzalez-Centeno et al. [12] and Veskoukis et al. [10] evaluated the total phenolic and total proanthocyanidin composition of different grape stem varieties, as well as their antioxidant potentials. Grape stem extracts also prevent ROS-induced DNA damage and have inhibitory activity against liver and cervical cancer cell growth, suggesting their potential as chemopreventive agents [13]. Using human epidermal keratinocytes, Domínguez-Perles et al. [14] observed a protective effect of grape stem extracts against oxidative stress. These researchers found a close correlation between the concentration of phenolic compounds in the extracts and the potential to regulate the redox balance in vitro, as well as the capacity of these extracts to efficiently modulate apoptosis in HaCaT keratinocytes. Cho et al. [15] studied the effect of the topical administration of grape stem extracts to mice skin before subjecting them to UVB radiation for three minutes, thrice a week for one month. These authors demonstrated that these extracts significantly inhibited oxidative damage induced by UVB radiation and observed decreased epidermal hyperplasia, melanin pigmentation, and collagen degradation in the skin of mice. In addition, grape pomace—consisting of peel, seed, stem, and pulps—is discarded during grape processing, including juice extraction and winemaking, despite its substantial phenolic content [8]. In this way, Del Pino-Garcia et al. [16] studied the chemopreventive potential of powdered red wine seasonings against colorectal cancer in HT-29 cells. Grape seed extracts have also been applied as photochemopreventive agents against UVB-induced skin cancer [17]. Likewise, Vitis vinifera extracts have shown an antidiabetic effect by inhibiting the enzyme glycogen phosphorylase [18].
On the other hand, grape stem extracts have significant antimicrobial activities that seem to be influenced by the structure and function of phenolic compounds, as well as by their interspecific relation with different bacterial strains [19,20]. For example, these types of extracts inhibited the growth of both Gram-positive (Listeria monocytogenes, Staphylococcus aureus and Enterococcus faecalis) and Gram-negative (Pseudomonas aeruginosa, Escherichia coli and Klebsiella pneumoniae) digestive pathogens under in vitro conditions [21], and their bioactive compounds are used in oral care [22].
In addition, the extracts could be used to control the presence of human pathogenic bacteria in fresh leafy vegetables [6]. Leal et al. [23] studied the potential of grape stem extracts from different white grape varieties as antimicrobial agents to reduce the use of antibiotics. These authors found that the bactericidal activity of the extracts was higher, in general, against Gram-positive than Gram-negative bacteria, although they used a different methodology from that of Dias et al. [21] to evaluate the antimicrobial activity (minimum inhibitory concentration vs. disc diffusion). Other studies have shown that grape stem extracts are highly effective against foot wound ulcers produced by Gram-positive bacteria, and they also have anti-inflammatory action, inhibiting the production of nitric oxide lipopolysaccharide-stimulated macrophages by up to 35.25% [24].
During the last few years, our research group has investigated the chemopreventive properties of extracts obtained from different plant matrices such as rosehips, fenugreek, pine bark, and artichoke waste on human colon cancer [3,25,26,27]. Though there have only been few publications on this subject, the anticarcinogenic potential of grape stem extracts has also been studied in different cell lines [13,28,29]. Additionally, grape stem extracts possess important bioactivities such as antiangiogenic properties [30].
However, the phenolic composition—and therefore the biological activity and efficacy—of a specific grape stem extract depends on the procedure used to obtain the extract. In a previous study, we selected an optimized extraction method for grape stems [3], and the extracts obtained by this method presented high antioxidant potential and were demonstrated to be good candidates for SO2 substitution in wines [4]. With this background, the aim of the present research was to complete the characterization of those grape stem extracts by studying their potential for the treatment of human colorectal adenocarcinoma (Caco-2) and human breast adenocarcinoma (MCF-7 and MDA-MB-231) cell lines. Thus, we measured the possible antiproliferative effects of these extracts on cancer cells and their mechanisms of action. Furthermore, the protective effects of these extracts, in a model of intestinal barrier (differentiated Caco-2 cells), were also tested through the measurement of the intracellular levels of ROS.
2. Phenolic Composition and Antioxidant Activity in Mazuelo Stem Extracts
The phenolic composition, as well as the total polyphenol and flavonoid contents, of Mazuelo stem extracts are presented in Table 1. In this extract, nine phenolic compounds were found, of which the most abundant were (+)-catechin and the quercetin-3-derivative. Likewise, Leal et al. [24] found that (+)-catechin was the most abundant phenolic compound in Portuguese grape stem extracts from different varieties (Tinta Roriz, Touriga Nacional, Castelão, Syrah, Arinto, and Fernão Pires). Anastasiadi et al. [11] also reported the presence of several phenolic compounds in grape stem extracts from six red and white varieties from Greece. In comparison to their results with our Mazuelo stem extract, trans-resveratrol, ε-viniferin, (+)-catechin, and caftaric acid coincide, (+)-catechin was found to be the most abundant in both studies. Regarding the concentrations of resveratrol and viniferin, these authors observed differences among varieties and vintages. Lambert et al. [41] analyzed the stilbene content of pruning canes of the Carignan variety, which is the name given in France to the Mazuelo variety. These authors found a higher amount of resveratrol and viniferin in their extracts (0.88 mg resveratrol/g extract and 0.97 mg viniferin/g extract), although it must be considered that grapevine canes are probably richer in stilbenes than grape stems [42,43]. In addition to the phenolic compounds found in the Mazuelo stems analyzed in this work, other compounds have been identified in grape stem extracts of different varieties. For instance, in stem extracts from Portuguese grapes, kaempferol and isorhamnetin were identified [2], and in grape stems from Greek varieties, ferulic, coumaric, caffeic, and syringic acids were identified [28].
Table 1. Phenolic composition (mg/g extract) and antioxidant capacity of the Mazuelo stem extract.
| Phenolic Composition & Antioxidant Capacity | Grape Stem Extract |
|---|---|
| Gallic acid | 0.21 ± 0.03 |
| Caftaric acid | 0.14 ± 0.03 |
| (+)-Catechin | 0.98 ± 0.20 |
| Quercetin | 0.05 ± 0.01 |
| Quercetin-derivative 1 | 0.91 ± 0.08 |
| Malvidin-3-glucoside | 0.10 ± 0.02 |
| Unknown anthocyanin 2 | 0.15 ± 0.02 |
| Trans-resveratrol | 0.26 ± 0.04 |
| Trans-ε-viniferin | 0.59 ± 0.09 |
| Total phenolic content 3 | 83 ± 2 |
| Total flavonoid content 4 | 2.6 ± 0.1 |
| Antioxidant capacity by DPPH 5 | 0.47 ± 0.04 |
1 Expressed as quercetin-3-glucoside; 2 expressed as malvidin-3-glucoside; 3 expressed as mg gallic acid/g extract; 4 expressed as mg quercetin/g extract; 5 expressed as mmol Trolox/g extract.
Regarding the antioxidant capacity measured by the DPPH assay (Table 1), the result of the Mazuelo stem extract was similar to that of the Syrah (0.44 ± 0.04 mmol Trolox/g) and Fernão Pires (0.55 ± 0.01 mmol Trolox/g) extracts and higher than that of the Castelão (0.31 ± 0.01 mmol Trolox/g) and Arinto (0.15 ± 0.01 mmol Trolox/g) varieties found by Leal et al. (2020).
3. Effect of Extracts From Grape Stem on Cancer Cells
3.1. Antiproliferative Activity
The toxicity of extracts from grape stems was evaluated on undifferentiated Caco-2, MCF-7, and MDA-MB-231 cells by an SRB assay. Initially, a range of concentrations of grape extracts (62.5, 125, 250, 500, and 1000 μg/mL) was tested. The concentrations chosen were in relation to previous work carried out by our research group with other plant extracts [25,36]. The IC50 was calculated in the different cell lines at two time-points of 48 and 72 h. However, in the MDA-MB-231 and MCF-7 cells, when treated for 72 h, this range was lethal in most concentrations and the IC50 could not be calculated, so the range was modified to decreased concentrations (range: 9, 18. 37.5, 75, and 200 μg/mL). These results suggest that cytotoxic effect of grape stem extracts (GSE) is concentration- and time-dependent and that Caco-2 cells are less sensitive to GSE at the highest incubation time. At 48 h, similar viability curves were obtained in the three different cell lines (Figure 1, Table 2).
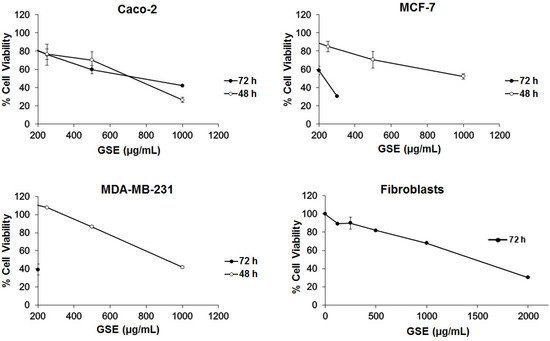
Table 2. IC50 (the concentration of compound that halves cell proliferation or viability) values of grape stem extracts on Caco-2, MCF-7, MDA-MB-231, and fibroblast cells after 72 and 48 h of incubation.
| IC50 (µg/mL) 72 h | IC50 (µg/mL) 48 h | Selectivity Index | |
|---|---|---|---|
| Caco-2 | 759 ± 51 | 661 ± 48 | 2.9 |
| MCF-7 | 203 ± 53 | 817 ± 52 * | 7.2 |
| MDA-MB-231 | 85 ± 9 | 911 ± 10 * | 17.0 |
| Fibroblast | 1454 ± 6 | - | - |
* p < 0.05; incubation time 48 vs. 72 h.
The results showed that the grape stem extracts were not selective for a single cancer line, but they produced a decrease in viability in the three tested cell lines (Figure 1). The effect was faster in Caco-2 cells, although their effectiveness was greater in breast cells (MCF-7 and MDA-MB-231) at longer times (72 h). In order to determine the action of these extracts on a noncancerous model, the IC50 was calculated on human fibroblast cells, after 72 h of incubation, where we observed a significantly lower effect. These data could be used to obtain a selectivity index (SI), as previously described by Badisa el al. [44]. The SI results are shown in Table 2, with the highest value being for the MDA-MB-231 cell line, according with the highest effective response of the extracts towards these cells after 72 h of incubation. The observed difference in the two breast cancer lines could have been due to the fact that the action of these extracts could be related to the receptors’ expression for estrogens, which are only present in MCF-7 cells [45].
3.2. Cell Death Studies
Since the grape stem extracts produce a reduction in cell viability, it was decided to determine what type of cell death occurred. Thus, flow cytometry analyses over 48 h were performed using biomarkers of cell death. The results showed that treatment for 48 h with the IC50 concentration corresponding to each cell line mainly produced early apoptosis in Caco-2 cells, while late apoptosis was mainly detected in MDA cells. However, no significant apoptosis was found in MCF-7 cells. Treatment with longer time (72 h) induced a significant death of these cells by late apoptosis (Figure 2). Therefore, the obtained results showed that grape stem extracts at their IC50 produced apoptosis in all tested cancerous cells by activating apoptotic pathways, thereby reducing their ability to non-selectively react with biological targets to cause necrosis and its related side effects.
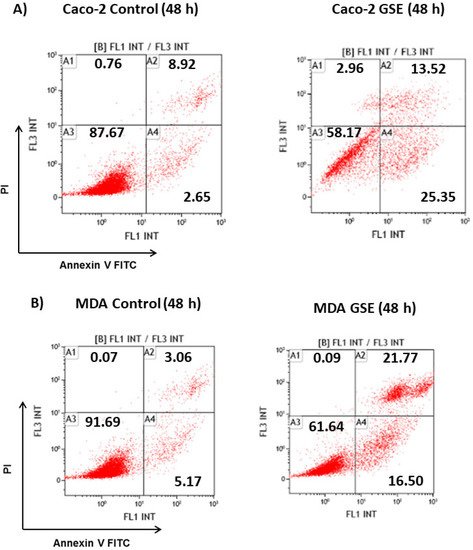
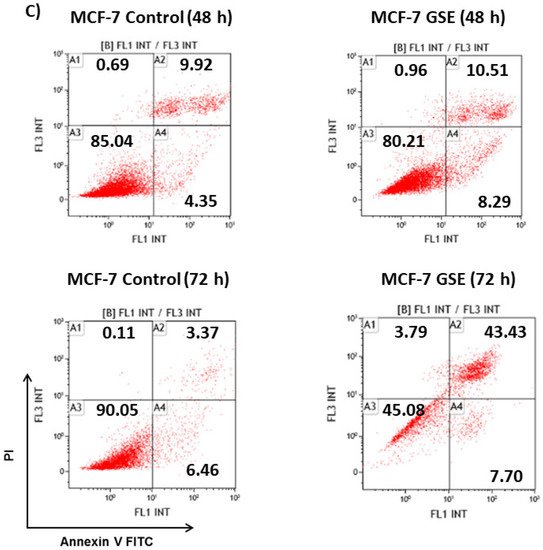
Figure 2. Analysis of the type of cell death induced on Caco-2 (A) MCF-7 (B), and MDA-MB-231 (C) after 48 or 72 h of incubation in control (untreated cells) and grape stem extracts (GSE) at IC50 (μg/mL) on Caco-2 (759), MCF-7 (203), and MDA-MB-231 (85). Percentages of live (A3), necrotic (A1), early apoptotic (A4), and late apoptotic (A2) cells are indicated.
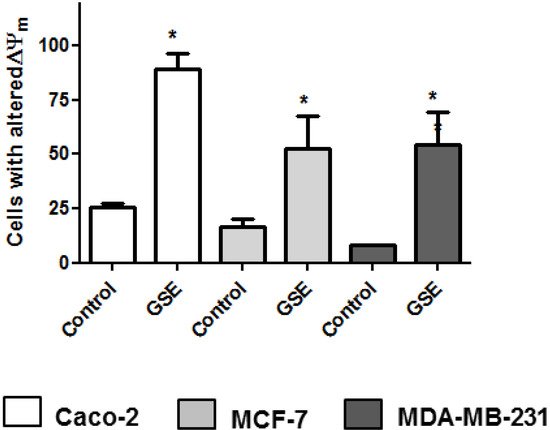
Since previous studies on plant extracts suggested mitochondrial dysfunction and intrinsic apoptosis induction [25,26], the mitochondrial membrane potential change was analyzed. Mitochondria play a pivotal role in life and cell death inasmuch as they produce the majority of the energy required for survival and regulate the intrinsic apoptosis pathway. The involvement of mitochondria in cell death is generally measured by following mitochondrial membrane depolarization [46]. The results showed that grape stem extracts significantly altered the mitochondrial potential of the tested cancer cells compared to the untreated ones (Figure 3); therefore, the changes in mitochondrial potential could be related to the observed apoptosis (Figure 2).
3.3. ROS Intracellular Levels
The oxidative stress imposed by ROS plays an important role in many chronic degenerative diseases and cancers. Higher levels of ROS are generated through an increase in metabolic activity of cancer cells including enhanced signaling pathways or mitochondrial dysfunction [47]. The ROS levels in the cells were determined based on the reaction between ROS and DCFH-DA. The assays were carried out by treating the cells with the grape stem extracts in the presence or absence of H2O2. Hydrogen peroxide is a widespread substance used to mimic the pro-oxidative environment that characterizes degenerative diseases such as cancer or neurodegenerative disorders on 2D cell cultures. The results showed that in Caco-2 cells, the extracts at a concentration of 750 μg/mL (IC50) were able to show a pro-oxidant effect after 24 h in both the absence and presence of hydrogen peroxide (Figure 4A,B). Therefore, this increase in oxidative stress caused by grape stem extracts, together with the significant change in the potential of the mitochondrial membrane, seems to be the cause of Caco-2 cell death by apoptosis. However, it must be considered that at low extract concentrations, a slight tendency to produce an antioxidant effect was observed, but there was no significant antiproliferative effect (data not shown). No modification of ROS levels was found in breast cells when they were treated with extracts at their respective IC50 and lower concentrations after 24 h (Figure 4A,B). Since breast cells seem to show a slower response to treatment with extracts after 24 h, (Table 2 and Figure 2) and previous results had shown a change in mitochondrial potential at 48 h (Figure 3), the ROS levels were measured at this time. The results showed a significant increase of ROS levels in both conditions (with/without H2O2) (Figure 4C,D).
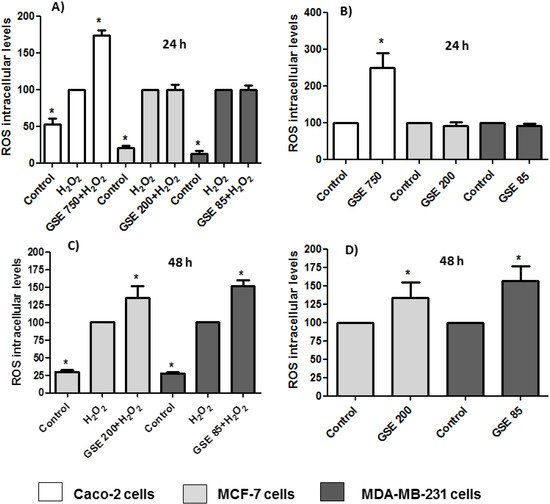
Figure 4. Measurements of reactive oxygen species (ROS) levels in the presence (A,C) or absence of H2O2 (80 mM, 20 min) (B,D) after 24 or 48 h of incubation with grape stem extracts (GSE) at IC50 (μg/mL) in Caco-2 (759), MCF-7 (203), and MDA-MB-231 (85). * p < 0.05 vs. respective control (untreated cells) (B,D) or vs. H2O2 (A,C).
The antioxidant effect of polyphenols has been extensively studied [38], although they may also have a pro-oxidant effect. These results have been mainly observed in tumor cells and have been related to pro-apoptotic action. The dual pro-oxidant and antioxidant behavior of phenolic plant compounds depends not only on the cell type but also on their concentration, chemical structure, and pH status [48,49].
3.4. Proteasome Activity
The ubiquitin/proteasome system (UPS) is a complex molecular machinery that constitute the main proteolytic pathway in eukaryotic cells. The UPS is involved in the regulation of basic biological process such as cell growth, proliferation, cell cycle, and apoptosis [50], and the dysregulation of these processes causes malignant transformation. Therefore, several cancer cells have a dysfunctional UPS with an increased activity of the proteasome [51], and various studies have shown that the inhibition of the proteasome in cancer cells may lead to the accumulation of inhibitors of cyclin-dependent kinases, pro-apoptotic proteins, and tumor suppressor proteins, leading to programmed cell death or apoptosis [52,53].
NF-kB proteins in the cytoplasm are associated with inhibitory proteins known as IkBs. The main activated form of NK-kB is a heterodimer composed of p65 and p50 subunits. NF-kB activation involves the phosphorylation of IkBs, after which it is ubiquitinated and degraded by the proteasome. Then the resulting free NF-kB is translocated to the nucleus, where it binds to kB-binding sites in the DNA and induces the transcriptions of several mediators.
To analyze whether the grape stem extracts were able to interact with the proteasome, which is involved in the activation of the NF-kB factor and its translocation to the nucleus, its activity was determined by a fluorometric assay. The results showed an increase in the cells’ proteasomal CT-L activity after 24 h of treatment with grape stem extracts (Figure 5). ROS often stimulates the NF-kB pathway in the cytoplasm but inhibits NF-kB in the nucleus [54]. In the cytoplasm, ROS have been shown to activate NF-kB through the alternative phosphorylation of IkBα, which may or may not result in the degradation of IkBα. Furthermore, ROS can influence the DNA-binding properties of the NF-kB proteins themselves. The oxidation of p50 in its DNA-binding domain has been shown to prevent its binding to DNA and, therefore, the activation of the NF-kB factor [55]. Therefore, the increase in ROS levels produced by grape stem extracts could oxidize the p50 subunit of the NF-kB factor, thus inhibiting its binding to nuclear DNA and causing an upregulation of the proteasome (Figure 5A).
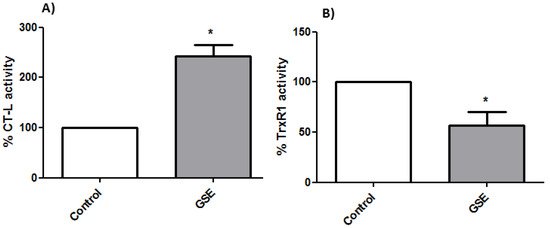
Figure 5. (A) Determinations of proteasomal chymotrypsin-like (CT-L) activity and (B) thioredoxin reductase 1 (TrxR1) activity from Caco-2 cells after 24 h of incubation with GSE to the IC50 concentration (759 μg/mL) * p < 0.05 vs. negative control (without treatment).
3.5. TrxR1 Activity
The thioredoxin system is one of the most important antioxidant systems in mammalian cells, and it is constituted by thioredoxin (Trx), the enzyme TrxR, and NADPH. Though the principal function of the thioredoxin system is controlling intracellular redox homeostasis and repairing oxidative damage, it is also implicated in cell growth and apoptosis control [56]. The overexpression of Trx has been shown to diminish NF-kB activation by inhibiting IkB degradation and can reverse the inhibition of p50 DNA binding caused by an increased amount of ROS [57]. However, in the present study, TrxR1 activity was found to be lower in treated cells, and this fact could explain the high levels of ROS found after treating the cells with the extracts (Figure 5B).
Therefore, the effect of the extracts on the viability of Caco-2 cells seemed to be related to an increase in ROS levels by an inhibition of TrxR1 that indirectly caused an upregulation in the proteasome due to the inhibition of NF-kB activity.
This entry is adapted from the peer-reviewed paper 10.3390/antiox10020243
