A relevant aspect for the development of novel chemotherapeutics to improve the currently used clinical protocols is to adopt reliable strategies that, in principle, could ensure a cheap and less time-consuming path toward advanced preclinical evaluations and clinical trials. In this view, on the basis of selected examples, we address four strategies we have pursued in recent years with the aim of improving metal-based chemotherapeutic treatments.
The Case of Auranofin
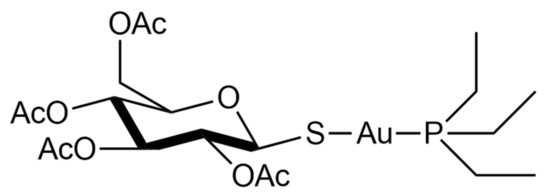
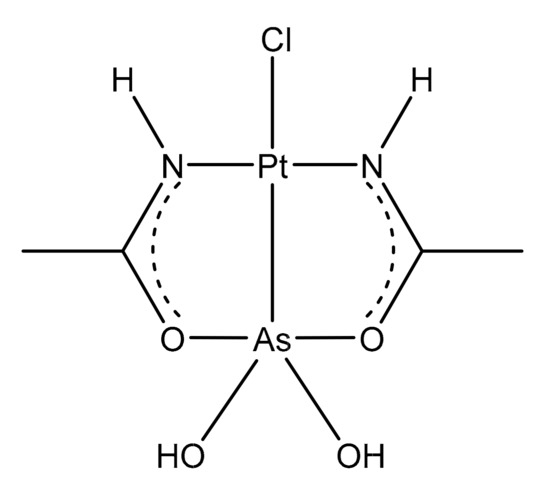
Auranofin, namely, [2,3,4,6-tetra-o-acetyl-L-thio-β-D-glycol-pyranoses-S-(triethyl-phosphine)-gold(I)], is a simple gold(I) compound, orally administered as an antirheumatic agent whose brand name is Ridaura® ().
Figure 1. Chemical structure of auranofin [2,3,4,6-tetra-o-acetyl-L-thio-β-D-glycol-pyranoses-S-(triethyl-phosphine)-gold(I)].
Auranofin was approved in 1985 and is reputed to be relatively safe and well-tolerated [
21]. Its mode of action is not fully understood, but it possesses anti-inflammatory properties and a high affinity to the specific biological target-bearing solvent-accessible sulfur or selenium residues [
22,
23,
24,
25]. In particular, it is a strong inhibitor of the TrxR system [
26]. This inhibition likely occurs through selective coordination of the gold(I) center at the level of the –Cys-Sec– redox-active motif of the enzyme [
25,
27,
28,
29]. Due to these features, it has been recently repurposed as an antiviral, antiparasitic, antibacterial, and anticancer agent [
21].
In recent decades, the discovery rate of novel antibiotics has slowed and the overall number of newly approved molecules has been low. As a result, in addition to the severe problem of the emergence of bacterial strains with multidrug-resistant (MDR) and extensively drug-resistant (XDR) phenotypes, the need for improved and readily available drugs is a critical issue in order to fight this global threat [
30].
The antibacterial properties of gold have been known for centuries [
31], and between the 19th and 20th centuries, its dicyanide complex, K[Au(CN)
2], was studied by the Nobel laureate Robert Koch for the treatment of tubercle bacillus infections. In subsequent years, he performed studies aiming to assess the antibacterial properties of gold and the possibility of application against several pathogens [
32]. More recently, the use of auranofin has been extensively reconsidered in view of its possible repurposing as an antibacterial agent [
33].
In 2013, we began to test and evaluate this drug against various strains, both Gram-positive and Gram-negative. In an early study, we evaluated auranofin against two reference strains, i.e.,
E. coli ATCC 25922 and
S. aureus ATCC 25923, being representative of Gram-negative and Gram-positive strains, respectively [
34]. The obtained results showed that auranofin possesses potent concentration-dependent antibacterial activity against
staphylococcus, providing the opportunity for further testing against, in particular, severe bacterial infections due to resistant
staphylococci. In the same study, we noted that auranofin was far less effective, or ineffective, in the case of the Gram-negative strain [
34]. Because the reduced activity in the case of the Gram-negative model is likely the result of a reduced internalization of the complex [
35,
36,
37], we carried out a second and more detailed study in which auranofin and a panel of its analogs bearing different ligands in place of thiosugar or silver in place of gold, respectively, were comparatively tested against clinical isolates including major Gram-positive, Gram-negative and other pathogens, such as fungal pathogens showing relevant resistance phenotypes [
38]. Additionally, to gather precise information about the mechanisms responsible for the reduced activity against the Gram-negative isolates, we performed experiments using auranofin and the other complexes in the presence of a permeabilizing agent, i.e., polymyxin B nonapeptide. Based on the overall integration of the obtained results, it was noted that auranofin possesses good stability in physiological-like conditions and the presence of thiosugar is not mandatory for the pharmacological effects, confirming the previous finding that the real pharmacophore is the cationic fragment [Au(PEt
3)]
+. Moreover, according to findings from other groups, we were able to confirm that the lower effect in the Gram-negative strains was likely related to a decreased drug uptake [
38].
An important paper by Jackson-Rosario and Self, published in 2009, reported that the antimicrobial action of auranofin against
Treponema denticola is likely mediated by the inhibition of selenium metabolism, which is crucial for the synthesis of selenoproteins [
39]. Basically, the high affinity of gold for selenium makes auranofin highly reactive toward this element. In turn, this reactivity subtracts the selenium available to the bacteria for the synthesis of key proteins with consequent inhibition of bacterial growth.
Beyond its recognized activity for the treatment of bacterial infections, auranofin entered phase II clinical trials (ClinicalTrials.gov Identifier: NCT02736968) for Giardia Protozoa (giardiasis) infection, whose treatment primarily relies on the use of 5-nitro imidazole antimicrobials such as metronidazole. However, even in this case resistance is a critical issue. Importantly, according to preclinical studies, auranofin is not only active against this pathogen, but it is also capable of overcoming resistance and is particularly active in metronidazole-resistant strains [
40].
Auranofin has also been investigated for the treatment of HIV infections. In preclinical models, it was able to interfere with several phases of the viral cycle. The high susceptibly to auranofin of various subsets of cells involved in both HIV production and persistence has been demonstrated. Overall, auranofin strongly perturbs, through its pro-apoptotic effects, the activation and differentiation stages of CD4+ T lymphocytes, whose role is strictly connected to the viral production, latency, and viral reactivation [
41]. Consequently, two clinical trials involving auranofin for HIV treatment have begun (ClinicalTrials.gov, Identifiers: NCT02961829 and NCT02176135). Furthermore, the antiviral activity of auranofin prompted us to suggest its evaluation for the treatment of COVID-19 infections within the wide drug repositioning program that was initiated to find effective drugs to counter the rapid spread of the pandemic [
5,
42].
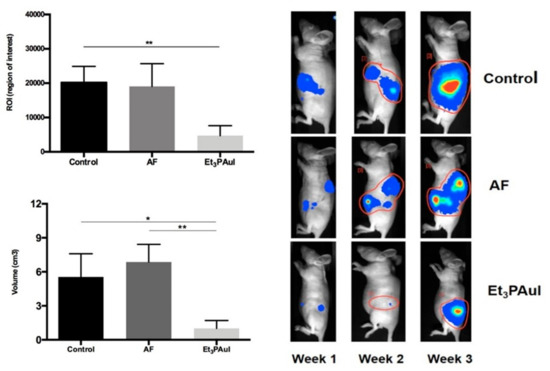
Another field in which gold compounds, and more precisely auranofin, are being repurposed with remarkable results is that of anticancer chemotherapy [
43,
44,
45,
46,
47]. Indeed, auranofin entered at least seven clinical trials as an anticancer drug for the treatment of ovarian, lung, glioblastoma, and chronic lymphocytic leukemia (ClinicalTrials.gov Identifiers: NCT01747798; NCT02126527; NCT03456700; NCT01737502; NCT02063698; NCT01419691; NCT02770378). Recently, we investigated the in vitro activity of auranofin in a panel of four colorectal cancer cell lines, namely, HCT8, HCT116, HT29, and Caco2. The compound was comparatively tested for its effects in the normal cell lines HDFa (human dermal fibroblast, adult) and HEK293 (human embryonic kidney) [
28]. Results revealed that auranofin exerts a potent cytotoxic effect against these cell lines, reducing the IC
50 values to the sub-micromolar range; in contrast, no cytotoxic effects were found in the normal cell lines, highlighting the selectivity for cancer cells. Overall, these results may warrant the assessment of auranofin in clinical trials for colorectal cancer.
Next, using high-resolution mass spectrometry, ethidium bromide displacement, and viscosity experiments, it was possible to demonstrate that the compound does not react toward single or double-stranded DNA models, strengthening the evidence that the targets for its anticancer action are likely non-genomic. This concept was further confirmed by inhibition experiments of TrxR. This enzyme is important for the maintenance of the redox balance of cells. Indeed, the impairment of the TrxR system may result in the loss of the redox homeostasis, which in turn may lead to oxidative stress and apoptosis [
28]. Results from the
in vitro screening highlighted a good correlation between the IC
50 values for the anticancer activity of auranofin in the colorectal cancer cells and the IC
50 for TrxR inhibition. This latter evidence can be explained based on the interaction between the [Au(PEt
3)]
+ cation and the TrxR redox-active site. This reaction pattern is in perfect agreement with those reported in the case of auranofin interaction towards other thiol-owning proteins and peptides [
23,
24].