Graphene quantum dots (GQDs) are small fragments of graphene with lateral dimensions less than 100 nm, with properties deriving from both graphene and carbon points.
- graphene quantum dots
- electrochemical sensors
- biomass
- green synthesis
1. Introduction
Over the last thirty years, both academic and industrial chemical research has increasingly oriented towards a holistic vision focused on pollution, use of renewable sources, and waste reduction, leading to the generation of a new concept of chemistry, called Green Chemistry, which with its 12 principles aims to redirect the chemical industry along paths of eco-sustainability. Indeed, sustainable development, which has become increasingly central to scientific and technological progress in the last century, requires chemistry to play a primary role in the conversion of old technologies into new “clean” processes and in the design of new products and new processes that are more eco-friendly, breaking the old paradigms based on the generation of large amounts of waste and the wide use of petrochemicals. One of the most important goals of green chemistry and resource efficiency, as stated in the seventh principle, is the design and development of synthetic approaches with low environmental impact, without the use of harmful solvents. On this account, renewable feedstocks, such as biomasses, constituted of a multifaceted array of low and high molecular weight products, such as sugars, hydroxy and amino acids, and biopolymers such as cellulose, hemicelluloses, or other raw materials easily obtainable from natural sources represent the right direction for sustainable production of fuels and novel advanced functional materials, as opposed to unsustainable production from non-renewable fossil resources such as oil, coal and natural gas [1].
Among advanced functional materials, carbon, one of the most abundant elements in the biosphere, plays a crucial role in the development of high-performance and sustainable materials. It is well known that carbon-based materials comprise the most effective properties among all the resources on the earth, such as light weight, high porosity, high-temperature resistance, acid and alkali resistance, good structural stability, and easy conductivity. The above-mentioned characteristics, together with the small background current, the wide potential window, and good electro-catalytic performance have made carbon materials effective in many applications and devices with unlimited possibilities for development [2].
GQDs are newly emerging members of the carbon materials family. GQDs are small fragments of graphene with lateral dimensions less than 100 nm, with properties deriving from both graphene and carbon points [3]. In addition to biocompatibility [4] and low toxicity [5,6]. GQDs have characteristics that make them ideal candidates for use in various fields. The high surface area and abundance of functional groups, as well as their easy functionalization with organic, inorganic, or biological molecules [7], has led to the use of GQDs as electrode modifiers. Moreover, they are chemically stable, water-soluble, robust, inert, and photo-stable against blinking and photo-bleaching [8]. Their solubility in water-based solvents has influenced their application in the field of bio-imaging [9,10] and targeted drug delivery [11]. GQDs exhibit attractive optical absorption properties with a peak between 260 and 380 nm making them ideal candidates for the fabrication of photodetectors or optoelectronic devices [3,12]. Another important feature is the excellent photoluminescence property (PL): normally, the quantum yield PL (QY) is high thanks to the crystallinity and the presence of layers in the structure of GQDs. GQDs have high-speed electron transport due to quantum confinement and an edge effect that directly affect electrical conductivity [13,14]. GQDs can act as a good sensing material due to their high electron movement with a high-speed reaction, making them excellent candidates for sensing applications. Moreover, GQDs possess peroxidase mimetic activity originating from their aromatic structure and this explains the strong interest in the development of electrocatalytic H2O2 detectors [15]. Therefore, taking into account the above mentioned electrochemical properties, great research interest in the use of GQDs for the design of novel electrode materials, not only in the field of fuel cells [16], supercapacitors [17] and photovoltaic cells [18], but also in the field of electrochemical immunosensors for biomedical applications [8] and biosensors [19], has been recently shown. As well as other carbonaceous materials, GQDs are conventionally synthesized from fossil feedstocks such as oil, coal, and petroleum coke and often require energy-intensive synthetic routes and severe process conditions [20]. On the contrary, biomasses or their constituents, such as carbohydrate or organic acids, characterized by their high availability, biodegradability, and low cost, are the only renewable carbon sources and crucial precursors of carbonaceous materials. Moreover, although to date only a few data are available regarding the costs of producing GQDs from renewable precursors, they are expected to have a much lower economic impact than conventional feedstocks (CNts, graphite, etc.), since the various functional groups already existing in the structure of biomass makes the fragmentation easier, related to the dense well-ordered single component graphene or CNTs. On the other hand, the conventional management of biomass waste involves noticeable economic and environmental problems, since traditional disposal strategies, such as incineration or landfilling, are insufficient in terms of environmental impacts, human health, and energy efficiency [21]. Indeed, the development of green routes to obtain GQDs, derived not only from renewable resources, such as lignocellulosic biomass waste, but also from other natural products present in food/agricultural waste (i.e., carbohydrates, lignin, proteins, etc.), not in competition with food suppliers, and without the use of any passivating, reducing, oxidizing agents or organic solvents, is a hot research topic of the 21st century.
2. GQDs from Eco-Friendly Raw Materials by Green Approaches
The synthesis methods of GQDs are generally classified into two groups, based on the reaction mechanism involved: top-down and bottom-up. The top-down approach consists of cutting down large graphene sheets, carbon nanotubes, carbon fibers, or graphite into small pieces of graphene sheet. Since top-down processes involve the conversion of macromolecules using physical forces into smaller ones, the main reaction mechanism involved is oxidative cleavage, although hydrothermal process is preferred, because it is simpler and faster than oxidative methods. Other top-down processes are electrochemical oxidation, microwave irradiation, and laser ablation. The strategy of the bottom–up method is the use of small molecules as starting materials for the production of the GQDs [22]. The bottom-up technique consists of the controllable synthesis of carbon sp2 from organic polymers, or of pyrolysis/carbonization processes starting from organic molecules. Typically, polycyclic aromatic hydrocarbon molecules are the most reliable precursors to form high-quality GQDs [23]. The first method results in a complicated process but has the advantage of obtaining products that can be controlled in terms of size and morphology. Carbonization, on the other hand, is an ecological, easy method, but the structure and the morphology of the GQDs are not controllable and the yield is lower. However, both top-down and bottom-up methods involve the use of very expensive non-renewable raw materials, such as CNTs, graphene, graphene oxide, or other graphene-based precursors, which, if prepared from bulk graphite, require the use of toxic chemicals and strong acidic treatment for the disintegration of the strong and well-ordered structure of graphene into small-sized GQDs, as well as high pressure, high-temperature equipment, resulting in low yields and limited production scalability [24].
The use of green synthetic routes is an emerging area in the field of nanotechnology and offers economic and environmental benefits as an alternative to conventional methods. As is well known, the preparation of graphene quantum dots often needs strong acids or organic solvents, and their green production via sustainable strategies, involving non-toxic and biosafe reagents, still faces important challenges; therefore, eco-friendly synthetic approaches, with easy separation and without complicated post-processes, should be designed and developed.
The synthesis of carbonaceous nanomaterials and the choice of precursor materials can be considered equally relevant as, depending on the process and the feedstock, the product will have different features affecting its future applicability. Furthermore, yields that guarantee large-scale production also depend on them. As mentioned in the introduction, in recent years interest has been growing in exploiting adequate renewable resources to decrease dependence on non-renewable resources and increase energy security and environmental safety [25], leading to the development of several attempts to exploit different natural carbon sources for the production of GQDs, by combining both top-down and bottom-up processes. The real advantage of bio based GQDs is the possibility of using a wide range of precursors and several technological approaches [26]. Several biomaterials have been already proposed for a wide range of electrochemical applications to obtain biomass-derived GQDs, ranging from simple and natural molecules to complex compounds, including wheat straw, wood charcoal, rice husk, coffee ground, forestry processing residue, livestock and poultry manure, organic waste from food processing, and municipal solid waste (Figure 1) [9,27,28]. Among these, citric acid (CA), a weak organic acid, and glucose, a carbohydrate, both available in nature, are undoubtedly the most popular carbon precursors, because of their biocompatibility, low cost and ease of supply. Furthermore, carbonaceous materials obtained via green synthesis from CA and glucose, show both photoluminescence from blue to red regions [29] and extremely high QYs (more than 80%) [30].
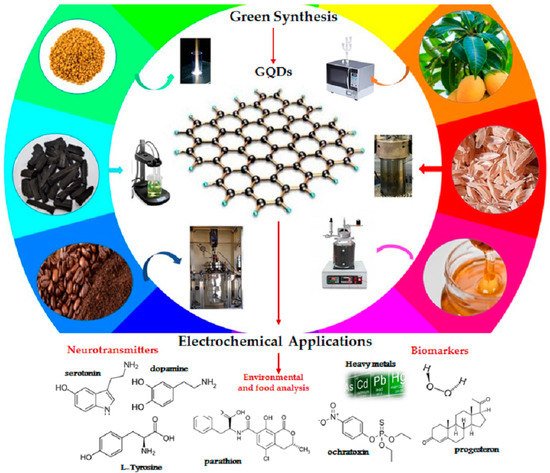
In the following paragraphs, a selection of the main innovative and interesting synthetic approaches proposed in the last years, as summarized in Table 1, involving the use of biomass waste or other natural starting materials, by green routes for the production of graphene quantum dots, focusing attention on their eco-compatibility and their perspectives for future development, will be discussed.
2.1. Oxidative Method
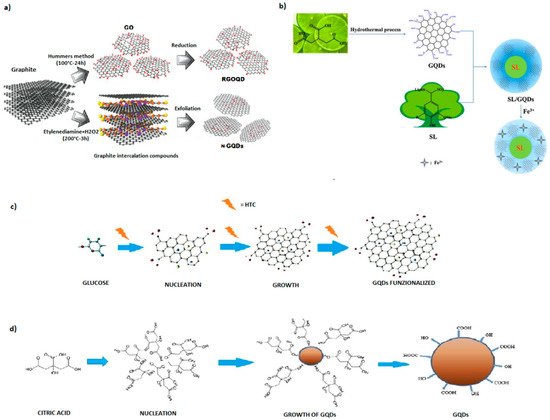
2.2. Laser Ablation
2.3. Controllable Synthesis
2.4. Pyrolysis
2.5. Hydrothermal Method
2.6. Microwave Irradiation Method
Microwaves are a form of electromagnetic radiation lying between infrared radiation and radio frequencies. Microwave heating is a simple, fast and economical process that is extensively used in the synthesis of different classes of materials, including GQDs [65]. Radiant energy is uniformly transferred to the substrate without direct interaction with the source, making the MW assisted approach more efficient than conventional heating. A one-pot microwave irradiation method was reported by Kumawat et al. [32] with mango leaves, exploited to synthesize GQDs, which were then used for in vivo imaging application. The mango leaves were minced, extracted in ethanol for 4 h, and mixed in water. The suspension was treated in the microwave for 5 min and, finally, centrifuged and filtered again. The obtained GQDs showed high biocompatibility and effectiveness for the detection of intracellular temperature. More recently, Abbas et al. [66] synthesized GQDs from tea waste by the microwave assisted oxidative process followed by a hydrothermal treatment in designing selective fluorescent sensors for the detection of the Fe3+ metal ion. The tea scraps were previously washed and dried for 12 h and, subsequently ground. Finally, they were subjected to pyrolysis in an oven at 500 °C for 3 h. The biochar obtained was subsequently subjected to oxidative cutting in a microwave reactor for 15–180 min. The material obtained was purified by hydrothermal treatment at 200 °C for 8 h. The prepared quantum dots showed high detection sensitivity. For the synthesis of quantum dots, plants have proved to be excellent precursors, thanks to their high carbon content. For this reason, the alcoholic extract of a climbing plant (Clitoria ternatea) was used by Tak et al. [67]. The Clitoria ternatea flower extract was mixed with HPLC water and subsequently heated to 900 W in a microwave oven for 5–10 min, then the resulting residue was distributed in absolute ethanol to form a GQD dispersion. The latter was filtered and the particles dried. The peculiar features of the GQDs obtained by this rapid synthesis were analyzed in vivo, evidencing the ability to significantly inhibit the enzyme acetylcholinesterase, and suggesting the possibility of exploiting GQDs in the treatment of Alzheimer’s disease. Dager et al. [33] have recently performed a synthesis of graphene nanoparticles using a one-step decomposition process, enhanced by microwave plasma. In a recent bottom-up method, Wu et al. [68] developed a synthetic alternative to GQDs for sensing applications by using microplasma, an innovative method in which the starting material is represented by fructose. Microplasma has already been used for the synthesis of semiconductor materials, electronic materials, or aerosols, but never for the synthesis of carbon-based quantum dots. This process does not find other sources in the bibliography, although the advantages of plasma treatment compared to other synthesis processes are already known: it increases the decomposition of the starting material, shortens the reaction time, and exploits the temperature produced during the reaction by not requiring an external power supply, therefore having excellent energy properties. The process takes place in a single step lasting 5 min. The pressure was monitored and kept constant and the internal temperature never exceeded 70 °C. A substrate holder, equipped with a halogen lamp heater, is placed under the plasma source. The carbon obtained is sonicated for 5 min, ultra-centrifuged for 10 min, and subsequently filtered. The yield of the described synthetic process was compared with that obtained with traditional synthetic methods, demonstrating its efficiency. Thakur et al. [69] proposed a microwave assisted heating one pot synthesis starting from pasteurized cow’s milk at different reaction times. The milk-derived multi-fluorescent GQDs, spherical in shape and with a lateral size of ca. 5 nm, were efficiently used in simultaneous bioimaging and drug delivery in cancer, using cysteamine hydrochloride as linker, demonstrating their possible use in drug delivery. GQDs functionalized with anti-cancer drug BHC using cysteamine hydrochloride as a linker molecule (GQDs@Cys-BHC) showed an 88% drug loading efficiency, and an in vitro drug release profile which was pH-responsive dependent. Moreover, GQDs have been demonstrated to be suitable for in vitro theranostic application in cancer therapy. By a similar approach, Li et al. [70] obtained GQDs O and S dual-doped (GCNQD) from citric acid and thiourea. The GCNQDs, with a luminescence behavior in the visible range highly dependent on the excitation wavelength and pH, denoting high fluorescence quantum yield (31.67%), strong resistance to the interference of high ionic strength environment, and good biocompatibility, were successfully used as fluorescent probes for HeLa cell imaging, suggesting a great potential in bioanalysis and related fields. Kumawat et al. [71] investigated a green method based on the use of alcoholic grape seed extract as a starting material. The extract was treated in a microwave after evaporation and dispersion in water obtaining GQDs that undergo “self-assembly” (sGQD) in the water, showing interesting cell proliferation activity in fibroblasts in vitro.
This entry is adapted from the peer-reviewed paper 10.3390/nano11051120
