Globally, colorectal cancer (CRC) ranks as one of the most prevalent types of cancers at the moment, being the second cause of cancer-related deaths. The CRC chemotherapy backbone is represented by 5-fluorouracil, oxaliplatin, irinotecan, and their combinations, but their administration presents several serious disadvantages, such as poor bioavailability, lack of tumor specificity, and susceptibility to multidrug resistance. To address these limitations, nanomedicine has arisen as a powerful tool to improve current chemotherapy since nanosized carriers hold great promise in improving the stability and solubility of the drug payload and enhancing the active concentration of the drug that reaches the tumor tissue, increasing, therefore, the safety and efficacy of the treatment.
1. Introduction
Globally, colorectal cancer (CRC) ranks as one of the most prevalent types of cancers at the moment, being the second cause of cancer-related deaths [
1,
2]. According to World Health Organization (WHO), CRC is positioned worldwide as the third most common type of cancer as incidence, and the second as mortality, with 1.93 million new cases and 935,000 deaths reported in 2020 [
2,
3]. The CRC incidence is directly correlated with the economic status of the countries, the most affected countries being low- and middle-income, where lifestyle habits and dietary patterns sustain the development of this malignancy [
4,
5,
6]. Despite the existence of screening programs that allow CRC diagnosis in early stages [
7], CRC morbidity and mortality are steadily increasing even in developed countries. This trend is correlated with the disease stage at the time of diagnosis that holds a great impact on the 5-year relative survival rate of CRC patients. The 5-year relative survival rate decreases from ~90% for stage I CRC to ~10% for stage IV metastatic CRC (mCRC) [
8], the disease stage being also a crucial factor in the establishment of a proper and effective therapeutic approach. Unfortunately, the onset of CRC is asymptomatic, a feature that is responsible for the large number of patients in advanced stages of the disease at initial diagnosis. While one-quarter of the CRC patients are diagnosed from the beginning with mCRC, more than half of the patients will develop metastasis along with CRC progression [
1,
9]. More, the initiation of the screening programs is typically recommended for adults with ages above 50 years, but the recent rising incidence among young adults could rewrite the current CRC screening guidelines [
10]. For example, the American Cancer Society already lowered the age threshold to 45 years for individuals with risk for CRC development [
11], but further adjustments need to be done at a global level to lower the CRC burden and ensure the detection of CRC in early stages.
At the moment, several therapeutic approaches are implemented in clinics for CRC management such as surgery [
12,
13,
14], radiotherapy [
15,
16], chemotherapy [
17,
18,
19], and targeted therapy if applicable [
20]. The choice of the approach is dependent on the stage of CRC at the initial diagnosis. While CRC patients diagnosed in the early stages of the disease by colonoscopy or sigmoidoscopy, which present a clean tumor with well-delimited margins, qualify for surgical resection of the tumor, the major challenge is represented by patients in stage III/IV, where the disease is spread to the lymph nodes and distant organs. For these patients, adjuvant chemotherapy is mandatory to control CRC and metastatic invasion, followed by tumor and metastases surgical resection if allowed [
21].
Despite the recent advances in cytotoxic chemotherapy and targeted therapy, the prognosis of CRC remains unsatisfactory, especially for mCRC patients [
22]. The severe side effects associated with chemotherapy and the development of multidrug resistance are critical issues that hinder the proper care of CRC patients by limiting treatment efficacy and leading to chemotherapy failure. In this view, nanomedicine has emerged as a powerful tool to improve the existing drug-based strategies for CRC treatment. Nanomedicines are nanosized carrier biomaterials that are used as shuttles to deliver drug cargos to the tumor tissue. These nanosized drug-delivery systems possess the potential to improve the stability and solubility of the drug payload and to enhance the active concentration of the drug that reaches the tumor tissue, increasing the safety and efficacy of the treatment [
23,
24]. In this context, the present review offers an overview of the most recent advances in the development of organic nanosized drug-delivery systems as smart therapeutic tools in CRC management.
2. Current Pharmacotherapy Available for CRC
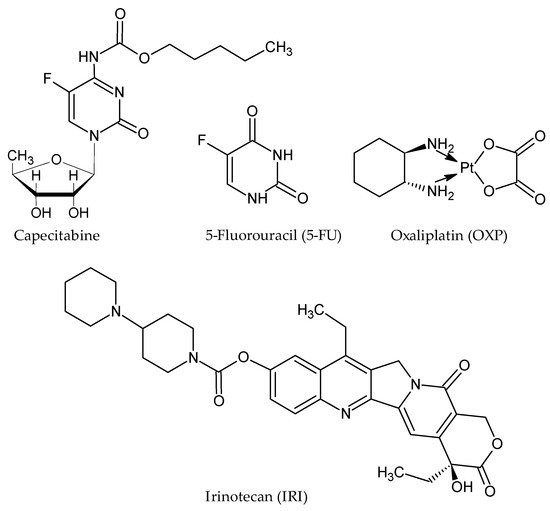
The existing pharmacotherapy available for CRC treatment relies on the administration of cytotoxic agents, targeted therapies, and their combinations. Among cytotoxic agents, CRC chemotherapeutic regiments are based on the administration of capecitabine or 5-fluorouracil (5-FU), oxaliplatin (OXP), and irinotecan (IRI) (), anticancer agents that are usually combinatorically administrated to amplify their antineoplastic potential: 5-FU/LV/OXP (FOLFOX), 5-FU/LV/IRI (FOLFIRI), and 5-FU/LV/IRI/OXA (FOLFIRINOX) [
25,
26]. These anticancer agents act on both DNA or RNA synthesis to exert their cytotoxic effects [
27,
28,
29], and although they still represent the backbone of CRC chemotherapy, their administration presents several serious disadvantages, such as poor bioavailability [
27,
30,
31], lack of tumor specificity, and susceptibility to multidrug resistance [
28,
32,
33].
Figure 1. Structure of conventional chemotherapeutic agents administrated in CRC.
One such example is 5-FU, a fluoropyrimidine analogue and a first-line drug in colorectal cancer chemotherapeutic regimens. The conversion of 5-FU into its inactive metabolite, 5,6-dihydro-5-fluorouracil (DHFU), is directly dependent on the dihydropyrimidine dehydrogenase (DPYD) enzyme, which is encoded by the DPYD gene [
34]. Several genotypic variants of DPYD were identified in the population that can be translated into individual variations in the drug’s clearance [
35], leading to 5-FU systemic toxicity and drug resistance [
36]. Consequently, 5-FU administration is associated with side effects that can vary from mild to severe, depending on the patient’s tolerance [
37,
38], severely limiting the dose of the drug and leading to poor antineoplastic results.
For a more specific approach to CRC pathology, the strategy relies on targeting molecules that play a central role in tumor development and progression, generally by using monoclonal antibodies [
39]. In this respect, anti-angiogenic drugs targeting the vascular endothelial growth factor (VEGF) pathway hold a key role in treating patients with metastatic CRC. Bevacizumab [
40,
41,
42], ramucirumab [
43], and aflibercept [
44,
45] are used for targeting angiogenesis, a culprit in tumor development and progression [
46,
47]. For CRC patients that do not harbor KRAS or NRAS mutations, cetuximab and panitumumab can be administrated as anti-EGFR antibodies [
48,
49,
50]. Epidermal growth factor receptor (EGFR) modulates CRC initiation and progression due to its key role in activating downstream signaling pathways that control tumor cell growth, differentiation, and proliferation [
51,
52,
53,
54]. More recently, immune checkpoint blockade agents such as pembrolizumab, nivolumab, and ipilimumab have been exploited as targeted therapy in CRC [
20]. Promising clinical studies raise the hope of survival improvement in CRC patients when administering bevacizumab and standard chemotherapy combined approach [
55,
56].
3. Targeting Strategies for Drug-Delivery Systems in CRC
An ideal drug-delivery system for anticancer agents should be designed and developed to significantly improve the efficacy of the drug-free traditional treatment by functioning as a protective shuttle for avoiding drug degradation, thus ensuring an increased drug concentration that reaches the tumor. More, in the preparation phase, the nanoparticles should be tailored to enhance their tumor selectivity and ensure accumulation to the tumor site, reducing therefore the cytotoxic effects on normal healthy tissues.
The physicochemical proprieties of the nanocarriers impact their effectiveness as drug-delivery systems in CRC management. The size and shape of the nanoparticles, as well as the charge of their surface, have a non-specific effect, which is determined by the interaction with the cell membrane [
57]. Since the surface of the cell membrane is negatively charged, positively charged particles have the highest penetration rate, while the lowest penetration rate is observed at nanoparticles with a negative surface charge [
58]. It has been shown that a high positive charge on the surface of particles leads to enhanced cellular uptake, while a negative charge contributes to reduced absorption of nanoparticles [
59]. It is noteworthy that the immobilization of some proteins on the surface of charged nanoparticles has a leveling effect on the charge factor, contributing to a similar rate of absorption of positively and negatively charged particles [
60].
The size of nanoparticles has a significant impact on the rate and mechanism of their absorption. The rate of nanoparticle absorption by cells increases with particle size [
61]. For particles with a diameter of more than 200 nm, absorption generally occurs through clathrin-dependent endocytosis. The mechanism of smaller particle absorption remains a subject of discussion, being probably associated with passive transport through the pores of the cell membrane or directly through membrane fusion [
62]. Lastly, the nanoparticles smaller than 100 nm may be absorbed through the nuclear membrane, enabling the transport of drugs into the cell nucleus. Thus, the size of the carrier particles determines the rate and mechanism of its absorption by the cell, as well as the character of drug distribution between the cell organelles [
63].
The penetration rate of nanoparticles into the cell also depends on their geometric shape. It has been shown that spherical particles are absorbed by cells at the highest rate [
64]. The interpretation of data on the effect of the size and shape of particles on the nature of their absorption by the cell should be carried out with caution since nanoparticles are simultaneously involved in at least three processes: diffusion, sedimentation, and agglomeration [
61].
Although these factors are common for the therapy of all types of cancers, CRC of various etiology [
65,
66] has special requirements for ensuring the bioavailability of pharmacologically active substances delivered through nanoparticles [
67]. The problem of increasing the bioavailability of drugs can be solved, at least partially, by introducing CRC-specific ligands on nanoparticle surfaces [
68,
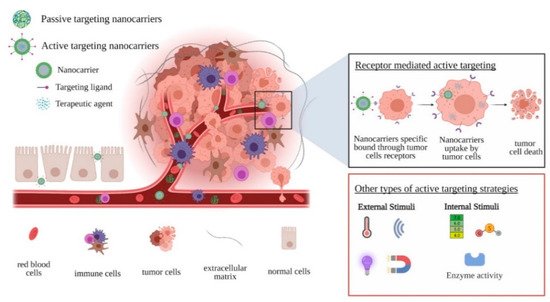
69]. Based on these observations, there are two strategies by which nanosized drug-delivery systems reach tumor cells (): (i) passive targeting, where nanoparticles take advantage of the abnormalities of tumor vasculature to accumulate at the tumor site, and (ii) active targeting, where nanoparticles are functionalized with moieties that directly target the tumor cells.
Figure 2. Schematic representation of drug-delivery systems targeting strategies.
This entry is adapted from the peer-reviewed paper 10.3390/ma14092440