The use of nanoparticles is a multidisciplinary approach to provide UV blocking, antimicrobial, water repellent, colorant, flame retardant, sensing, and self-cleaning properties to textiles. Particularly, the antimicrobial textiles with improved functionalities find several applications, namely, in health and hygiene products, infection control, and barrier material. Depositing nanoparticles in textiles have been a promising strategy to achieve multifunctional materials. Particularly, antimicrobial properties are highly valuable due to the emergence of new pathogens and the spread of existing ones. Various techniques have been used by researchers to functionalize natural and synthetic fibers with AuNPs such as sputtering, electrostatic assembly, chemical reduction in solution, dip-coating, electroless plating, drop and dry, biosynthesis, and print pasting method.
- gold nanoparticles
- in situ
- textiles
- antibacterial properties
1. Gold Nanoparticles
Metal nanoparticles have demonstrated unique physical and chemical properties unlike those in their bulk state. This is attributed to the quantum size effect resulting in specific electronic structures [1][2]. For antimicrobial purposes, silver nanoparticles (AgNPs) have presented particular interest. However, in vitro studies have demonstrated their toxic effects on liver, neuronal, epithelial, and murine stem cells. AgNPs have also shown toxicity in aquatic organisms and accumulation in plants, which allow their introduction into the food chain. These facts triggered the research for other metals to obtain antimicrobial effects [3]. Gold nanoparticles (AuNPs) emerged as an alternative due to their higher biocompatibility and facility of surface functionalization [4]. Additionally, AuNPs present intense plasmon resonance and suitable electrical, magnetic, and thermal conductivity and chemical stability (either in atmospheric conditions or living tissues, being resistant to oxidation) [5]. In pharmacology, they present attractive anti-HIV, anti-angiogenesis, anti-malarial, anti-arthritic, and some antimicrobial activity. Biomedical applications include drug delivery, gene therapy, catalysts for medical therapy, and diagnostics [6][7]. Regarding the antimicrobial properties of the AuNPs, studies have demonstrated that several parameters condition its activity [8]. The abovementioned properties/activities of AuNPs are dependent on their physicochemical characteristics provided by the surface composition, size, and shape. During the AuNPs synthesis, variables such as the reaction temperature, stirring rate, the ratio of the gold to a reducing agent, and the use of stabilizing agents and surfactants influences the final characteristics of nanoparticles [9]. Specifically, the AuNPs surface, where the external atoms are bonded to the internal atoms, opens opportunities for interaction with donor–acceptor species or ligands, promoting variable surface charges. In addition, the use of low concentrations of surfactants is beneficial to obtain the desired morphologies and avoid agglomeration [9][10][11]. Another common approach is the AuNPs functionalization with molecules attached to their surface by chemisorption, electrostatic attraction, hydrophobic interaction, or chemical bonds. The head group of the ligands are able to improve the interaction of AuNPs and the external environment to improve the desired effects [12]. Thus, synthesizing AuNPs with specific characteristics is highly valued.
Synthesis of gold nanoparticles dates to 1951 where Turkevich used sodium citrate as reducing agent for producing gold nanoparticles. Since then, researchers have used other reducing agents such as gallic acid, hydrogen peroxide, hydrazine etc. Later, a two-phase method was proposed by Brust-Schiffrin in 1994. However, usage of citric acid, sodium borohydride (NaBH4), polyethylene glycol (PEG), hexadecyltrimethylammonium bromide (CTAB), trioctyl-phosphine (TOPO), and oleyl amine (OAm) showed to be toxic, harmful, irritating, flammable, or hazardous to the environment. Therefore, green synthesis methods were introduced recently, where chemical reducing agents are being replaced by plant extracts, bacteria, yeasts, fungi, and enzymes [13][14].
2. Methods to Prepare Textile Materials Functionalized with AuNPs
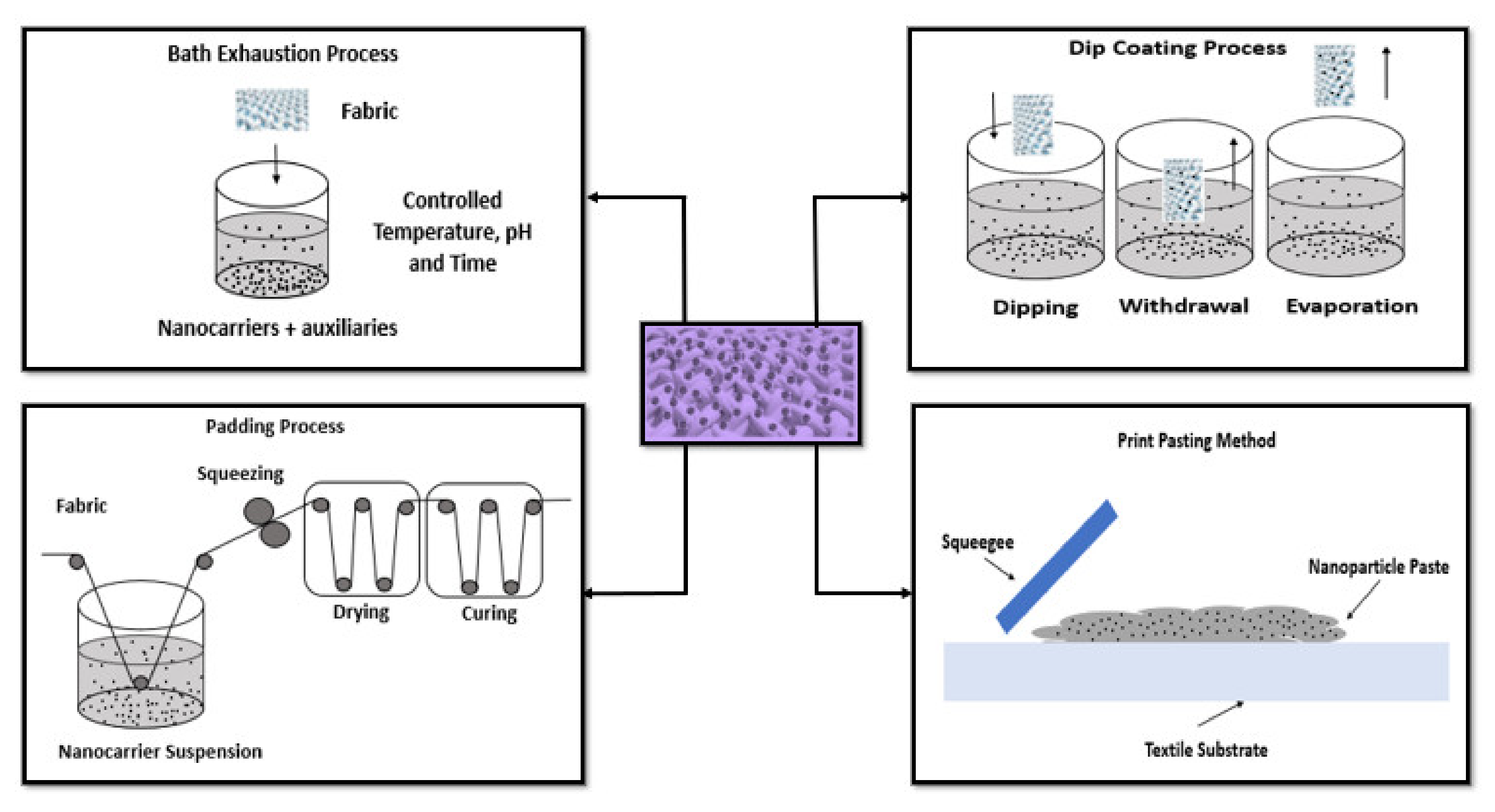
Textile industry has benefited from the development of new concepts of applications, particularly by their conjugation with nanomaterials. Textile materials are included in several advanced and smart applications encompassing woven, knitted, and non-woven fabrics; fibers; yarns; threads; nanofibers; scaffolds; and membranes. The incorporation of AuNPs is interesting to obtain fabrics with innovative coloration, filtration, antimicrobial, conductive, UV protection, sensory, and catalytic properties as presented in this section (Figure 1). Driven by these excellent properties, during the last years, several scientific publications have reported methods to perform this combination.
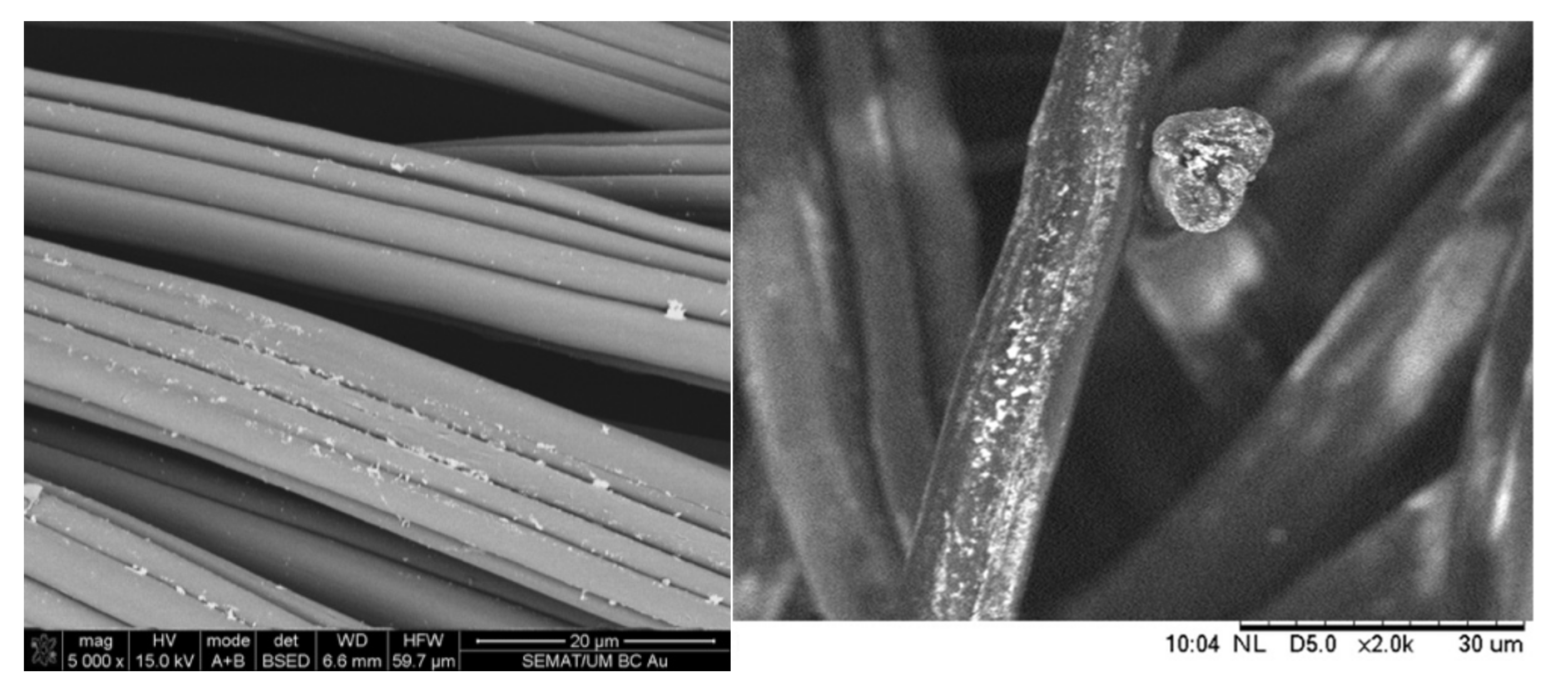
Figure 1. Gold nanoparticles (AuNPs) deposited onto synthetic fibers.
The functionalization of textile surfaces with AuNPs have been performed by several methods including exhaustion, padding, dip coating, electroless, screen printing, dropwise, immersion, sonication, and electrospinning (Figure 2).
Exhaustion is one of the older process, in which the textile material is placed in the nanomaterial’s solution and the nanomaterials are adsorbed onto the surface of the fabric. Parameters such as exhaustion temperature, time, pH, concentration of nanomaterial solution, and addition of auxiliary agents play a crucial role in this process. Once the fabric is taken out from the solution, it is washed and dried [15][18]. The padding process is a traditional method and involves the use of a pair of rollers where the textile is immersed in a nanomaterial dispersion and then it is directed to move in between the rollers. This facilitates the penetration of nanomaterial into the textile and removes the excess of the solution from the textile. The pressure of the rollers and the speed of the fabric moves through the roller are vital in this procedure. Once the textile is passed out of the roller, it is then dried and cured, which helps to keep the nanomaterial on to the surface [15][19][20]. Dip coating is another impregnation method to coat nanoparticles on fibers or fabrics. The materials are dipped into the coating bath. The fibers/fabrics obtained are dried and may be cured, which enhance the adhesion between nanomaterial and the fibers/fabrics [15][21]. Electroless deposition is a common method used to deposit metal on nonconductive surfaces. It is based on surface charge activation and covers the layer with a catalyst. This process includes reduction of metallic ions to pure metal in the presence of a catalyst on to a surface. The electroless deposition will be carried out, as long as it is immersed in the bath. It is a simple technique and facilitates the adhesion of the nanoparticles onto the surface [15][22][23]. The print method was used by very few authors using a AuNPs paste made up of thickener, binder, and nanomaterial. The mixture can be applied via flat-to-flat method using a squeegee or screen printing on a curved surface using a rotating cylinder [15][24]. In the drop-wise deposition, the AuNPs are dispersed in a volatile liquid that is deposited by drop-on-demand ink jet heads. After that, the materials pass through a heating and evaporation process. In this final step, the particles deposition is promoted [25]. Immersion is a simple technique based on the direct dipping of the material into nanoparticles solution and subsequent diffusion. This process is time dependent and may require several minutes. The success of the deposition depends on the affinity between the materials surface and nanoparticles [26]. Sonication deposition is based on ability to generate acoustic cavities that undergo implosive collapse, discharging enormous energy in the form of high temperature and pressure. These facts produce shock waves, microjets, turbulence and shear forces that enhance the diffusion of nanoparticles and increase the kinetic energy of the system to overcome their ionic repulsion forces. Thereby, it reduces the deposition time and increases the surface coverage of nanoparticles [27]. Lastly, electrospinning also can be used to introduce nanoparticles into textile materials by mixing them with the polymer solution before the fiber formation. The polymer solution is charged and ejected through a spinneret under a high-voltage electric field that solidifies or coagulates to form filaments [28][29].
Herein, the studies and methods evolving AuNPs and textile materials are underlined (Table 1). The studies were grouped first depending on the textile materials type and second depending on the method to synthesize the AuNPs. The main aim was to emphasize the studies that focus on textiles functionalized with AuNPs to understand the developed techniques according to this purpose to promote novel studies. Despite some of these studies are not connected with the development of antimicrobial textiles, it will be possible to comprehend other approaches, applied in other fields, to open new possibilities in the development of antimicrobial textiles materials. Thus, the most important methods and cost-competitive studies were considered.
Table 1. Studies about the functionalization of fabrics (F), knitted fabrics (KF), and non-woven fabrics (NW) with AuNPs. (n.a. = not available).
| Method for Synthesis of AuNPs | Deposition Method | Fabric/Textile | Precursor Salt | Reducing Agent | Stabilizing Agent | Additional Information | Size of NPs | Application | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Chemical reduction | Drop-wise deposition | Cotton, silk, wool, polyester and nylon—F | Chloroauric acid (0.001 M) | Sodium borohydride solution of (0.1 M, 3 mL) | Sodium citrate 3 mL solution of 0.001 M | Sodium citrate also act as capping agent | n.a. | Wearable sensors | [30] |
| Chemical reduction | Padding | Cotton—F | Chloroauric acid (0.01 Wt %, 50 mL) | Trisodium citrate (1 wt%) | No stabilizing agent | Keratin coating (360 mL of keratin solution (10 mg/mL concentration). | 71.8 nm | Antimicrobial textiles | [31] |
| Chemical reduction | Printing and paste method | Polyester—F | Gold (III) chloride hydrate | Sodium citrate | No stabilizing agent | One step green procedure | 13–20 nm | Coloration, UV protection | [24] |
| Chemical reduction, seed-mediated growth | Immersion | Silk and cotton—F | Tetrachloroauric acid (0.01 M, 0.25 mL) Tetrachloroauric acid (0.01 M, 20 mL) |
Sodium borohydride (0.01 M, 0.6 mL) Ascorbic acid (0.1 M, 3.2 mL) |
CTAB (0.1 M) 9.75 mL CTAB (0.1 M) 400 mL |
Au nanorods with spherical shape. | 19 nm | Textile’s collation, UV protection, and antibacterial | [32] |
| In situ chemical reduction | Immersion and heating | Nylon—F | Tetrachloroauric (III) acid (0.05, 0.10, 0.15 and 0.20 mM) | Trisodium citrate | Trisodium citrate | pH value is in the range of 5.0–6.5 as per the concentration. | n.a. | UV blocking textiles | [33] |
| Chemical reduction | Exhaustion | Soybean—KF | Tetrachloroauric acid 0.01% W/V | Sodium citrate dihydrate (1% W/V, 2 mL) | Chitosan | Treatment with chitosan; spherical shape. | 34.6 ± 0.5 nm | UV blocking and antimicrobial textiles | [34] |
| In situ chemical reduction | Impregnation | Cotton, silk, and wool—F | Hydrogen tetrachloroaurate (III) trihydrate | Sodium borohydride (1.3 g/L) | n.a. | AuNPs are mixed with other nanoparticles such as Ag and Pt; AuNPs have spherical shape. | 6.64 nm | Antimicrobial textiles | [35] |
| In situ synthesis, groups on silk | Immersion and constant shaking at 85 °C | Silk—F | Gold (III) chloride (0.5–2 mM) | Silk macro molecular chains | Silk fabric | Hydrogen peroxide used for activation of silk macro molecules. | 22–66 and 18–49 nm | Fabric coloration and antimicrobial properties | [36] |
| In situ synthesis, sericin from silk | Soaking and sonication | Silk—F | Tetrachloroauric (III) acid (10 mg/mL, 500 mL) | Reduction by sericin from silk | n.a. | Spherical and ellipsoidal; pH = 12. | 11 ± 4 nm | Antimicrobial textiles | [37] |
| Chemical reduction | Immersion | Polypropylene—NW | Tetrachloroauric Acid (1 mM) | Gallic acid (0.5 mM) | Without stabilizer | Surface activation by dielectric barrier discharge (DBD) and diffuse coplanar surface barrier discharge (DCSBD). | 20 nm | Antimicrobial textiles | [38] |
| n.a. | Deposition-precipitation | Poly(ethylene terephthalate—NW | Tetrachloroauric acid (5 mmol/L) | n.a. | n.a. | Fabric coated with ZrO2 fine particles before deposition of AuNPs at pH = 7 | n.a. | Air filter | [39] |
| In situ chemical reduction | Immersion | Cotton—F | Tetrachloroauric acid (10 mM) | Polydopamine (2 mg/mL) | n.a. | Treated with polydopamine before depositing nanoparticles; AgNPs were deposited prior to AuNPs at pH = 8.5. | n.a. | Catalysis | [40] |
| In situ chemical reduction | Immersion and stirring | Cotton—F | Hydrogen tetra-chloroaurate (III) trihydrate HAuCl4 (1 mL, 20 mM) | N-vinyl pyrrolidone (0.1 mL) | 1-Hexadecylamine | Surface modification by ATS is crucial for the formation of gold nano particles; spherical shaped. | 2–7 nm | Textile coloration | [41] |
| Chemical reduction | Dip coating | Cotton—F | Sodium tetrachlorocuprate (III) dihydrate (1%, 90 µL) | Trisodium citrate (1%, 2.7 mL) | n.a. | Cotton fabric precoated with Zn nanorods before deposition of AuNPs. | 18.5 ± 2.8 nm | Photocatalysis | [42] |
| In situ chemical reduction | Soaked in solution | Polyethylene-coated polypropylene—NW | Chloroauric acid | Amine groups grafted in textile surface | Amine groups grafted in textile surface | PE-coated PP fabric was used as a ligand and template; fabric is treated by the electron beam; spherical shape. | 5–20 nm | n.a. | [43] |
| Chemical reduction | Soaked in solution of AuNPs solution | Nylon-6—F | Tetrachloroauric acid (0.0863 g) | Oleylamine | n.a. | – | 14.6 ± 1.4 nm | Catalytic systems | [44] |
| In situ synthesis, tyrosine groups on silk fiber | Immersed in solution and heating | Silk—F | Tetrachloroauric (III) acid Various concentrations |
Tyrosine groups on silk fiber | Tyrosine groups on silk fiber | Different shapes were observed according to the Wt % of the precursor solution. | 21.3 ± 3.4 nm | Textile colorations and antimicrobial effect | [45] |
| In situ synthesis | Immersion and heating | Silk—F | Tetrachloroauric acid (0.1–0.6 mM, 50 mL) | n.a. | n.a. | Spherical, triangular nanoplates, truncated nanoprisms, and polygonal; depend on concentration of the precursor solution. | n.a. | Fabric coloration | [46] |
| In situ synthesis, sericin from silk | Immersion and heating | Silk—F | Tetrachloroauric (III) acid (0.3 mM) | n.a. | n.a. | The pH value of solutions was adjusted to 3; spherical and platelike shape. | n.a. | Fabric coloration and antimicrobial properties | [47] |
| Biological reduction | Pad-dry-cure | Cotton—F | Chloroauric acid (0.001 M, 2.5 mL) | Acorus calamus rhizome extract (2.5 mL) | Plant extract | Small spherical ball and bigger spherical ball, and it depends on the concentration at pH = 4, 7 and 9.2. | (0.001 M) below 100 nm (0.01 M) 100–500 nm |
Antibacterial and UV blocking | [48] |
| Biological reduction |
Sonication | Cotton and viscose—KF | Tetrachloroauric acid (3 mM, 100 mL) | Bacterial isolates (Streptomyces Sp) | Streptomyces Sp | Plasma treatment along with the combination of TiO2NPs and ZnONPs spherical shape. | 4–13 nm | Antimicrobial and UV- blocking textiles | [49] |
| In situ synthesis, hydroxyl groups on cellulose | In situ synthesis | Cotton—KF | Tetrachloroauric acid (0.025, 0.05, 0.075, 0.10, and 0.125 mM) | Hydroxyl groups from cellulose | n.a. | Cotton also acted as a reducing agent; spherical and triangular nanoplates. | 8.7 ± 1.2, 8.6 ± 1.3, 14.1 ± 3.0, 17.4 ± 3.0, and 20.5 ± 3.8 nm | Catalytic, UV blocking, and antibacterial textiles | [50] |
| In situ synthesis, hydroxyl groups on cellulose | Dip and dry | Cotton and polyester—F | Tetrachloroauric (III) acid (5.88 × 10−4 M, 198 mL,) | Hydroxyl groups on cellulose | Natural rubber latex | – | 31 nm | Catalytic textiles | [51] |
| Biological reduction | Pad dry cure | Cotton—F | Tetrachloroauric acid (0.001 M and 0.1 M, 2.5 mL) | Coleus aromaticus leaf extract (2.5 mL) | Coleus aromaticus leaf extract | Spherical, rod, and triangular shapes, pH = 7. | Different sizes (<20 nm) | Antimicrobial textiles | [52] |
| Biological reduction |
Pad-dry-cure | Cotton—F | Chloroauric acid (0.001 M) | Croton sparsiflorus leaves Extract | Croton Sparsiflorus leaves extract | Low concentration: bulbous shape high concentration: spherical shape; the fabric was pre-treated through scouring and bleaching. |
12.2–12.7 High concertation 16.6 nm. |
UV protection, antibacterial, and anticancer | [53] |
| Biological reduction | Dry-jet wet spinning process | Cellulose—NW | Tetrachloroauric acid (0.03 mL/g, 50 mM) | Bleached birch pre-hydrolyzed kraft pulp | n.a. | – | n.a. | UV blocking | [54] |
| In situ biological reduction | Immersion | Silk and cotton—F | Tetrachloroauric acid (2.00 × 10−4 M, 80 mL) | Ginkgo biloba Linn leaf powder extract | n.a. | Rectangular, spherical, hexagonal with smooth edges, or roughly circular in shape. | 10–75 nm | Textile colorations and antimicrobial effect | [55] |
| In situ chemical reduction | Immersion | Silk and cotton—F | Tetrachloroauric acid (2.00 × 10−4 M, 80 mL) | Potassium borohydride | n.a. | – | n.a. | ||
| In situ biological and chemical reduction | Immersion | Silk and cotton—F | Tetrachloroauric acid (2.00 × 10−4 M, 80 mL) | Ginkgo biloba Linn leaf powder extract and potassium borohydride | n.a. | – | n.a. | ||
| In situ photoreduction | Immersion | Silk—F | Tetrachloroauric acid (0.2, 0.3, 0.4, 0.5, 0.6, and 0.7 mM) | n.a. | n.a. | In situ synthesized AuNPs on through the induction of sunlight; spherical shape. | 16.9 ± 1.2, 24.1 ± 1.7, 23.0 ± 2.1, 20.6 ± 1.2, 19.9 ± 1.3, and 28.4 ± 1.6 nm | Fabric coloration | [56] |
| Heating and photochemical | Exhaustion | Wool—F | Tetrachloroauric acid (0.2 mM) | Trisodium citrate, D-Malic acid disodium salt or disodium tartrate (1 mM) |
Trisodium citrate | Spherical and egg shapes were observed for heating method and photochemical synthesis at pH 4, respectively. | Various sizes | Textile coloration | [57] |
| n.a. | Electrodeposition | Polyester—F | Gold (III) chloride trihydrate (20 mM) | n.a. | n.a. | Coating single-walled carbon nanotubes on the polyester textile substrate before AuNPs deposition. | 50 nm | Fuel cells—conductive fabrics | [58] |
2.1. Functionalization of Fabrics with AuNPs
2.1.1. Chemical Reduction Method without Pre-Treatments on Fabrics
Within the methods to produce AuNPs to functionalize textiles, chemical reduction is one of the most applied. This method is preferred due to its simplicity, but the current trend is to replace it with more environmental-friendly and inexpensive methods. Researchers often use the traditional two-step functionalization (synthesis and posterior deposition) but also in situ methodologies, where the synthesis and deposition of gold nanoparticles on fabrics takes place in single step. The common reducing agents used in the two-step process are NaBH4 and sodium citrate. When the two-step method is applied, different methodologies were reported for deposition such as drop-wise deposition, exhaustion, padded, impregnation, and printing of the fabrics with AuNPs dispersion. Kam Ling Chan et al. (2016) have synthesized AuNPs using chloroauric acid (HAuCl4) as precursor salt, NaBH4 as reducing agent, and sodium citrate as a capping agent. They were coated on fabrics made with cotton, silk, and wool using drop-wise deposition [30]. Coating of keratin protein in combination with gold nanoparticles onto the cotton fabric by padded method was attempted by O Shanmugasundaram et al. (2018) to improve the antibacterial property. Porosity and water absorbency of fabric was decreased with the coating of keratin and AuNPs. AuNPs were synthesized using a chemical reduction method where HAuCl4 and trisodium citrate were used as precursor salt and reducing agent, respectively. The synthesized nanoparticles were measured and found to be in the size of 8–30 nm with mean size as 14 nm [31]. T. Abou Elmaaty et al. (2018) have used a simple method, printing and paste, to coat the AuNPs on to the polyester and cotton fabrics. The gold nanoparticles were synthesized using gold (III) chloride hydrate and sodium citrate. Thereafter, the solution was made into paste, and it was printed using flat screen technique. TEM observations revealed that the average diameter was observed in the range between 13 and 20 nm [24]. Yidan Zheng et al. (2013) have produced silk and cotton fabrics coated with gold nanorods for coloring. Au nanorods with the size of 19 nm were synthesized using seed-mediated growth method where they have used HAuCl4, NaBH4, and CTAB as precursor solution, reducing agent, and stabilizing agent, respectively. The Au nanorods were deposited onto the fabrics by immersing them in the Au nanorod solutions [32].
The in situ approach allowed to remove one step to functionalize the fabrics. Here, chemical reducing agents are added to the gold salt solution or the chemical groups on the fiber surface may act as reducing agent itself. Xia Lin et al. (2017) have produced AuNPs via reduction method using HAuCl4 as a precursor solution and trisodium citrate as a reducing agent, where pH value was maintained at 5.0–6.5. The nylon fabrics was functionalized with gold nanoparticles by immersion and heating process. It was observed that the color of the fabric became lighter with an increase in the concentration of the reducing agent [33]. Chitosan being nontoxic and cationic in nature, Iris O. Silva et al. (2019) treated knitted fabric to improve the binding effect of nanoparticles. The AuNPs were chemically synthesized, and NPs with spherical shape and approximately 35 nm in size were obtained. Then, the team immobilized the AuNPs onto the soybean knitted fabric treated with chitosan by exhaustion method. In this process, HAuCl4, sodium citrate dihydrate, and chitosan acted as precursor salt, reducing agent, and stabilizing agents, respectively. The fabrics developed showed improved thermal stability [34]. Liheng Gao et al. (2020) have produced single-layered Ag-Au-Pt nanocrystal ternary coated biomass textiles using polymer-driven self-assembly strategy. In order to improve the water solubility, oxidative resistance, and affinity to biomass, metal nanoparticles, are transformed into homogenous hyperbranched poly(amide-amine) (HBPAA) encapsulated nanoparticles. In this study, the AuNPs were produced by in situ synthesis, where HAuCl4.4H2O and NaBH4 were used as precursor salt and reducing agent, respectively. AuNPs with a diameter of 6.64 nm were mixed with other nanoparticles, and the textiles were impregnated in the solution [35]. Hanan B. Ahmed et al. (2017) produced AuNPs and coated a silk fabric by immersion with constant shaking and temperature to enhance NPs adhesion and, consequently, the antibacterial properties. In the process of synthesizing AuNPs by in situ reaction, AuCl3 was used as a precursor salt, silk macro molecular chains were used as reducing agent, and silk fabric itself acted as stabilizing agent. Hydrogen peroxide was used to activate the silk macro molecules, and the size of the AuNPs was in the range of 22–66 and 18–49 nm [36]. Ultrasonic treatment was used by Lin Zhou et al. (2019) for the better deposition of gold nanoparticles on to the silk fabric. HAuCl4 and sericin in silk were used as precursor salt and reducing agent, respectively, to produce AuNPs. The AuNPs were deposited on the fabric by soaking the fabric in the solution and using ultrasonic treatment. The AuNPs found to have spherical and ellipsoidal shape in the size of 11 ± 4 nm [37]. A few works in the literature exist with this last method, which can be a promising approach to reduce the chemicals during the NPs synthesis, the preparation time, and the energy required. More studies are needed to provide toxicological tests and adapt to industrial processes.
2.1.2. Chemical Reduction Method with Pre-Treatments on Fabrics
Pre-treatments of fabrics are vital to enhance the reactivity onto their surface and enhance the AuNPs deposition rate or to act in synergy with AuNPs improving a specific property. Two different studies emerged in the literature, the first one was the fabric surface activation using plasma treatments and the other one was the pre-functionalization with minerals, by silanization with alkoxysilane molecules or by cross-linking agents. It was reported by Nina Radic et al. (2012) that dielectric barrier discharge and diffuse coplanar surface barrier discharge was used to enhance the deposition of AuNPs. The AuNPs were synthesized by the reduction of HAuCl4 solution by gallic acid without stabilizer and coating onto the fabric by immersion of the fabric in the solution containing AuNPs. The size of the particles deposited onto the fabrics was in the range of 30–60 nm, and it was observed that they were smaller in solution, which indicates some agglomeration on the fabric [38]. Another methodology was reported by Makoto Ikegami et al. (2012), where the PET (polyethylene terephthalate) non-woven fabric was coated with zirconium dioxide (ZrO2) fine particles prior to the deposition of AuNPs to enhance the catalytic activity. An in situ reaction method was used to produce and deposit AuNPs by the deposition-precipitation method [39]. Deshan Cheng et al. (2019) synthesized AuNPs via in situ synthesis reduction method and deposited them on cotton fabric by dip-coating. In this work, HAuCl4 was used as the precursor salt and polydopamine (PDA) acted as reducing agent. AgNPs were deposited prior to the deposition of AuNPs, which acted as catalytic hotspots for enhancing the deposition of AuNPs [40]. Hongjun Liu et al. (2013) used HAuCl4 to synthesize AuNPs on cotton fibers via in situ synthesis using the amine stabilizers and N-vinyl pyrrolidone (PVP) reductant in an aqueous medium. Fibers of cotton surface were first modified with (3-aminopropyl) triethoxy silane (ATS). The pre-treatment of the surface was found to be essential, without which a small amount of nonuniformly adsorbed AuNPs was formed on the fabric. The size of the nanoparticles was in the range of 2–7 nm and attained spherical shape [41]. Bharat Baruah et al. (2019) have focused on improving the catalytic activity of the fabrics combining zinc oxide (ZnO) and AuNPs. Hence, the fabric was coated with ZnO nanorods prior to the deposition of AuNPs. AuNPs was obtained by ex situ synthesis and citrate reduction method. AuNPs of the size 18.5 ± 2.8 nm were then deposited on cotton fabric coated with ZnO nanorods by the dip-coating method [42]. Hongjuan Ma et al. (2013) have functionalized PE/PP non-woven fabric by gold nanostructured microtubes, where AuNPs were in situ prepared after the grafting of 4-hydroxybutyl acrylate glycidyl ether onto the shell PE layer and posterior reaction with diethylamine. The tertiary amine groups covalently bonded onto the fabric are designed as the adsorbent for metal ions and reducing agent. Spherical-shaped nanoparticles with size of 5–20 nm were obtained in this study [43]. Richard P. Padbury et al. (2015) produced 14.6 ± 1.4 nm gold nanoparticles using HAuCl4 as precursor salt. The sheets made up of nonwoven nylon-6 were soaked in NP solution and AuNPs are settled on the fabric [44]. It was observed that size and shape of nanoparticles depend upon reducing agent and concentration of the solution. These strategies produced functional textiles with improved properties, opening new perspectives for studies using combined approaches, able to provide the maximum AuNPs effect using low concentrations. Thus, improved methods may be obtained with reduced costs and environmental impact.
2.1.3. Chemical Reduction Method Using Thermal Treatment
Some works were found in the literature that described the potential of functional groups in the composition of fibers to act as reducing agents of gold salts under heating. These works synthesized AuNPs in situ of silk fibers due the presence of tyrosine amino acid. Bin Tang et al. (2014) functionalized the pristine silk fabric by in situ synthesis of AuNPs. The silk fabrics with tetracholoroaurate ion (AuCl4−) were heated at 85 °C in solution, and the AuNPs were in situ synthesized on silk fabric. Nanoparticles of size 21.3 ± 3.4 nm and different shapes were observed, and it was noticed that the concentration of the precursor in solution have influenced the shape of the nanoparticles and also improved thermal conductivity [45]. Jun Liu et al. (2016) have produced silk fabrics coated with gold nanoparticles. Gold nanoparticles were in situ synthesized on silk fabrics by heating precursor salt (HAuCl4.3H2O). The AuNPs were deposited on the silk fabrics by immersion of fabrics in the solution and heating the solution at 85 ℃ for 60 min. Concentration of the precursor solution had an influence on the shape of the AuNPs and attained spherical, triangular nanoplates, truncated nano prisms, and polygonal shapes with varying concentrations [46]. Zhanyu Zhang et al. (2019) have synthesized AuNPs from HAuCl4 by in situ process and maintaining pH value at 3. AuNPs coated the silk fabrics by immersion of fabric in solution and applying heat process [47]. These works showed the importance of functional groups in the fiber composition and how they can be used to obtain functional textiles and suggested novel studies using other protein fibers.
2.1.4. Green-Bio Synthesis
To facilitate the use of gold nanoparticles in the health sector and minimize the environmental impact, it is essential not to use any toxic or harmful chemical during the synthesis. Hence, researchers have been shifted towards green synthesis methods, where the reducing and stabilizing agents are obtained from plants or a few at times bacteria. All the works that evolve biological methods for AuNPs are quite recent, being this approach a high research tendency. R.M. Ganesan et al. (2015) have synthesized AuNPs using HAuCl4 as a precursor and extract of Acorus calamus rhizome as a reducing agent. Then, the cotton fabrics were coated by the pad-dry-cure method. The synthesized AuNPs were small and big spherical shape and had different sizes depending upon concentration of the solution [48]. Nabil A. Ibrahim et al. (2016) have biosynthesized AuNPs using HAuCl43H2O as a precursor salt and bacterial isolates (Streptomyces sp.) as a reducing agent. The surfaces of cotton and viscose knitted fabrics were modified using plasma treatment before the functionalization with gold nanoparticles in combination with TiO2NPs or ZnONPs. The AuNPs were observed to have spherical shape with size in the range of 4–13 nm. The nanoparticles were deposited on to the knitted fabrics by sonication [49]. Bin Tang et al. (2017) have used in situ synthesis method to prepare AuNPs onto a cotton fabric using HAuCl4 solutions at the concentrations of 0.025, 0.05, 0.075, 0.10, and 0.125 mM. Cellulose acted as reducing and stabilizing agents. The obtained AuNPs have exhibited different shapes depending on the content of gold such as spherical and triangular nanoplates with different sizes. The coated fabric displayed catalytic activity and improved UV protection [50]. To obtain a green approach, Jinlon Tao et al. (2018) functionalized cotton and polyester fabric by hybrid colloids of AuNPs and NRP (natural rubber particles), which was obtained through in situ synthesis of AuNPs in NRL (natural rubber latex) matrix. In this process, NRL act as both reducing and capping agent. HAuCl4 solution and NRL was employed for obtaining AuNP@ NRP hybrid latex. The team induced the hierarchical nature into the material by the addition of NRL, which lead to the phenomena of hydrophobicity of the treated fabrics. The fabric surface was coated with AuNPs of size 31 nm using the dip and dry method [51]. P. Boomi et al. (2019) have functionalized cotton fabric using gold nanoparticles produced by green synthesis reduction method by maintaining pH value equal to 7. In this study, AuNPs were synthesized by reducing HAuCl4 with Coleus aromaticus leaf extract. The AuNPs were coated on the cotton fabric by immersion of the fabric in the colloidal solution. The obtained nanoparticles were of spherical and triangular shape and different sizes were measured [52]. Pandi Boomi et al. (2020) have synthesized AuNPs and deposited them on the cotton fabric to improve their antibacterial and anticancer properties. The gold nanoparticles were synthesized by green synthesis, where Croton sparsiflorus leaves extract acted as both reducing and stabilizing agent and HAuCl4 as precursor salt. The cotton fabric was coated with pristine leaf extract through the pad-dry-cure method. Different sizes between 16.6 and 17 nm were obtained using high concentration and low concentration solution, respectively [53]. Simone Haslinger et al. (2019) reported a novel strategy that was attempted for the first time. Noble metal nanoparticles were added into the cellulose pulp by a hydrothermal approach and subsequently subjected to dry-wet spin process. They have functionalized cellulose-based textiles with Au and AgNPs. In this study, bleached birch prehydrolyzed kraft pulp acted as a reducing agent and HAuCl4 as precursor salt to synthesize AuNPs. These nanoparticles were incorporated into the textile by dry-jet wet spinning process, which improved the UV protection and helped to achieve bright colors [54]. Some attempts to use the combination of both chemical and green methodologies to study the synergetic effect of both methodologies have been performed. Velmurugan et al. (2016) synthesized AuNPs using in situ synthesis method onto leather, silk, and cotton fabrics by three different methods that include green, chemical, and a combination of green and chemical synthesis. Ginkgo biloba Linn leaf powder extract, HAuCl4, and potassium borohydride (KBH4) were used in green and chemical synthesis. For the combination of green and chemical synthesis, Ginkgo biloba Linn leaf powder extract and KBH4 were used, and the obtained nanoparticles were deposited by immersion of the fabrics in the solution. TEM observations had revealed nanoparticles in the range of 10–75 nm with either rectangular, spherical, hexagonal with smooth edges, or roughly circular in shape [55]. The use of biological methods showed several advantages, but more studies are needed to solve reproducibility issues, understand the influence of AuNPs attached groups in the assigned properties, and implement them at the commercial level.
2.1.5. Electrochemical Synthesis
Electrochemical synthesis is a simple and inexpensive method to obtain NPs but a few studies were found in the literature in the textile field. Mauro Pasta et al. (2012) have produced a conductive textile by coating single-walled carbon nanotubes on a polyester textile substrate. Subsequently, AuNPs were prepared using HAuCl3 in the presence of HCl electrolyte. Later, AuNPs were electrodeposited onto the conductive textiles and the size of the nanoparticles was found with a mean diameter of 50 nm [58].
2.2. Functionalization of Fibers/Yarns/Threads with AuNPs
Similarly, to the fabrics, the functionalization of fibers, yarns, and threads is performed mostly using chemical methods. A few reports were found in the literature using the other strategies (Table 2). AuNPs were deposited in these textile materials by commonly used methods, namely, exhaustion, immersion, soaking, sonication, and electrodeposition. Here, the use of most recent approaches such as in situ and biological methods to prepare AuNPs are limited and numerous researcher studies can be developed.
Table 2. Studies about the functionalization of Fibers (Fb), Yarns (Y) or Threads (T). (n.a. = not available).
| Method for Synthesis of AuNPs | Deposition Method | Fabric/Textile | Precursor Salt | Reducing Agent | Stabilizing Agent | Additional Information | Size of NPs | Application | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Photochemical reduction | Exhaustion | Silk and nylon—Fb | Tetrachloroauric acid (0.2 mM) | Trisodium citrate, D-malic acid disodium slat, and disodium tartrate (1 mM) | Trisodium citrate | Spherical and egg shapes were observed for heating method and photo chemical synthesis at pH = 4, respectively | Various sizes | Textile coloration | [57] |
| Chemical reduction | Soaked in solution | Cotton—T | Hydrogen tetrachloroaurate (0.65 mM) | Sodium citrate tribasic dihydrate | n.a. | SERS technique was used to detect and analyze adsorbed gold nanoparticles. | 20 and 60 nm | Diagnostics for surface-enhanced Raman scattering (SERS) spectroscopy | [59] |
| Chemical reduction | Immersion and capillary action | Cotton—T | Tetrachloroauric acid solution 2% (V/V) | Sodium citrate (2% (M/V)) | n.a. | Spherical shape; used HCl and NaOH for pH. | 20–40 nm | Surface-enhanced Raman scattering detection | [60] |
| Chemical reduction | Sonication | Cellulose—Fb | Tetrachloroauric (III) acid (0.5 mM, 20 mL) | Sodium squarate in water | Sodium squarate in water | AuNPs synthesized in water; Spherical in shape. | 21.01 nm | Catalysis | [61] |
| Chemical reduction | Immersion and stirring | Cotton—Y | Tetrachloroauric (III) acid (1 mM) | Trisodium citrate dihydrate (4 mM) | Citrate | pH 3–4. | 13 nm | Human motion sensor/wearable sensor | [62] |
| In situ chemical reduction | Soaking in solution | Silk fibroin—Fb | Tetrachloroauric (III) acid (10 mmol L−1) | Sulfonated polyaniline (20 mL of 5 wt %) | n.a. | Sulfonated polyaniline modified fibers catalytic reduction reaction of p-nitrophenol by NaBH4 |
50–100 nm | Catalysis | [63] |
| In situ chemical reduction | Immersion | Ramie—Fb | Tetrachloroauric (III) acid with different concentrations | Sodium borohydride | n.a. | AuNPs were synthesized in acidic condition, pH = 2–6. | n.a. | Textile coloration and antimicrobial textiles | [64] |
| In situ chemical reduction | Immersion and stirring | Cellulose—Fb | Tetracholoroaurate (0.5 mM) | Sodium rhodizonate | Sodium rhodizonate | Size depends on temperature; spherical shape. | 11 nm at 23 °C and 7 nm at 80 °C | Catalysis | [65] |
| Chemical reduction | Immersion and stirring | Cellulose—Fb | Gold chloride (AuCl3) | p-nitro-aniline (2 mM) Sodium borohydride (150 mM) |
Cellulosic macromolecules | n.a. | 26.1 nm | Catalysis | [66] |
| Chemical reduction | Electroless deposition | Gold/graphene—Y | Tetrachloroauric (III) acid (1.6 mM) | Hydroxylamine | n.a. | Spherical to plate; dependent on reaction time. | 40 nm | Wearable electronics | [67] |
| Chemical reduction | Immersion | Regenerated cellulose—Fb | Gold (III) chloride triydrate (1 mM) | Trisodium citrate (1%, 2.2 mL) | Trisodium citrate | Fibers were grafted with positive charge; spherical shape | 40–50 nm | colorimetry and surface-enhanced Raman scattering (SERS) assays | [68] |
| Chemical reduction | Sonication | Cotton—T | Tetrachloroauric (III) acid (0.01%, W/V) | Trisodium citrate | n.a. | AuNPs coated on CNTs CNTs were functionalized with PDDA; homogenous surface. |
15 nm | Immunological chromatographic sensor | [69] |
| Chemical reduction | Centrifugation | Cotton—T | Tetrachloroauric (III) acid (0.01%, W/V) | Trisodium citrate | n.a. | AuNPs coated on CNTs CNTs were functionalized with PDDA; homogenous surface. |
15 ± 3 nm | Immunological chromatographic sensor | [70] |
| In situ green synthesis | Immersion | Cotton—Fb | Hydrogen tetrachloroaurate (III) hydrate (0.05 mM) | Osmanthus fragrans 10% (m/v) | Osmanthus fragrans | Spherical and hexagonal shape. | 40 and 60 nm | Heterogeneous catalyst | [71] |
| Biological reduction | Soaking | Silk—Fb | Tetrachloroauric acid (10−3 M) | Citrus paradisi extract | n.a. | Quasi-spherical | 30 nm | Textile coloration | [72] |
2.2.1. Chemical Reduction Method without Pre-Treatments on Fabrics
David R. Ballerini et al. (2014) have synthesized AuNPs by the Turkevich method using HAuCl4 as precursor salt and sodium citrate tribasic dihydrate as a reducing agent. AuNPs of size of 20 and 60 nm were obtained and deposited on the cotton thread by soaking them individually in the solution of AuNPs. The cotton threads were treated with cationic polyacrylamide (CPAM) before the deposition of AuNPs [59]. Cristina Battesini Adamo et al. (2020) have synthesized spherical-shaped gold nanoparticles of size 20–40 nm using the Turkevich method. In this study, HAuCl4 solution (30 wt%) and sodium citrate were used. The AuNPs were wicked on to the cotton thread by capillary action when threads were immersed in the solution [60]. Md. Tariqul Islam et al. (2016) have produced AuNPs with average size of 21.01 nm where HAuCl4 was used as a precursor salt and sodium squarate in water was used as a reducing and stabilizing agent. The gold nanoparticles were synthesized in water and attached to the cellulose fibers by sonication [61]. Hyung Ju Park et al. (2016) have fabricated cotton yarns decorated with Au core-shell nanoparticle by a solution-based approach. The AuNPs were synthesized by a chemical reduction method where HAuCl4 was used as a precursor salt solution and trisodium citrate dihydrate was used as a reducing agent and stabilizing agent. The solution was maintained at pH between 3 and 4, and the obtained nanoparticles were of the size 13 nm [62].
Some reports conducted with in situ methods have been reported, but in all studies, additional chemical reducing agents were used. Youvi Xia et al. (2011) have produced sulfonated polyaniline-modified silk fibroin fibers coated with AuNPs. AuNPs of size in the range of 50–100 nm were synthesized using HAuCl4 via in situ reduction method where sulfonated polyaniline acted as reducing agent. They were deposited by soaking fibers in the solution that contains AuNPs [63]. Bin Tang et al. (2015) have synthesized AuNPs through in situ synthesis using HAuCl4 with different concentrations and NaBH4. The pH value of the solution was maintained such that it had acidic condition. They were deposited on to the ramie fibers by immersion of fibers in the solution [64]. Tariqul Islam et al. (2017) have used in situ synthesis where HAuCl4 was used as precursor salt and sodium rhodizonate was used as a reducing and stabilizing agent to produce AuNPs. The AuNPs were found to have spherical shape and the size of 7 and 11 nm. The stabilized AuNPs were readily adsorbed on cellulose fibers [65]. Hossam E. Emam et al. (2017) have used one-pot fabrication of AgNPs onto a cellulosic solid support. AuNPs of 26.1 nm were synthesized through green synthesis using gold chloride (AuCl3), NaBH4 as reducing agent, and cellulosic macromolecules as stabilizing agent. They were deposited onto cellulose solid support by one-step method [66]. Yong Ju Yun et al. (2017) have fabricated gold/graphene yarns using a solution-based process to potentially use them for flexible and wearable electronics. HAuCl4 was used as precursor salt and hydroxylamine was used as a reduced agent for the synthesis of AuNPs. They were deposited uniformly on the surface by electroless deposition and found to have from spherical shape to plate and grow vertically with time, the size of the AuNPs was 40 nm in diameter [67]. Qian Yu et al. (2018) have produced multifunctional cellulose fiber Au composites via decorating regenerated cellulose fiber with AuNPs. HAuCl4 was used as a precursor salt and trisodium citrate was used as a reducing and stabilizing agent to produce gold nanoparticles following Natan’s method. The obtained spherical AuNPs with size of 40–50 nm were decorated on to the surface of cellulose fiber by the immersion of fibers in the solution of Au colloids. The fibers were grafted with positive charge according to the method proposed by Tabba and co-workers with some modifications [68].
2.2.2. Chemical Reduction Method with Pre-Treatments
Yan Liu et al. (2017) produced AuNPs with size of 15 nm using HAuCl4 solution and trisodium citrate by chemical reduction method. AuNPs were coated on the CNTs, which were priorly functionalized with poly(diallyldimethylammonium chloride) (PDDA) using sonication method, and these were deposited on to the cotton thread by soaking cotton threads in the solution that contains AuNPs/CNTs. To improve the wicking function of the cotton thread, chemical treatments are necessary to eliminate the surface contaminants [69]. Xiaobo Jia et al. (2017) have synthesized AuNPs with size of 15 ± 3 nm using HAuCl4 solution and trisodium citrate by chemical reduction method. These nanoparticles are mixed with CNT and subsequently, the cotton thread device was constructed for carcinoembryonic antigen (CEA) detection. It was constructed by soaking the cotton thread in the solution containing AuNPs-coated CNTs that were previously functionalized with PDAA [70].
2.2.3. Reduction with Photochemical Treatments
Bin Tang et al. (2011) have synthesized AuNPs using HAuCl4 as precursor salt and citrate, malate, and tartrate as reducing agents by heating and photochemistry processes. The solution was used to color silk and nylon fibers, and AuNPs were deposited using the exhaustion process. Nanoparticles were observed to have egg, spherical shape and various sizes depending on the type of reducing agent [57].
2.2.4. Green Synthesis
Naseeb Ullah et al. (2019) have used HAuCl4 as a precursor salt and Osmanthus fragrans leaves as reducing and capping agent irradiated by natural sunlight to produce AuNPs. The cotton fibers were then immersed in the AuNPs solution, and they were deposited on the fabrics through in situ reduction in the presence of direct sunlight. Nanoparticles of size 40 and 60 nm in diameter were observed in TEM and appeared to have spherical and hexagonal shapes [71]. Victor Nolasco-Arizmendi et al. (2012) have synthesized AuNPs using HAuCl4 as the base solution and citrus paradise extract as the reducing agent, and the AuNPs were deposited on the silk fabric by impregnating the fabric in the solution. Quasi-spherical-shaped nanoparticles were observed [72].
2.3. Functionalization of Nanofibers/Scaffolds/Membranes with AuNPs
The progress in synthetic or artificial fibers has created several opportunities to develop novel multifunctional textiles. Spinning methods, particularly, the electrospinning, have provided fibers from different polymers and have been widely used to obtain nanofibers and scaffolds conjugated with AuNPs. Very limited literature is available in recent years that used green synthesis methods to produce AuNPs and deposited them on nanofibers/scaffolds/membranes. In the case of chemical methods, most studies perform the in situ AuNPs synthesis directly in the electrospinning solution. In addition, in very specific cases, the AuNPs are previously synthesized and mixed in the solution before electrospinning. In the case of membrane functionalization, the AuNPs are deposited by soaking or immersion (Table 3).
Table 3. Studies about the functionalization of Nanofibers (NF), Scaffolds (S) and Membranes (M). (n.a. = not available).
| Deposition Method | Fabric/Textile | Precursor Salt | Reducing Agent | Stabilizing Agent | Additional Information | Size of NPs | Application | Reference |
|---|---|---|---|---|---|---|---|---|
| Method for Synthesis: Biological reduction | ||||||||
| Electrospinning solution | Polyacrylonitrile—NF | Tetrachloroauric (III) acid (0.001 M) | Banana peel extract, phenolic compounds and flavonoids | n.a. | Spherical shape. | 9 nm | Electrochemical sensors | [2] |
| Method for Synthesis: Chemical reduction | ||||||||
| Electrospinning solution | Polyimide—NF | Gold (III) chloride hydrate | Thermal treatment at 200 °C in polyimide nanofibers | n.a. | Thermally reduced Au3+ to Au0. | n.a. | n.a. | [73] |
| Immersion | Crystalline cellulose—NF | Tetrachloroauric acid | Sodium borohydride | n.a. | - | <5 nm | Catalysis | [74] |
| Immersion | Bacterial cellulose—NF | Tetrachloroauric acid | Poly (ethyleneimine) | Poly(ethyleneimine) | Spherical shape. | ≈9 nm | Biosensors | [75] |
| Immersion | Bacterial cellulose—NF | Tetrachloroauric acid (20 mM) | Poly (ethyleneimine) | n.a. | BC nanofibers are produced by ultrasonic cell disruption system at room temperature. | ≈9 nm | Biosensors | [76] |
| Immersion | Cellulose acetate (mats)—NF | Tetrachloroauric (III) acid (1 Wt %, 3 mL) | Trisodium citrate | Trisodium citrate | LBS self-assembly technique. | – | Antimicrobial textiles | [77] |
| Immersion | Thermoplastic polyurethane—NF | Hydrogen tetrachloroaurate | Trisodium citrate | Chitosan | Precoated with chitosan reduction in 4-nitrophenol by sodium borohydride, pH value 3–11. |
16 nm | Catalysis | [78] |
| Electrospinning solution | PCL/Gelatin—NF/S | Tetrachloroauric (III) acid | Sodium borohydride | n.a. | Process is done in the presence of antibiotic intermediates. | 3 nm | Antimicrobial textiles, wound treatment | [79] |
| Soaking | Bacterial cellulose—M | Tetrachloroauric (III) acid (40 mg mL−1) | Sodium borohydride | n.a. | Gold nanoparticles modified with 4,6-Diamino-2-Pyrimidinethiol (DAPT). | ≈3 nm | Antimicrobial textiles, wound treatment | [80] |
| Electrospinning solution | PEI/PVA—NF | Chloroauric acid (0.5 mM) | Sodium borohydride | n.a. | The fibrous were cross-linked via glutaraldehyde (GA) vapor to produce water-stable fibrous mats; round shaped. | 11.8 nm | Catalysis | [81] |
| Electrospinning solution | Polycaprolactone—NF | Chloroauric acid (0.5 mM) | Sodium borohydride (1.25 mM) | n.a. | Round shape. | 5–6 nm | Heterogeneous catalysis and SERS | [82] |
| Immersion and shaking | PET track-etched micro-porous—M | Tetrachloroauric (III) acid (1 mg mL−1) | Dopamine on membranes | n.a. | Coated with dopamine. | n.a. | Catalysis | [83] |
| Dropping | Polyamide—NF/M | Hydrogen tetrachloroaurate (III) (1.00 mM) | Trisodium citrate dihydrate (0.30 M) | Trisodium citrate dihydrate | Spherical | 20.1 ± 1.76 nm | Colorimetric sensor | [84] |
| Immersion | Bacterial cellulose—NF | Chloroauric acid (0.4 mM) | Sodium borohydride (6 mM) | n.a. | 2,2,3,3-tetramethylpiperidine-l-oxyl (TEMPO)-oxidized (TOBCNS); spherical shape. | 4.30 ± 0.97 nm | Catalysis | [85] |
| Immersion | Cellulose—NF | Tetrachloroauric (III) acid solution (0.2 mM) | Hydrazine hydrate | n.a. | Unsupported AuNPs. | 18.3 ± 3.5 nm | Catalysis | [86] |
| Method for Synthesis: In situ chemical reduction | ||||||||
| Electrospinning solution | Polyacrylonitrile—NF | Hydrogen tetrachloroaurate (III) trihydrate | 4-(Dimethylamino) benzaldehyde | n.a. | – | 6 nm | Biosensors | [87] |
| Soaking | Cellulose Acetate—M | Tetrachloroauric (III) acid | Dithiothreitol (DTT) | Porous fiber network | DTT capped AuNPs. | 2.5 ± 0.5 nm | Sensors | [88] |
| Electrospinning solution | Zein—NF | Tetrachloroauric (III) acid (20 mM, 0.25 mL) | Poly(ethyleneimine) | n.a. | Spherical shape; value of pH 3.0–7.0. | 90.9 nm | Biosensors | [89] |
| Electrospinning solution | Polyacrylonitrile—NF | Tetrachloroauric (III) acid (0.30 mmol) | n.a. | n.a. | Au-PANF was prepared and electrospun to form mats; spherical shape. | 2.3 ± 0.5 nm | Sensors | [90] |
| Electrospinning solution | Polyimide—NF | Tetrachloroauric (III) acid (0.5, 1, and 3 Wt %) | Polyimide and high temperature (200 °C) | Polyimide | Reduction takes place due to thermal reactions. | 9–22 nm | High temperature end-of-service indicators | [91] |
| Immersion | Cellulose—M | Potassium gold (III) chloride | Sodium borohydride | Poly(diallyl-dimethylammonium chloride) poly(sodium-p-styrenesulfonate) |
Membranes previously coated with titania gel. | 3.5 ± 0.7 nm | n.a. | [92] |
| Immersion and continuous shaking | PET track-etched microporous—M | Tetrachloroauric (III) acid (1 mg mL−1) | Sodium borohydride | n.a. | Coated with dopamine. | n.a. | Catalysis | [83] |
| Method for Synthesis: Photoreduction | ||||||||
| Electrospinning solution | Polystyrene (mats)—NF | Tetrachloroauric (III) acid (3% (W/W) | Ultraviolet irradiation | n.a. | Undergone electrospinning and then mats exposed to UV light. | n.a. | n.a. | [93] |
| Method for Synthesis: In situ photoreduction | ||||||||
| Electrospinning solution | Polyacrylonitrile—NF | Gold (III) chloride hydrate(0.044 M, 0.022 M) | Sodium alginate | n.a. | UV light has been used during electrospinning; spherical shape. | 21.4 nm (0.044 M) 5.8 nm (0.022 M) |
n.a. | [94] |
| Electrospinning solution | Polyacrylonitrile—NF | Tetrachloroauric (III) acid | N,N-dimethylformamide | n.a. | Undergone electrospinning and then mats exposed to UV light. | 4.7–5.4 nm (5 days of UV radiation) | n.a. | [95] |
| Immersion | Cellulose—NF | Tetrachloroauric (III) acid solution (0.2 mM, 20 mL) | Cellulose nanocrystals | Cellulose nanocrystals | CNs were produced from microcrystalline cellulose. | 30.5 ± 13.4 nm | Catalysis | [86] |
| Immersion | Bacterial cellulose—M | Tetrachloroauric acid (0.2, 0.4, 0.6 mM) | n.a. | n.a. | Xenon lamp was used in the process of synthesis of AuNPs; spherical shape. |
n.a. | Sensors | [96] |
| Method for Synthesis: Laser ablation | ||||||||
| Electrospinning solution | Polyacrylonitrile—NF | Gold plate | n.a. | n.a. | Spherical shape; face centered cubic crystal structure with crystallite size of 8 nm. | 17 nm | Glucose sensors | [97] |
2.3.1. Chemical Reduction Method without Pre-Treatments
The two-step chemical reduction method, the AuNPs synthesis and posterior textiles functionalization, was widely applied. Wei Wang et al. (2010) have produced AuNPs–Bacterial cellulose (BC) nanocomposites. In this study, AuNPs were synthesized by the reduction of HAuCl4 in the presence of NaBH4 that acted as reducing agent, mixed with BC nanofibers. Produced AuNPs have measured approximately 9 nm. The AuNPs were deposited on fibers by immersing them in the solution of BC nanofibers and applying ultrasonic cell disruption system at room temperature. [76]. Hirotaka Koga et al. (2010) have synthesized AuNPs on crystalline cellulose single nanofibers (CSNFs). Preparation of AuNPs includes the addition of CSNFs in the solution of HAuCl4 followed by reduction with NaBH4. The nanoparticles of size less than 5 nm were deposited because of immersion of fibers in nanoparticles in solution [74]. Taiji Zhang et al. (2010) have obtained spherical-shaped AuNPs of approximately 9 nm by the reduction of HAuCl4 in the presence of polyethyleneimine (PEI) that played the role of both reducing and stabilizing agent, and it also acted as a linking agent to uniformly coat the AuNPs on to the BC nanofibers. AuNPs were coated on to the BC nanofibers by suspending them in the solution [75]. Bin Zhou et al. (2014) have produced antibacterial multilayer films coated with gold nanoparticles on nanofibers. Gold nanoparticles were synthesized using chemical reduction method. HAuCl4 solution and trisodium citrate were used as precursor salt, reducing and stabilizing agent for the synthesis of AuNPs, and they were coated on to the electrospun cellulose acetate fiber mats by immersion of mats into colloidal gold nanoparticles solution [77].
Hui-Hui Cheng et al. (2016) have fabricated thermoplastic polyurethane electrospun fiber mat and deposited the gold nanoparticles after the functionalization of the mat with chitosan by dip-coating method. The AuNPs of size 16 nm were produced using HAuCl4, trisodium citrate as reducing agent, and chitosan as stabilizing agent [78]. Xinglong Yang et al. (2017) have produced antibiotic intermediate capped AuNPs by chemical reduction of HAuCl4 by NaBH4. The process was done in the presence of antibiotic intermediates. These nanoparticles of size approximately 3 nm have been embedded on to the polycaprolactone (PCL)/gelatin nanofibers in the form of scaffolds during electrospinning [79]. Ying Li et al. (2017) have produced a bacterial cellulose (BC) membrane decorated by AuNPs and modified with 4,6-diamino-2-pyrimidinethiol (DAPT) for treating bacterially infected wounds. In this research work, AuNPs of size 3 nm were produced via in situ synthesis method where HAuCl4 and DAPT solution was used. The AuNPs were deposited on to the BC membranes by soaking them in the solution [80]. Xu Fang et al. (2010) have produced electrospun poly(ethyleneimine)/polyvinyl alcohol nanofibers and immobilized them with round-shaped gold nanoparticles of size 11.8 nm. In this study, the AuNPs were synthesized and deposited on to the electrospun fibers via in situ reduction of AuCl4− ions using NaBH4 as reducing agent [81]. Zhen Liu et al. (2011) have produced electrospun porous polyacrylonitrile nanofibers and functionalized them with AuNPs. AuNPs were synthesized by in situ reduction of HAuCl4 to Au (0) by 4-(dimethylamino) benzaldehyde. The electrospun membrane was immersed into the buffered hydrogen tetrachloroaurate (III) trihydrate solution and then, AuNPs of size 6 nm, were adsorbed onto the nanofibers [87]. Anitha Senthamizhan et al. (2014) have produced DTT capped AuNPs by in situ synthesis using the gold solution and dithiothreitol. AuNPs of the size 2.5 ± 0.5 nm were deposited on the cellulose acetate fibrous membranes by soaking them in the solution [88].
Xiaodong Chen et al. (2014) have functionalized zein ultrafine fibers by AuNPs. The zein ultrafine fibers were produced by electrospinning and, subsequently, AuNPs were added on to the fibers by one step reduction method (in situ polymerization) using HAuCl4 as precursor salt and poly(ethyleneimine) (PEI) as reducing agent and cross-linking agent. The pH was maintained between 3 and 7, and the size of the AuNPs were observed to be 90.9 nm in diameter [89]. Han Zhu et al. (2014) have synthesized AuNPs embedded polyacrylonitrile nanofibers by the combination of in situ reaction and electrospinning. AuNPs were produced using the HAuCl4 solution via in situ reduction method. The nanofibrous mats with AuNPs embedded in it were achieved by the electrospinning technique and spherical-shaped AuNPs with size of 2.3 ± 0.5 nm [90]. Diana Serbezeanu et al. (2015) have produced gold-containing polyimide fibers. AuNPs were synthesized using an in situ electrospinning approach where HAuCl4.3H2O was used as precursor salt and polyimide was used as stabilizing agent. The reduction takes place due to the thermal reactions and the SEM observations displayed nanoparticles of the size 9–22 nm [91]. Simón Yobanny Reyes-López et al. (2015) have proposed a facile method to produce gold-coated polycaprolactone nanofibers. Reduction method was employed to synthesize AuNPs, round-shaped 5–6 nm nanoparticles, using HAuCl4 and NaBH4 as a reducing agent. The gold nanoparticles were separated in a rotavapor, were added into the viscous solution of PCL, and subjected to electrospinning [82].
2.3.2. Chemical Reduction Method with Pre-Treatments
Tao Niu et al. (2014) have produced cellulose-based membranes and coated the membranes with AuNPs. In this research study, AuNPs were synthesized with KAuCl4 and NaBH4 and these nanoparticles were deposited on membranes previously coated with Titania gel [92]. Riyas Subair et al. (2016) synthesized gold nanoparticles on the PET track-etched micro-porous membranes. Before synthesis on nanoparticles, these membranes were coated with dopamine and poly(ethyleneimine). The authors followed two types of methodologies for the synthesis of AuNPs. AuNPs were synthesized by self-reduction of AuCl4− immobilized on the surface of dopamine membrane, and AuNPs were synthesized via in situ reduction with NaBH4 on PEI membranes. In both the cases, AuNPs were deposited by immersion of the membranes into nanoparticles solution and maintaining continuous shaking condition [83]. Mohammed Awad Abedalwafa et al. (2019) have functionalized polyamide nanofiber membranes using AuNPs. The AuNPs were synthesized via citrate reduction technique where HAuCl4 and trisodium citrate dihydrate were used as precursor salt and reducing agent, respectively. AuNPs were functionalized with melamine (MA) and were immobilized on polyamide 6 nanofiber electrospun membranes by dropping MA@AuNPs on the membranes. AuNPs and MA@AuNPs were found to have the size of 20.1 ± 1.7 and 28.4 ± 2.3 nm, respectively [84]. Yan Chen et al. (2016) have deposited AuNPs on bacterial cellulose nanofibers treated with 2,2,3,3-tetramethylpiperidine-l-oxyl (TEMPO)-oxidized (TOBCNS). AuNPs were produced by chemical reduction method using HAuCl4 and NaBH4, and simultaneously, they were deposited on to the TOBCNS by immersing nanofibers in the solution. It was observed from TEM that nanoparticles were spherical having size of 4.30 ± 0.97 nm [85].
2.3.3. Chemical Reduction by Photo Reduction/UV Radiation/Photo Reduction
Fadwa H. Anka et al. (2012) have produced polyacrylonitrile nanofibers using electrospinning, and they have been functionalized with AuNPs. The AuNPs were synthesized via in situ photoreduction. In this study, HAuCl4 was the precursor salt and sodium alginate acted as reducing agent. UV light has been used during electrospinning. Spherical shape was observed with a size of 21.4 and 5.8 nm [94]. Koichi Sawada et al. (2013) have produced PAN (Polyacrylonitrile) nanofibrous fabrics and deposited AuNPs onto the fabrics. PAN fabrics were produced by electrospinning of PAN solution and HAuCl4. AuNPs were formed on the fabrics by in situ gold formation using UV irradiation. The average size of the gold nanoparticles obtained was 4.7–5.4 nm [95].
Xiaodong Wu et al. (2014) have used one-pot and green synthesis to synthesize and deposit the gold nanoparticles on to cellulose nanocrystals. By means of hydrothermal reaction AuNPs were deposited on to the cellulose nanocrystals. In this work, they have used HAuCl4 solution and cellulose nanocrystals as a both reducing and stabilizing agent to synthesize AuNPs with size of 30.5 ± 13.4 nm. These cellulose nanocrystals are derived from cotton fabrics. In the same study, unsupported AuNPs were synthesized, using hydrazine hydrate as reducing agent, with size of 18.3 ± 3.5 nm [86]. Xu Zhou et al. (2018) have synthesized AuNPs on BC using the photoinduction method. In this study, HAuCl4 was used as precursor salt and xenon lamp was used as reducing agent. AuNPs were deposited by immersing the BC hydrogels in the HAuCl4 solution [96].
2.3.4. Green Synthesis
Moeng G. Motitswe et al. (2020) have used the green technique for the synthesis of AuNPs where banana (Musa paradisiaca) was used as a reducing agent and HAuCl4 was used as the precursor salt. The spherical-shaped 9 nm AuNPs were deposited on to the polyacrylonitrile nanofibers using electrospinning technology [2].
2.3.5. Reduction with Thermal Treatments
Maggalena Aflori et al. (2015) have incorporated gold nanoparticles into electrospun polyimide fibers. In this work, HAuCl4 was used as gold precursor and treated thermally at 200 °C for 6 h to reduce Au3+ to Au0. The electrospinning technique was used to incorporate the AuNPs into polyimide fibers [73].
2.3.6. Reduction with Other Treatments
Amir Shahin Shamsabadi et al. (2019) have produced AuNPs by laser ablation in the solution of PAN in dimethyl formamide. After that, spherical-shaped AuNPs were mixed with the PAN solution to subject to electrospinning and produce PAN nanofibers incorporated with AuNPs [97].
This entry is adapted from the peer-reviewed paper 10.3390/nano11051067
References
- Zhao, P.; Li, N.; Astruc, D. State of the Art in Gold Nanoparticle Synthesis. Coord. Chem. Rev. 2013, 257, 638–665.
- Motitswe, M.G.; Fayemi, O.E.; Drummond, H.P. Electrochemical and Spectroscopic Properties of Green Synthesized Gold Nanoparticles Doped in Polyacrylonitrile Nanofibers. J. Clust. Sci. 2020, 32, 683–692.
- Stensberg, M.C.; Wei, Q.; McLamore, E.S.; Porterfield, D.M.; Wei, A.; Sepúlveda, M.S. Toxicological Studies on Silver Nanoparticles: Challenges and Opportunities in Assessment, Monitoring and Imaging. Nanomedicine 2011, 6, 879–898.
- Okkeh, M.; Bloise, N.; Restivo, E.; De Vita, L.; Pallavicini, P.; Visai, L. Gold Nanoparticles: Can They Be the next Magic Bullet for Multidrug-Resistant Bacteria? Nanomaterials 2021, 11, 312.
- Roy, S.; Das, T.K.; Maiti, G.P.; Basu, U. Microbial Biosynthesis of Nontoxic Gold Nanoparticles. Mater. Sci. Eng. B 2016, 203, 41–51.
- Baruah, D.; Goswami, M.; Yadav, R.N.S.; Yadav, A.; Das, A.M. Biogenic Synthesis of Gold Nanoparticles and Their Application in Photocatalytic Degradation of Toxic Dyes. J. Photochem. Photobiol. B Biol. 2018, 186, 51–58.
- Shamaila, S.; Zafar, N.; Riaz, S.; Sharif, R.; Nazir, J.; Naseem, S. Gold Nanoparticles: An Efficient Antimicrobial Agent against Enteric Bacterial Human Pathogen. Nanomaterials 2016, 6, 71.
- Andra, S.; Balu, S.; Jeevanandam, J.; Muthalagu, M. Emerging Nanomaterials for Antibacterial Textile Fabrication. Naunyn Schmiedeberg’s Arch Pharmacol. Modifying 2021, 1–28.
- Toma, H.E.; Zamarion, V.M.; Toma, S.H.; Araki, K. The Coordination Chemistry at Gold Nanoparticles. J. Braz. Chem. Soc. 2010, 21, 1158–1176.
- Bakshi, M.S. How Surfactants Control Crystal Growth of Nanomaterials. Cryst. Growth Des. 2016, 16, 1104–1133.
- Daruich De Souza, C.; Ribeiro Nogueira, B.; Rostelato, M.E.C.M. Review of the Methodologies Used in the Synthesis Gold Nanoparticles by Chemical Reduction. J. Alloys Compd. 2019, 798, 714–740.
- Zhao, J.; Stenzel, M.H. Entry of Nanoparticles into Cells: The Importance of Nanoparticle Properties. Polym. Chem. 2018, 9, 259–272.
- Kalimuthu, K.; Cha, B.S.; Kim, S.; Park, K.S. Eco-Friendly Synthesis and Biomedical Applications of Gold Nanoparticles: A Review. Microchem. J. 2020, 152, 104296.
- Fan, J.; Cheng, Y.; Sun, M. Functionalized Gold Nanoparticles: Synthesis, Properties and Biomedical Applications. Chem. Rec. 2020, 20, 1474–1504.
- Pereira, C.; Pereira, A.M.; Freire, C.; Pinto, T.V.; Costa, R.S.; Teixeira, J.S. Nanoengineered Textiles: From Advanced Functional Nanomaterials to Groundbreaking High-Performance Clothing. In Handbook of Functionalized Nanomaterials for Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2020.
- Massella, D.; Argenziano, M.; Ferri, A.; Guan, J.; Giraud, S.; Cavalli, R.; Barresi, A.A.; Salaün, F. Bio-Functional Textiles: Combining Pharmaceutical Nanocarriers with Fibrous Materials for Innovative Dermatological Therapies. Pharmaceutics 2019, 11, 403.
- Sanchez-Herencia, A.J. Water Based Colloidal Processing of Ceramic Laminates. Key Eng. Mater. 2007, 333, 39–48.
- Engates, K.E.; Shipley, H.J. Adsorption of Pb, Cd, Cu, Zn, and Ni to Titanium Dioxide Nanoparticles: Effect of Particle Size, Solid Concentration, and Exhaustion. Environ. Sci. Pollut. Res. 2011, 18, 386–395.
- Ribeiro, A.I.; Senturk, D.; Silva, K.S.; Modic, M.; Cvelbar, U.; Dinescu, G.; Mitu, B.; Nikiforov, A.; Leys, C.; Kuchakova, I.; et al. Efficient Silver Nanoparticles Deposition Method on DBD Plasma-Treated Polyamide 6,6 for Antimicrobial Textiles. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 460, p. 0120070.
- Boomi, P.; Ganesan, R.; Prabu Poorani, G.; Jegatheeswaran, S.; Balakumar, C.; Gurumallesh Prabu, H.; Anand, K.; Marimuthu Prabhu, N.; Jeyakanthan, J.; Saravanan, M. Phyto-Engineered Gold Nanoparticles (AuNPs) with Potential Antibacterial, Antioxidant, and Wound Healing Activities Under in Vitro and in Vivo Conditions. Int. J. Nanomed. 2020, 15, 7553–7568.
- Magura, J.; Zeleňáková, A.; Zeleňák, V.; Kaňuchová, M. Thiol-Modified Gold Nanoparticles Deposited on Silica Support Using Dip Coating. Appl. Surf. Sci. 2014, 315, 392–399.
- Tang, J.; Ou, Q.; Zhou, H.; Qi, L.; Man, S. Seed-Mediated Electroless Deposition of Gold Nanoparticles for Highly Uniform and Efficient SERS Enhancement. Nanomaterials 2019, 9, 185.
- Preda, N.; Enculescu, M.; Zgura, I.; Socol, M.; Matei, E.; Vasilache, V.; Enculescu, I. Superhydrophobic Properties of Cotton Fabrics Functionalized with ZnO by Electroless Deposition. Mater. Chem. Phys. 2013, 138, 253–261.
- Elmaaty, T.A.; El-Nagare, K.; Raouf, S.; Abdelfattah, K.; El-Kadi, S.; Abdelaziz, E. One-Step Green Approach for Functional Printing and Finishing of Textiles Using Silver and Gold NPs. RSC Adv. 2018, 8, 25546–25557.
- Dietzel, M.; Bieri, N.R.; Poulikakos, D. Dropwise Deposition and Wetting of Nanoparticle Suspensions. Int. J. Heat Fluid Flow 2008, 29, 250–262.
- Ranzoni, A.; Cooper, M.A. The Growing Influence of Nanotechnology in Our Lives. In Micro- and Nanotechnology in Vaccine Development; Skwarczynski, M., Toth, I., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 1–20.
- Basarir, F.; Yoon, T.H. Sonication-Assisted Layer-by-Layer Deposition of Gold Nanoparticles for Highly Conductive Gold Patterns. Ultrason. Sonochem. 2012, 19, 621–626.
- Zhong, W. Nanofibres for Medical Textiles. In Advances in Smart Medical Textiles: Treatments and Health Monitoring; van Langenhove, L., Ed.; Woodhead Publishing, Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 57–70.
- Jose Varghese, R.; Sakho, E.H.M.; Parani, S.; Thomas, S.; Oluwafemi, O.S.; Wu, J. Introduction to Nanomaterials: Synthesis and Applications. In Nanomaterials for Solar Cell Applications; Thomas, S., Sakho, E.H.M., Kalarikkal, N., Oluwafemi, S.O., Wu, J., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 75–95.
- Chan, K.L.; Fawcett, D.; Poinern, G.E.J. Gold Nanoparticle Treated Textile-Based Materials for Potential Use as Wearable Sensors. Int. J. Sci. 2016, 2, 82–89.
- Shanmugasundaram, O.L.; Ramkumar, M. Characterization and Study of Physical Properties and Antibacterial Activities of Human Hair Keratin–Silver Nanoparticles and Keratin–Gold Nanoparticles Coated Cotton Gauze Fabric. J. Ind. Text. 2018, 47, 798–814.
- Zheng, Y.; Xiao, M.; Jiang, S.; Ding, F.; Wang, J. Coating Fabrics with Gold Nanorods for Colouring, UV-Protection, and Antibacterial Functions. Nanoscale 2013, 5, 788–795.
- Lin, X.; Zou, F.; Chen, X.; Tang, B. Functional Modification of Nylon Fabrics Based on Noble Metal Nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2017, 231, 012175.
- Silva, I.O.; Ladchumananandasivam, R.; Nascimento, J.H.O.; Silva, K.K.O.S.; Oliveira, F.R.; Souto, A.P.; Felgueiras, H.P.; Zille, A. Multifunctional Chitosan/Gold Nanoparticles Coatings for Biomedical Textiles. Nanomaterials 2019, 9, 1064.
- Gao, L.; Feng, J.; Xu, S.; Shi, M.; Yao, L.; Wang, L.; Yang, Z. General Strategy to Prepare Single-Layered Ag–Au–Pt Nanocrystal Ternary-Coated Biomass Textiles through Polymer-Driven Self-Assembly. Nanomaterials 2020, 10, 495.
- Ahmed, H.B.; El-Hawary, N.S.; Emam, H.E. Self-Assembled AuNPs for Ingrain Pigmentation of Silk Fabrics with Antibacterial Potency. Int. J. Biol. Macromol. 2017, 105, 720–729.
- Zhou, L.; Yu, K.; Lu, F.; Lan, G.; Dai, F.; Shang, S.; Hu, E. Minimizing Antibiotic Dosage through in Situ Formation of Gold Nanoparticles across Antibacterial Wound Dressings: A Facile Approach Using Silk Fabric as the Base Substrate. J. Clean. Prod. 2020, 243, 118604.
- Radić, N.; Obradović, B.M.; Kostić, M.; Dojčinović, B.; Hudcová, M.; Kuraica, M.M.; Černák, M. Deposition of Gold Nanoparticles on Polypropylene Nonwoven Pretreated by Dielectric Barrier Discharge and Diffuse Coplanar Surface Barrier Discharge. Plasma Chem. Plasma Process. 2013, 33, 201–218.
- Ikegami, M.; Matsumoto, T.; Kobayashi, Y.; Jikihara, Y.; Nakayama, T.; Ohashi, H.; Honma, T.; Takei, T.; Haruta, M. Air Purification by Gold Catalysts Supported on PET Nonwoven Fabric. Appl. Catal. B Environ. 2013, 134–135, 130–135.
- Cheng, D.; Bai, X.; He, M.; Wu, J.; Yang, H.; Ran, J.; Cai, G.; Wang, X. Polydopamine-Assisted Immobilization of on Cotton Fabrics for Sensitive and Responsive SERS Detection. Cellulose 2019, 26, 4191–4204.
- Liu, H.; Goh, W.; Norsten, T.B. Aqueous-Based Formation of Gold Nanoparticles on Surface-Modified Cotton Textiles. J. Mol. Eng. Mater. 2013, 1, 1250001.
- Baruah, B.; Downer, L.; Agyeman, D. Fabric-Based Composite Materials Containing ZnO-NRs and ZnO-NRs-AuNPs and Their Application in Photocatalysis. Mater. Chem. Phys. 2019, 231, 252–259.
- Ma, H.; Chi, H.; Wu, J.; Wang, M.; Li, J.; Hoshina, H.; Saiki, S.; Seko, N. A Novel Avenue to Gold Nanostructured Microtubes Using Functionalized Fiber as the Ligand, the Reductant, and the Template. ACS Appl. Mater. Interfaces 2013, 5, 8761–8765.
- Padbury, R.P.; Halbur, J.C.; Krommenhoek, P.J.; Tracy, J.B.; Jur, J.S. Thermal Stability of Gold Nanoparticles Embedded within Metal Oxide Frameworks Fabricated by Hybrid Modifications onto Sacrificial Textile Templates. Langmuir 2015, 31, 1135–1141.
- Tang, B.; Sun, L.; Kaur, J.; Yu, Y.; Wang, X. In-Situ Synthesis of Gold Nanoparticles for Multifunctionalization of Silk Fabrics. Dye. Pigment. 2014, 103, 183–190.
- Liu, J.; Zhou, J.; Tang, B.; Zeng, T.; Li, Y.; Li, J.; Ye, Y.; Wang, X. Surface Enhanced Raman Scattering (SERS) Fabrics for Trace Analysis. Appl. Surf. Sci. 2016, 386, 296–302.
- Zhang, Z.; Lv, X.; Chen, Q.; An, J. Complex Coloration and Antibacterial Functionalization of Silk Fabrics Based on Noble Metal Nanoparticles. J. Eng. Fiber. Fabr. 2019, 14.
- Ganesan, R.M.; Gurumallesh Prabu, H. Synthesis of Gold Nanoparticles Using Herbal Acorus Calamus Rhizome Extract and Coating on Cotton Fabric for Antibacterial and UV Blocking Applications. Arab. J. Chem. 2019, 12, 2166–2174.
- Ibrahim, N.A.; Eid, B.M.; Abdel-Aziz, M.S. Green Synthesis of AuNPs for Eco-Friendly Functionalization of Cellulosic Substrates. Appl. Surf. Sci. 2016, 389, 118–125.
- Tang, B.; Lin, X.; Zou, F.; Fan, Y.; Li, D.; Zhou, J.; Chen, W.; Wang, X. In Situ Synthesis of Gold Nanoparticles on Cotton Fabric for Multifunctional Applications. Cellulose 2017, 24, 4547–4560.
- Tao, J.; Tang, B.; Li, P.; He, D.; Liao, L.; Peng, Z.; Wang, X. Natural Rubber Particle Modified Fabrics with Catalytic Activity and Hydrophobicity. Compos. Sci. Technol. 2018, 162, 123–130.
- Boomi, P.; Ganesan, R.M.; Poorani, G.; Gurumallesh Prabu, H.; Ravikumar, S.; Jeyakanthan, J. Biological Synergy of Greener Gold Nanoparticles by Using Coleus Aromaticus Leaf Extract. Mater. Sci. Eng. C 2019, 99, 202–210.
- Boomi, P.; Poorani, G.P.; Selvam, S.; Palanisamy, S.; Jegatheeswaran, S.; Anand, K.; Balakumar, C.; Premkumar, K.; Prabu, H.G. Green Biosynthesis of Gold Nanoparticles Using Croton Sparsiflorus Leaves Extract and Evaluation of UV Protection, Antibacterial and Anticancer Applications. Appl. Organomet. Chem. 2020, 34, 1–13.
- Haslinger, S.; Ye, Y.; Rissanen, M.; Hummel, M.; Sixta, H. Cellulose Fibers for High-Performance Textiles Functionalized with Incorporated Gold and Silver Nanoparticles. ACS Sustain. Chem. Eng. 2020, 8, 649–658.
- Velmurugan, P.; Shim, J.; Bang, K.S.; Oh, B.T. Gold Nanoparticles Mediated Coloring of Fabrics and Leather for Antibacterial Activity. J. Photochem. Photobiol. B Biol. 2016, 160, 102–109.
- Yao, Y.; Tang, B.; Chen, W.; Sun, L.; Wang, X. Sunlight-Induced Coloration of Silk. Nanoscale Res. Lett. 2016, 11, 293.
- Tang, B.; Tao, J.; Xu, S.; Wang, J.; Hurren, C.; Xu, W.; Sun, L.; Wang, X. Using Hydroxy Carboxylate to Synthesize Gold Nanoparticles in Heating and Photochemical Reactions and Their Application in Textile Colouration. Chem. Eng. J. 2011, 172, 601–607.
- Pasta, M.; Hu, L.; La Mantia, F.; Cui, Y. Electrodeposited Gold Nanoparticles on Carbon Nanotube-Textile: Anode Material for Glucose Alkaline Fuel Cells. Electrochem. Commun. 2012, 19, 81–84.
- Ballerini, D.R.; Ngo, Y.H.; Garnier, G.; Ladeeig, B.P.; Wei, S. Gold Nanoparticle-Functionalized Thread as a Substrate for SERS Study of Analytes Both Bound and Unbound to Gold. AIChE J. 2012, 59, 215–228.
- Adamo, C.B.; Junger, A.S.; Bressan, L.P.; da Silva, J.A.F.; Poppi, R.J.; de Jesus, D.P. Fast and Straightforward In-Situ Synthesis of Gold Nanoparticles on a Thread-Based Microfluidic Device for Application in Surface-Enhanced Raman Scattering Detection. Microchem. J. 2020, 156, 104985.
- Islam, M.T.; Padilla, J.E.; Dominguez, N.; Alvarado, D.C.; Alam, M.S.; Cooke, P.; Tecklenburg, M.M.J.; Noveron, J.C. Green Synthesis of Gold Nanoparticles Reduced and Stabilized by Squaric Acid and Supported on Cellulose Fibers for the Catalytic Reduction of 4-Nitrophenol in Water. RSC Adv. 2016, 6, 91185–91191.
- Park, H.J.; Kim, W.J.; Ah, C.S.; Jun, Y.; Yun, Y.J. Solution-Processed Au-Ag Core-Shell Nanoparticle-Decorated Yarns for Human Motion Monitoring. RSC Adv. 2017, 7, 10539–10544.
- Xia, Y.; Wan, J.; Gu, Q. Silk Fibroin Fibers Supported with High Density of Gold Nanoparticles: Fabrication and Application as Catalyst. Gold Bull. 2011, 44, 171–176.
- Tang, B.; Yao, Y.; Li, J.; Qin, S.; Zhu, H.; Kaur, J.; Chen, W.; Sun, L.; Wang, X. Functional Application of Noble Metal Nanoparticles In Situ Synthesized on Ramie Fibers. Nanoscale Res. Lett. 2015, 10, 366.
- Islam, M.T.; Dominguez, N.; Ahsan, M.A.; Dominguez-Cisneros, H.; Zuniga, P.; Alvarez, P.J.J.; Noveron, J.C. Sodium Rhodizonate Induced Formation of Gold Nanoparticles Supported on Cellulose Fibers for Catalytic Reduction of 4-Nitrophenol and Organic Dyes. J. Environ. Chem. Eng. 2017, 5, 4185–4193.
- Emam, H.E.; El-Zawahry, M.M.; Ahmed, H.B. One-Pot Fabrication of AgNPs, AuNPs and Ag-Au Nano-Alloy Using Cellulosic Solid Support for Catalytic Reduction Application; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 166.
- Yun, Y.J.; Ah, C.S.; Hong, W.G.; Kim, H.J.; Shin, J.H.; Jun, Y. Highly Conductive and Environmentally Stable Gold/Graphene Yarns for Flexible and Wearable Electronics. Nanoscale 2017, 9, 11439–11445.
- Yu, Q.; Kong, X.; Ma, Y.; Wang, R.; Liu, Q.; Hinestroza, J.P.; Wang, A.X.; Vuorinen, T. Multi-Functional Regenerated Cellulose Fibers Decorated with Plasmonic Au Nanoparticles for Colorimetry and SERS Assays. Cellulose 2018, 25, 6041–6053.
- Liu, Y.; Song, T.; Jia, X.; Meng, L.; Mao, X. Gold Nanoparticles Decorated Carbon Nanotube Probe Based Immunochromatographic Assay on Cotton Thread. Sens. Actuators B Chem. 2017, 251, 1112–1118.
- Jia, X.; Song, T.; Liu, Y.; Meng, L.; Mao, X. An Immunochromatographic Assay for Carcinoembryonic Antigen on Cotton Thread Using a Composite of Carbon Nanotubes and Gold Nanoparticles as Reporters. Anal. Chim. Acta 2017, 969, 57–62.
- Ullah, N.; Odda, A.H.; Li, D.; Wang, Q.; Wei, Q. One-Pot Green Synthesis of Gold Nanoparticles and Its Supportive Role in Surface Activation of Non-Woven Fibers as Heterogeneous Catalyst. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 571, 101–109.
- Nolasco-Arizmendi, V.; Morales-Luckie, R.; Sánchez-Mendieta, V.; Hinestroza, J.P.; Castro-Longoria, E.; Vilchis-Nestor, A.R. Formation of Silk–Gold Nanocomposite Fabric Using Grapefruit Aqueous Extract. Text. Res. J. 2013, 83, 1229–1235.
- Aflori, M.; Serbezeanu, D.; Carja, I.D.; Fortunato, G. Gold Nanoparticles Incorporated into Electrospun Polyimide Fibers. Chem. Lett. 2015, 44, 1440–1442.
- Koga, H.; Tokunaga, E.; Hidaka, M.; Umemura, Y.; Saito, T.; Isogai, A.; Kitaoka, T. Topochemical Synthesis and Catalysis of Metal Nanoparticles Exposed on Crystalline Cellulose Nanofibers. Chem. Commun. 2010, 46, 8567–8569.
- Zhang, T.; Wang, W.; Zhang, D.; Zhang, X.; Yurong, M.; Zhou, Y.; Qi, L. Biotemplated Synthesis of Cold Nanoparticle-Bacteria Cellulose Nanofiber Nanocomposites and Their Application in Biosensing. Adv. Funct. Mater. 2010, 20, 1152–1160.
- Wang, W.; Zhang, T.J.; Zhang, D.W.; Li, H.Y.; Ma, Y.R.; Qi, L.M.; Zhou, Y.L.; Zhang, X.X. Amperometric Hydrogen Peroxide Biosensor Based on the Immobilization of Heme Proteins on Gold Nanoparticles-Bacteria Cellulose Nanofibers Nanocomposite. Talanta 2011, 84, 71–77.
- Zhou, B.; Li, Y.; Deng, H.; Hu, Y.; Li, B. Antibacterial Multilayer Films Fabricated by Layer-by-Layer Immobilizing Lysozyme and Gold Nanoparticles on Nanofibers. Colloids Surfaces B Biointerfaces 2014, 116, 432–438.
- Cheng, H.H.; Chen, F.; Yu, J.; Guo, Z.X. Gold-Nanoparticle-Decorated Thermoplastic Polyurethane Electrospun Fibers Prepared through a Chitosan Linkage for Catalytic Applications. J. Appl. Polym. Sci. 2016, 134, 1–9.
- Yang, X.; Yang, J.; Wang, L.; Ran, B.; Jia, Y.; Zhang, L.; Yang, G.; Shao, H.; Jiang, X. Pharmaceutical Intermediate-Modified Gold Nanoparticles: Against Multidrug-Resistant Bacteria and Wound-Healing Application via an Electrospun Scaffold. ACS Nano 2017, 11, 5737–5745.
- Li, Y.; Tian, Y.; Zheng, W.; Feng, Y.; Huang, R.; Shao, J.; Tang, R.; Wang, P.; Jia, Y.; Zhang, J.; et al. Composites of Bacterial Cellulose and Small Molecule-Decorated Gold Nanoparticles for Treating Gram-Negative Bacteria-Infected Wounds. Small 2017, 13, 1–10.
- Fang, X.; Ma, H.; Xiao, S.; Shen, M.; Guo, R.; Cao, X.; Shi, X. Facile Immobilization of Gold Nanoparticles into Electrospun Polyethyleneimine/Polyvinyl Alcohol Nanofibers for Catalytic Applications. J. Mater. Chem. 2011, 21, 4493–4501.
- Reyes-López, S.Y.; Cornejo-Monroy, D.; González-García, G. A Novel Route for the Preparation of Gold Nanoparticles in Polycaprolactone Nanofibers. J. Nanomater. 2015, 2015.
- Subair, R.; Tripathi, B.P.; Formanek, P.; Simon, F.; Uhlmann, P.; Stamm, M. Polydopamine Modified Membranes with in Situ Synthesized Gold Nanoparticles for Catalytic and Environmental Applications. Chem. Eng. J. 2016, 295, 358–369.
- Abedalwafa, M.A.; Li, Y.; Ni, C.; Yang, G.; Wang, L. Non-Enzymatic Colorimetric Sensor Strip Based on Melamine-Functionalized Gold Nanoparticles Assembled on Polyamide Nanofiber Membranes for the Detection of Metronidazole. Anal. Methods 2019, 11, 3706–3713.
- Chen, Y.; Chen, S.; Wang, B.; Yao, J.; Wang, H. TEMPO-Oxidized Bacterial Cellulose Nanofibers-Supported Gold Nanoparticles with Superior Catalytic Properties. Carbohydr. Polym. 2016, 160, 34–42.
- Wu, X.; Lu, C.; Zhou, Z.; Yuan, G.; Xiong, R.; Zhang, X. Green Synthesis and Formation Mechanism of Cellulose Nanocrystal-Supported Gold Nanoparticles with Enhanced Catalytic Performance. Environ. Sci. Nano 2014, 1, 71–79.
- Liu, Z.; Zhou, C.; Zheng, B.; Qian, L.; Mo, Y.; Luo, F.; Shi, Y.; Choi, M.M.F.; Xiao, D. In Situ Synthesis of Gold Nanoparticles on Porous Polyacrylonitrile Nanofibers for Sensing Applications. Analyst 2011, 136, 4545–4551.
- Senthamizhan, A.; Celebioglu, A.; Balusamy, B.; Uyar, T. Immobilization of Gold Nanoclusters inside Porous Electrospun Fibers for Selective Detection of Cu(II): A Strategic Approach to Shielding Pristine Performance. Sci. Rep. 2015, 5, 15608.
- Chen, X.; Li, D.; Li, G.; Luo, L.; Ullah, N.; Wei, Q.; Huang, F. Facile Fabrication of Gold Nanoparticle on Zein Ultrafine Fibers and Their Application for Catechol Biosensor. Appl. Surf. Sci. 2014, 328, 444–452.
- Zhu, H.; Du, M.; Zhang, M.; Zou, M.; Yang, T.; Wang, L.; Yao, J.; Guo, B. Probing the Unexpected Behavior of AuNPs Migrating through Nanofibers: A New Strategy for the Fabrication of Carbon Nanofiber-Noble Metal Nanocrystal Hybrid Nanostructures. J. Mater. Chem. A 2014, 2, 11728–11741.
- Serbezeanu, D.; Popa, A.M.; Sava, I.; Carja, I.D.; Amberg, M.; Rossi, R.M.; Fortunato, G. Design and Synthesis of Polyimide—Gold Nanofibers with Tunable Optical Properties. Eur. Polym. J. 2015, 64, 10–20.
- Niu, T.; Xu, J.; Xiao, W.; Huang, J. Cellulose-Based Catalytic Membranes Fabricated by Deposition of Gold Nanoparticles on Natural Cellulose Nanofibres. RSC Adv. 2014, 4, 4901–4904.
- Sakai, S.; Kawa, S.; Sawada, K.; Taya, M. Electrospun Polystyrene Fiber-Templating Ultrafine Gold Hollow Fiber Production. Gold Bull. 2013, 46, 97–101.
- Anka, F.H.; Perera, S.D.; Ratanatawanate, C.; Balkus, K.J. Polyacrylonitrile Gold Nanoparticle Composite Electrospun Fibers Prepared by in Situ Photoreduction. Mater. Lett. 2012, 75, 12–15.
- Sawada, K.; Sakai, S.; Taya, M. Polyacrylonitrile-Based Electrospun Nanofibers Carrying Gold Nanoparticles in Situ Formed by Photochemical Assembly. J. Mater. Sci. 2014, 49, 4595–4600.
- Zhou, X.; Zhao, Z.; He, Y.; Ye, Y.; Zhou, J.; Zhang, J.; Ouyang, Q.; Tang, B.; Wang, X. Photoinduced Synthesis of Gold Nanoparticle–Bacterial Cellulose Nanocomposite and Its Application for in-Situ Detection of Trace Concentration of Dyes in Textile and Paper. Cellulose 2018, 25, 3941–3953.
- Shamsabadi, A.S.; Ranjbar, M.; Tavanai, H.; Farnood, A. Electrospinning of Gold Nanoparticles Incorporated PAN Nanofibers via In-Situ Laser Ablation of Gold in Electrospinning Solution. Mater. Res. Express 2019, 6, 055051.