Rechargeable power sources are an essential element of large-scale energy systems based on renewable energy sources. One of the major challenges in rechargeable battery research is the development of electrode materials with good performance and low cost. Carbon-based materials have a wide range of properties, high electrical conductivity, and overall stability during cycling, making them suitable materials for batteries, including stationary and large-scale systems.
- energy storage
- carbon
- nanomaterials
- lead–acid batteries
- lithium-ion batteries
- lithium–sulfur batteries
- sodium-ion batteries
- supercapacitors
1. Introduction
The development of technology, exponentially accelerating in modern times, is closely related to rising energy needs. Moreover, the human population is constantly rising, reaching 7.8 billion in 2020 according to the UN [1]. The changes in population are leading to a further increase in energy demands. This growing demand is not without influence on the natural environment. Recent increases in pollution and CO2 emissions are causing a gradual, global shift toward more sustainable energy sources than currently widespread fossil fuels. For example, the European Union aims to be climate-neutral by 2050, as presented in the European Commission’s European Green Deal [2]. This transition to renewable energy sources requires efficient energy storage. Energy production by renewable sources is characterized by a variable output and inconsistent behavior. As a result of these properties, there is a growing demand for fast and cheap energy storage, which can be used to capture the excess energy generated during the periods of high production. This stored energy can then be released when required by a drop in production or a rise in energy usage.
In recent times, there has been great interest in developing materials that will allow effective and economically viable energy storage. This goal can be realized by improvements in currently known power sources, as well as by introducing completely new solutions. One of the materials that is often used during development of such technologies is carbon. It is used in various energy storage technologies, including primary and secondary batteries, fuel cells, flow batteries, and capacitors [3,4,5,6,7,8]. Carbon is a very versatile element, capable of forming various types of bonds and compounds, leading to its very rich organic chemistry. At the same time, even in its elemental form, it can create many different allotropic forms with interesting physical and chemical characteristics. Moreover, recent advances in nanotechnology have opened new avenues of carbon usage [9,10,11]. Carbon nanomaterials have very unique properties, further broadening the possibilities of carbon application in energy storage. The versatility of carbon allows it to find use in a wide range of electrochemical power sources.
2. Lead–Acid Batteries

3. Lithium-Ion Batteries
Lithium-ion batteries are one of the most widely used secondary batteries with a dynamically increasing market share. The worldwide market for lithium-ion batteries was over 55 billion USD in 2017 [12]. In Li-ion batteries, the positive electrode material is an intercalated lithium compound, e.g., LiCoO2, LiMn2O4, lithium nickel manganese cobalt oxides (NMC, layered compositions with different Co:Ni:Mn ratios), or LiFePO4 [15,68,69]. Most commercial Li-ion batteries have carbon materials as a negative electrode. When a battery is charged, the lithium ion from the positive electrode get inserted into the negative electrode, while the opposite occurs during discharge. During discharge, the carbon material electrode works as an anode. The basic parameters determining the choice of the anode material are the lithium intercalation and de-intercalation reversibility, chemical, thermal, and electrochemical environment, electrical conductivity, cyclic stability, and cost. Due to its good electrical properties and long cycle life, graphite is the most commonly used anode material [70,71]. The stoichiometry of lithium bonding (one Li+ can be intercalated per six C atoms—the limiting composition is LiC6) limits the theoretical specific capacity of graphite to around 370 mAh/g [70,72].
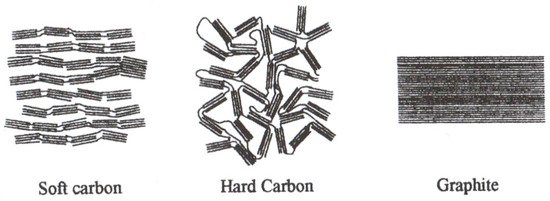
Non-graphitic carbon materials are also used as the negative electrode material. These materials are characterized by the appearance of amorphous areas together with more crystalline ones. Non-graphitic carbons are broadly classified into soft carbons (graphitizable carbons, where crystallites are stacked in the same direction) and hard carbons (non-graphitizable carbons, where crystallites have disordered orientation) [68]. Schematics of the structure of selected carbon materials are presented in Figure 3.
The main advantages of soft carbons are long cycle life, coulombic efficiency over 90%, and highly reversible specific capacity [70]. One of the first soft carbon materials applied in lithium-ion batteries was coke, which offered a capacity around 180 mAh/g and stability in the presence of propylene carbonate-based electrolytes. Currently, various carbon types are used in negative electrodes e.g., graphitic spheres and natural graphite [15,70,73]. Additionally, the improvement of cyclic stability of soft carbons can be achieved by modifying the surface of the carbon material, e.g., by a silane coating [74]. However, the specific capacity of these materials is similar to that of graphite. These properties result quite often in the use of soft carbons in small, low-power devices (portable electronics) but not in hybrid electric vehicles and electric vehicles.
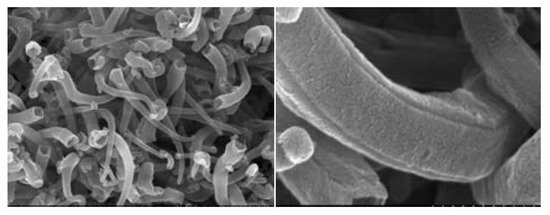
The second group involves hard carbons. These materials offer higher capacity, typically around 500–1000 mAh/g [15,70]. The relatively high capacity of hard carbons compared to graphite can be explained by the fact that lithium in hard carbons additionally occupies the nearest neighbor sites between pairs of graphene sheets [75]. Another mechanism proposed is that lithium may also bind to the hydrogen-containing regions of carbon [76,77]. Hard carbons show a random alignment of graphene sheets and slow lithium diffusion inside the carbon structure. In turn, the disadvantages of hard carbons are its low initial coulombic efficiency and high loss in initial capacity. In connection with these problems, numerous works on hard carbons modification were carried out [78,79,80,81,82,83]. One of the proposed solutions is carbon surface modification by oxidation. It was observed that a mild oxidation of carbon leads to pore production, which is a crucial process in obtaining higher capacity [78]. Another solution is the application of a soft carbon layer on hard carbon. This modification resulted in a high-power performance and good cycling stability [79]. An improvement of the working parameters of negative electrodes based on hard carbons can also be achieved by using high-porosity carbons. Hu et al. [80] obtained and applied hard carbon spherules as a porous carbon material. A scanning electron microscope (SEM) image illustrating the morphology of such a material is presented in Figure 4. The proposed electrode showed an improvement of electrochemical performance and a capacity of almost 390 mAh/g.

Similar capacity (344 mAh/g) was shown by the material obtained by Rao et al. [81]. Hard carbon in this study was prepared by calcination of polypropylene cyanide. The obtained material showed an initial coulombic efficiency over 87% and a superior cycle stability at different current rates compared to graphite. Li et al. [82] examined a microporous hard carbon prepared from potato starch. The electrochemical tests revealed a good cyclic life and high reversible capacity (around 530 mAh/g). Similarly, nanoporous hard carbon obtained from pyrolyzed sucrose exhibited a specific capacity close to 500 mAh/g (at 0.2 C rate) and an equally good capacity, around 330 mAh/g at 5 C rate [83].
Hard carbons in general are characterized by a faster decrease in capacity compared to soft carbons but higher specific capacity (above 500 mAh/g), especially in the case of highly porous materials. At the same time, the price of hard carbons is only about 20% higher than soft carbons. Nevertheless, due to their high energy and power density, they are widely used in batteries in hybrid-electric and electric vehicles.
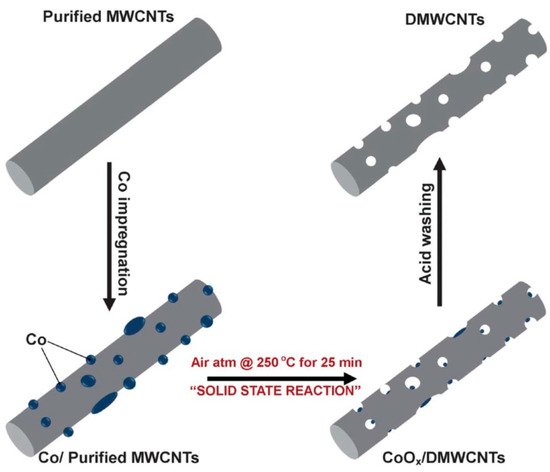
Recently, carbon nanomaterials (such as carbon nanotubes, nanofibers, and graphene) have become very promising candidates for use as anodes in lithium-ion batteries. Compared to other materials, carbons nanomaterials have a higher specific theoretical capacity, e.g., 1116 mAh/g for single-walled nanotubes in LiC2 stoichiometry [84,85,86]. Nanomaterials also offer other advantages, such as stable cyclic behavior and increased lithium ions diffusion rate. The use of carbon nanotube composites with other carbon materials allowed for the improvement of many parameters, such as electrical conductivity, transport properties, and thermal and mechanical stability. On the other hand, the basic disadvantages of nanosized particles are high surface reactivity, low coulombic efficiency during the first cycle, tendency to aggregation during cycling, and higher price [72,87]. Moreover, in practice, it is difficult to achieve the theoretical capacity, and the properties of carbon nanomaterials depend on the preparation methods and pretreatments (e.g., ball milling, acid treatment) [85]. Various morphological modifications of the nanotubes are known, which allow them to reach a capacity of approximately 1000 mAh/g [88,89]. Appropriate modification of parameters such as the tube diameter, wall thickness, or porosity allows improving the working parameters of such anode materials. Even the shape of the nanotubes can be changed. Bamboo-shaped carbon nanotubes produced by using a cresol precursor are characterized by an improved cycle stability and electric conductivity [88]. Quadrangular carbon nanotubes have a high specific capacity and good high-rate performance [89]. High reversible capacity was also found in the case of chemically nano-drilled multiwalled carbon nanotubes [90]. The structures of such materials are presented in Figure 5 and Figure 6.
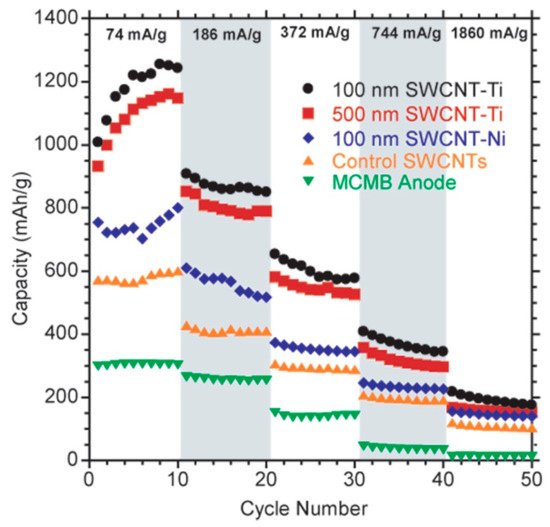
A considerable number of studies investigated composites of carbon nanomaterials (nanotubes, nanofibers) with semi-metals, metals, and metal oxides such as Si, Ti, TiO, MnO, Fe, Fe2O3, Fe3O4, Co, Ni, Cu, Ge, Nb2O7, Sn, SnSb, and SnO2 [91,92,93,94,95,96,97,98,99]. The use of composite material allows for the improvement of many parameters. It has been found that the use of a nickel and titanium contact in a free-standing single-walled carbon nanotube electrode increased both the reversible lithium ion capacity and the rate capacity [92]. Another example is the Fe3O4–carbon nanotube composite presented by Wu et al. [95], which exhibited a high reversible capacity, high rate capability and remarkable capacity retention. Graphene is yet another material of interest for use as the anode material in lithium-ion batteries with a specific capacity at the level of 780–1120 mAh/g, depending on its structure [100,101,102]. Anode materials based on graphene sheets exhibit a high specific capacity (around 1000 mAh/g) and long cycling capability, up to 3000 cycles [100,103,104]. Graphene composite materials are also known to be used as anodes. Good anode properties, highly reversible capacity, and small loss from initial capacity were exhibited by graphene composites with silicon [93], SnO [105], or Fe3O4 [106]. Figure 7 illustrates how the introduction of nanomaterials and composite materials can lead to marked improvements in characteristics of the anode.
In recent years, lithium titanate with a spinel structure (LTO) has been studied as a potential anode material characterized by a good cycling stability [107,108]. Over the last 10 years, there has been a continuous increase in the number of studies from about 50 publications per year in 2010 to almost 300 in 2020 [13]. Carbon materials are also used as additives to LTO anodes. Carbon nanotubes and graphene used as additives provide good contact between particles and conductivity at the interfaces [109,110,111]. Additional solutions for improving electrochemical properties and capacity retention are carbon additives with a large surface area such as carbon black, mesoporous carbon, and amorphous carbon [112,113,114].
Another new research area involves lithium-ion capacitors, a combination of a a faradaic lithium-ion battery anode and a supercapacitor cathode, which offers rapid charging–discharging capability and long cycle life. In this promising technology, carbon materials can be used in both the positive and the negative electrode [115,116,117]. Lithium-ion capacitors combine high energy density with high power density and excellent durability. Due to this, an important potential application for lithium-ion capacitors is represented by energy recovery systems in industrial machinery and transportation systems. Hybrid ion capacitors can be successfully used in regenerative braking energy harvesting from trains, heavy automobiles, and ultimately electric and hybrid-electric vehicles.
The carbon materials used during the earlier phases of lithium-ion battery development were mainly graphite and soft and hard carbons. With maturing of the technology and the introduction of new available techniques and concepts, other carbon materials were employed. In particular, the use of carbon nanomaterials is very promising. They can lead to large improvements of the capacity and the cycle life of lithium-ion batteries. Their successful and economically viable introduction into mass-produced battery models would provide lithium-ion batteries with a very dominant position in the current market. Some of the disadvantages of currently used lithium-ion batteries are their temperature limitations, limited safety, and comparatively high cost. To address these problems, many potential solutions are being researched, including technologies beyond the scope of this review, e.g., solid-state electrolytes or new electrode compositions. The recent growth of portable electronic devices and electric vehicles creates a very attractive market for lithium-ion batteries with proper characteristics and provides strong incentives for developing further improvements in these batteries.
This entry is adapted from the peer-reviewed paper 10.3390/en14092649
