Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Genetics & Heredity
Circulating tumor DNA (ctDNA) is tumor-derived fragmented DNA in the bloodstream that was shed from primary and/or metastatic tumors.
- circulating tumor DNA
- liquid biopsy
- metastatic colorectal cancer
- metastasectomy
1. Introduction
Colorectal cancer (CRC) is the third most common cancer in the world and Korea [1]. Approximately 25% patients with CRC present with overt metastasis and 25–35% patients develop metastasis during the course of the disease [2]. The majority of these patients have a poor prognosis. However, some patients with metastatic CRC (mCRC) are diagnosed with isolated liver and lung metastases. Surgical resection is the treatment of choice, with a survival rate of 25–50% [3]. Metastasectomy can be performed if the liver or lung volume after resection is sufficient to maintain organ function while removing all macroscopic disease with microscopically negative margins and preserving adequate vascular inflow and outflow [4]. Patients with widely metastatic disease may be suitable candidates for local treatment depending on the systemic chemotherapy response. mCRC treatment strategies aim at converting irresectable disease into resectable disease. The 5-year survival after conversion ranges from 35% to 50% [5,6], which is similar to that for patients who underwent resection at presentation.
Although the survival of patients with resectable stage IV cancer has significantly improved with effective chemotherapy regimens and advances in surgical techniques, 75% patients who undergo metastasectomy developed recurrence within 18 months of surgery [7]. Moreover, there is no consensus on standard treatment guidelines regarding the role of postoperative chemotherapy, treatment period, and additional intervention for preventing recurrence after metastasectomy. In addition, difficulty in detecting tiny lesions during postmetastasectomy follow-up, lack of reliable longitudinal surveillance methods, and multiple genetic changes because of clonal evolution and corresponding intratumoral heterogeneity are limitations for customizing treatment strategies to mCRC.
Liquid biopsy has emerged as a novel method for tumor mutation profiling. Various tumor-derived products such as circulating tumor cells, circulating cell-free DNA, and circulating tumor DNA (ctDNA) can be detected in the blood, which has been increasingly used in clinical practice [8]. The DNA fragments from tumor cells are released into the circulation through apoptosis, necrosis, and secretion. Furthermore, tumor-specific genetic alterations such as driver mutations, chromosome copy number alterations, and methylation can be detected in ctDNA, which can be of high value for cancer detection, prognostication, and treatment monitoring [9]. Several studies have confirmed the predictive value of ctDNA levels in mCRC and the prognostic significance of postoperative ctDNA levels in early and locally advanced CRC [10,11,12,13,14]. The presence of ctDNA after radical surgery correlates with recurrence, and elevated ctDNA levels in R0 and R1 resections may signal the occurrence of micrometastases [15,16,17].
2. ctDNA Detection before and after Metastasectomy
Data on 98 metastasectomies were collected; however, data on 31 of 98 metastasectomies (3 cases showing no cancer cells after metastasectomy, 10 cases that failed quality control during the sample preparation process, and 18 cases lacking pathogenic variants in the metastatic tumor tissue; Figure 1) were excluded. The clinical data on every metastasectomy and pathogenic variants in ctDNA are presented in Table S2. Overall, ctDNA was detected in 16 of 67 (24%) cases before metastasectomy and in 4 of 67 (6%) cases with rapid progressive disease immediately after metastasectomy.
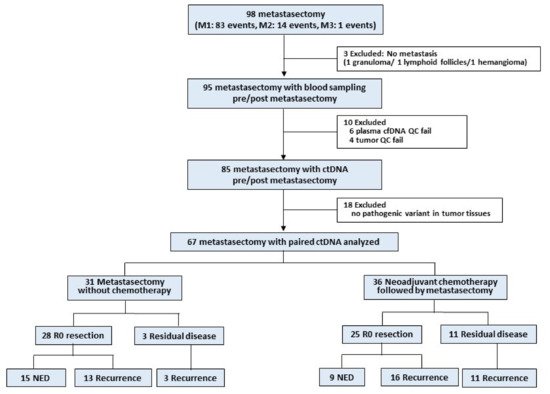
Figure 1. Workflow of the exploratory prospective study.
In the clinical context, metastasectomy of mCRC can be performed in 2 situations. First, if the metastatic lesion is isolated and small, upfront metastasectomy without systemic chemotherapy can be performed (groups 1 and 2). Second, if the metastatic disease is extensive and not resectable, systemic chemotherapy is preferred and, thereafter, the decision to perform metastasectomy can be reassessed depending on the chemotherapy response (groups 3 and 4).
3. Longitudinal Tracking with Serial ctDNA Analysis
We conducted longitudinal ctDNA tracking in 14 cases. During serial sampling, the VAF in ctDNA increased before metastasis was radiologically confirmed in the case of recurrence (case number 6, 8, 20, 25, 26, 37, 40, 51, 53, 61, 65, 79, 92; all cases are listed in Supplementary Table S1); however, ctDNA was not detected after local interventions such as repeated surgery or radiofrequency ablation for metastatic lesions (case number 6, 20, 25, 37, 40, 51, 79, 92).
Moreover, although gross lesions are present, ctDNA might not be detected if the effective chemotherapy and radiotherapy are continued. In case number 26, ctDNA was not detected during maintenance chemotherapy for lung metastasis, but when a new brain metastasis occurred, ctDNA was detected. An increase in the VAF in ctDNA during treatment is highly associated with tumor growth and is detectable postmetastasectomy.
In case number 19, advanced gastric cancer occurred after 4.5 years of the diagnosis of mCRC with a novel pathogenic variant (TP53) from the gastric cancer tissue, which was not found in colon cancer tissue. During follow-up after gastrectomy, this pathogenic variant was repeatedly detected in the blood, confirming the recurrence of gastric cancer in the supraclavicular lymph nodes.
The detection rate of ctDNA differed based on the size and location of the metastatic lesion. Among R0 resection cases, the detection of ctDNA was less in small tumors (<1 cm) and lung metastasis and was high in large tumors (≥1 cm) and liver metastasis (Table 6; Figure 2). As demonstrated in this study, biological factors such as metastatic milieu shedding ctDNA other than tumor burden should be considered. In case of concurrent chemoradiotherapy or neoadjuvant chemotherapy for primary tumor, the VAF in the tumor was extremely low or under the detection level. This could be related to the lack of ctDNA detection in group 1 (case number 2, 7, 34, 40, 58, 60, 68, 72). Although not statistically presented, ctDNA was detected in several cases before several months of bone metastasis (case number 6) or confirmation of disease progression with radiological imaging (case number 40, 53).
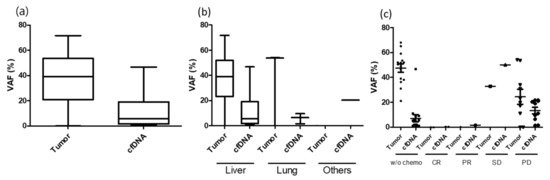
Figure 2. VAF difference between tumor and plasma under various conditions. (a) Mean VAF difference between tumor and plasma; (b) VAF difference between tumor and plasma under metastatic organ; (c) VAF difference between tumor and plasma under neoaduvant chemotherapy response.
Table 6. ctDNA Detection before Metastasectomy (Nevents = 67).
| Clinical Condition | ctDNA Positive (N = 16) |
ctDNA Negative (N = 51) |
p Value | |
|---|---|---|---|---|
| Neoadjuvant chemotherapy before R0 resection |
Yes | 7 | 29 | 0.3587 |
| No | 9 | 22 | ||
| Metastasectomy organ | Liver | 14 | 21 | 0.0045 |
| Lung | 1 | 24 | ||
| Other | 1 | 6 | ||
| Tumor burden (tumor diameter) | >1 cm | 15 | 28 | 0.0183 |
| ≤1 cm | 1 | 23 | ||
(The editor will correct the format and rearrange the refernce after the entry is online)
This entry is adapted from the peer-reviewed paper 10.3390/cancers13092231
This entry is offline, you can click here to edit this entry!
