Gastric cancer (GC) is the third cause of cancer-related death worldwide; the prognosis is poor especially in the case of metastatic disease; bone metastases are rare. Their impact on prognosis is still under debate.
- metastatic gastric cancer
- target therapy
- bone flare
- treatment
- RANK-L
1. Introduction
Gastric cancer (GC) is the third cause of cancer-related death worldwide [1]. In particular, even today, survival is dismal, and only 5.5% of patients diagnosed with metastatic GC are alive at 5 years [2]. The treatment algorithm for metastatic GC is often painted according to the patient’s (age, PS, comorbidities, and nutritional assessment) or tumor’s (tumor burden, symptomatic disease, metastatic sites) characteristics. Thus, a multidisciplinary evaluation of each patient is crucial in the treatment decision process.
In this context, it is unclear how the sites of metastases may affect the prognosis. In fact, if the presence of peritoneal disease or of multiple metastatic sites is considered a well-known worse prognostic factor [3], the knowledge about the role of bone metastases or other visceral sites, such as lung, is scant. This could be related to the rarity of bone involvement in GC, representing the fifth metastatic site after liver, peritoneum, lymph nodes, and—according to the series—lung. Additionally, they are often underestimated at the diagnosis due to the lack of specific recommendation for their detection. Thus, bone metastases have been typically searched for only in case of appearance of new symptoms (e.g., pain), and a consistent fraction of them have been recognized only post-mortem during autopsy in case of non-symptomatic disease [4].
2. The Biological Basis of Bone Involvement in Metastatic Gastric Cancer
Several steps are required for bone metastases development: first of all, cells from the primary tumor should reach bones through systemic circulation; then, they enter the bone microenvironment, especially in areas of the skeleton characterized by high vascularization, such as bones with red marrow [5]. The hypothesis of vascular niches in the bone microenvironment, which promotes the skeletal localization of tumor cells through their extravasation and docking, is supported by the discovery of biological and molecular mechanisms, such as cytokines, adhesion molecules, and skeletal endothelial cells properties [6]. Additionally, the dormancy of disseminated tumor cells, followed by their reactivation and proliferation, is a poorly understood process involved in bone metastases development [7].
Regarding GC, it is still not very clear how the tumor cells colonize the bone microenvironment. Since bone metastases are the results of hematogenous spreading of cancer cells, preferential ways among venous systems, potentially used by GC cells to reach the skeleton, have not been identified [8]. Angiogenesis, which is involved in gastric carcinogenesis and tumor progression, could play a primary role in bone metastasis growth [9]. Among the actors of tumoral angiogenesis, mast cells positive to tryptase (MCPT) have demonstrated to be positively associated with neovascularization in bone metastases from GC, identifying them as a new potential anti-tumor target [10].
3. Bone Metastases from Gastric Cancer
The skeleton has been considered a typical metastatic site in some kind of tumors, such as breast or prostate cancer [11][12]. However, bone involvement is considered unusual in GC. Table 1 summarizes the most significant data reported in the literature regarding the descriptive evaluation of bone metastases in GC, including time of onset, type, and distribution.
There are few reports in the literature regarding the appearance of bone lesions related to early GC [13][14]. When the primary tumor is an early lesion, the presence of metastases to the bone is often underdiagnosed, because bone involvement is investigated only in case of symptoms (e.g., pain). Park et al. focused on the incidence and risk factors of bone recurrence in 1683 GC patients who received a curative resection between 1989 and 2008 [15]. Therefore, this retrospective study analyzed only metachronous bone disease. The incidence of bone involvement was 1.8% in the entire study population, with a higher rate in case of advanced primary tumor at diagnosis (0.4% in case of early GC versus 3.4% in the more advanced stages). The median time from the surgery to the detection of bone metastases was 28 months (range: 4–111 months) and the majority of patients had multiple metastases located to the axial skeleton (spine: 93.3%, pelvic bone: 40% and ribs: 36.6%).
Table 1. Clinical overview about bone metastases characteristics in gastric cancer patients.
| Author and Year * | Study Design, Timeline, Country | N Patients Analyzed | N Patient with Bone Metastasis (%) | Onset | Type | Distribution | Main Patients’ Characteristics | Main Tumor’s Characteristics | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Yoshikawa et al., 1983 [4] |
Retrospective Monoinstitutional 1970–1979 Japan |
1945 | 23 (1.2%) | NR | NR | Thoracic vertebrae: 69.6%; Lumbar vertebrae: 69.6%; Pelvic bones: 26.1%; Ribs: 21.7% |
Young (age <60: 78.3%); male: 56.5% |
NR | NR |
| Park et al., 2011 [16] |
Retrospective Monoinstitutional 1998–2008 Korea |
8633 | 203 (2.4%) | Synchronous: 62%; Metachronous: 38% |
NR | NR | Median age: 51 years; multiple metastatic sites (bone and visceral): 84.7%; multiple bone metastasis: 88.7%; ECOG PS 0–2: 82% |
Poorly differentiated: 72% | mOS: 3.4 months |
| Park et al., 2013 [17] | Retrospective Monoinstitutional 1989–2008 Korea |
1683 | 30 (1.8%) | Metachronous: 100% | NR | Vertebrae: 93.3%; pelvic: bones: 40%; ribs: 33.3% |
young (median age 53.1 years old) male: 63.3% |
Undifferentiated: 73% N3: 43.3% |
mOS after bone recurrence: 6 months |
| Silvestri N et al., 2013 [18] |
Retrospective Multicenter 1998–2011 Italy |
2000 | 208 (10%) | Synchronous: 28%; Metachronous: 62% |
Osteolytic: 52%; mixed: 25%; osteoblastic: 23% | Long bones: 52%; Hip: 38%; Spine: 20% |
young (median age 61 years old): 52.9%; male: 66%; ECOG PS 0–1: 43.9%; multiple metastatic sites (bone and visceral): 86.3%; multiple bone metastasis: 68.6% |
Intestinal: 38.9%; G3: 81.3%; N2: 41.5%. |
mOS: 14 months; mOS from the diagnosis: 6 months; mOS SRE versus no SRE: 3 versus 5 months |
| Nakamura et al., 2014 [19] |
Retrospective Monoinstitutional 2000–2010 |
1837 | 31 (1.7%) | Synchronous: 25.8%; Metachronous: 74.2% |
NR | NR | Age <65: 51.6%; multiple metastatic sites (bone and visceral): 79.5%; multiple bone metastasis: 79.5%; ECOG PS 0–1: 58.1% |
Undifferentiated: 67.8% | mOS: 3.3 months |
| Mikami et al., 2017 [20] |
Retrospective Monoinstitutional 2010–2015 |
NR | 34 (100%) | Synchronous: 29.4%; Metachronous: 70.6% |
NR | Thoracic vertebrae: 55.9%; Pelvic bones: 41.2%; Lumbar vertebrae: 38.2%; Ribs: 29.4% |
multiple metastatic sites (bone and visceral): 76.5%; multiple bone metastasis: 64.7% |
Undifferentiated: 55.9% | mOS: 7.5 months |
| Qiu et al., 2018 [21] |
Retrospective Multicenter 2010–2014 |
19022 | 966 (5.1%) | NR | NR | NR | NR | Intestinal: 62%; G3: 60.7%; located to the cardia: 38% |
mOS: 4 months; 5 year CSS: 1.27% |
| Wen L et al., 2019 [22] |
Retrospective Monoinstitutional 2008–2018 China |
884 | 66 (11.3%) | Synchronous: 45.5%; Metachronous: 54.5% |
NR | Spine: 78.5%; pelvic bones: 68.2%; ribs: 47.0%; lower extremity: 34.8%; sternum:33.3%; scapula: 31.8%; upper extremity: 21.2%; skull: 19.7% |
young (median age 53 years old) male: 68.2%; ECOG PS 0–1: 68.2%; multiple metastatic sites (bone and visceral): 84.9%; multiple bone metastasis: 84.8% |
G3/mucinous/signet ring cells: 71.2%; located to the antrum: 30.3% |
mOS: 6.5 months; mOS metachronous: 11.8 months synchronous: 4.1 months |
| Liang C et al., 2020 [23] |
Retrospective Multicenter 2010–2016 |
42966 | 1798 (4.2%) | NR | NR | NR | multiple metastatic sites (bone and visceral): 52.6% | Intestinal: 60.8%; G3: 62.2%; located to the cardia: 38.4% |
mOS: 3 months |
| Imura et al., 2020 [24] |
Retrospective Monoinstitutional 2005–2017 |
NR | 60 (100%) | NR | NR | NR | Age >60: 56.7%; multiple metastatic sites (bone and visceral): 61.7%; multiple bone metastasis: 83.3%; ECOG PS 0–2: 70% |
NR | mOS: 9 months |
* listed by year. Abbreviations: N: number; ECOG PS: performance status according to ECOG scale; G3: grade 3; mOS: median overall survival; SRE: skeletal-related events; NR: not reported.
For a summary of the incidence of bone involvement according to different skeletal sites, see Figure 1.
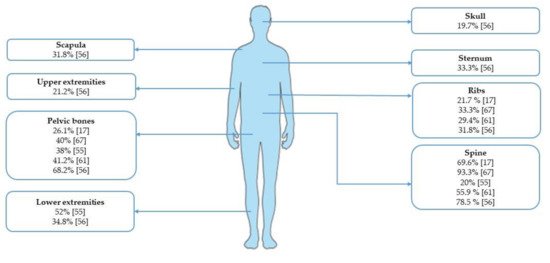
Figure 1. Incidence of bone metastases from gastric cancer according to skeletal sites.
The presence of bone metastases seems to be related to worse prognosis in metastatic GC patients. However, due to the retrospective design of the analysis, the heterogeneity of the study populations, and the rarity of bone involvement, further investigations are needed in order to evaluate the prognostic role of bone metastases and to confirm these findings.
4. Clinical Management of Metastatic Gastric Cancer Patients with Bone Involvement
4.1. Radiological Assessment
A “Tricky” Evaluation of the Response on Bone Metastases during Treatment: Focus on the Bone Flare
Even if rare, bone flare should be taken into account in metastatic GC patients with osteoblastic lesions who show progression of disease only to the skeleton. In these cases, especially if the patients do not show a clinical worsening, the clinicians should be aware about this phenomenon, since it could be misinterpreted as progression of disease, leading to a change in the chemotherapy regimen. Therefore, a multidisciplinary framework in distinguishing the two conditions is critical also in GC patients.
4.2. Treat Metastatic Gastric Cancer Patients with Bone Metastases
The treatment of GC patients with metastases to the skeleton can be distinguished into two areas: treatment of metastatic GC disease per se and bone-related treatments.
5. Future Perspectives
The bone metastases from GC represent still a challenge for the research in this field. In fact, they are rare and often underdiagnosed due to the lack of specific recommendation for their detection according to international guidelines [25][26]. However, bone involvement should be evaluated not only in patients with bone pain or neurological symptoms but also in metastatic GC patients with risk factors, such as aggressive disease or lung metastases. Additionally, there is a lack of prospective evidences regarding specific treatments for patients with bone metastases as well as data showing the outcomes of patients with skeletal metastases from GC or the response of those lesions to standard therapies. Therefore, since the majority of the data in the literature are retrospective and based on a very heterogeneous populations, further prospective studies are needed in order to define the best treatment for GC with bone metastases. Additionally, a better understanding of the underlying molecular mechanisms, by analyzing tumor cells as well as inflammatory tumor infiltrating cells or bone matrix compounds into the bone lesions specimens, could be useful in order to design specific trials.
This entry is adapted from the peer-reviewed paper 10.3390/jcm10081777
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Surveillance, Epidemiology, and End Results (SEER) Database. Available online: (accessed on 19 December 2020).
- Chau, I.; Norman, A.R.; Cunningham, D.; Waters, J.S.; Oates, J.; Ross, P.J. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer—Pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J. Clin. Oncol. 2004, 22, 2395–2403.
- Yoshikawa, K.; Kitaoka, H. Bone metastasis of gastric cancer. Jpn. J. Surg. 1983, 13, 173–176.
- Bussard, K.M.; Gay, C.V.; Mastro, A.M. The bone microenvironment in metastasis; what is special about bone? Cancer Metastasis Rev. 2008, 27, 41–55.
- Kusumbe, A.P. Vascular niches for disseminated tumour cells in bone. J. Bone Oncol. 2016, 5, 112–116.
- Sosa, M.S.; Bragado, P.; Aguirre-Ghiso, J.A. Mechanisms of disseminated cancer cell dormancy: An awakening field. Nat. Rev. Cancer 2014, 14, 611–622.
- D’Amico, L.; Satolli, M.A.; Mecca, C.; Castiglione, A.; Ceccarelli, M.; D’Amelio, P.; Garino, M.; De Giuli, M.; Sandrucci, S.; Ferracini, R.; et al. Bone metastases in gastric cancer follow a RANKL-independent mechanism. Oncol. Rep. 2013, 29, 1453–1458.
- Hsieh, H.-L.; Tsai, M.-M. Tumor progression-dependent angiogenesis in gastric cancer and its potential application. World J. Gastrointest. Oncol. 2019, 11, 686–704.
- Ammendola, M.; Marech, I.; Sammarco, G.; Zuccalà, V.; Luposella, M.; Zizzo, N.; Patruno, R.; Crovace, A.; Ruggieri, E.; Zito, A.F.; et al. Infiltrating mast cells correlate with angiogenesis in bone metastases from gastric cancer patients. Int. J. Mol. Sci. 2015, 16, 3237–3250.
- Brook, N.; Brook, E.; Dharmarajan, A.; Dass, C.R.; Chan, A. Breast cancer bone metastases: Pathogenesis and therapeutic targets. Int. J. Biochem. Cell Biol. 2018, 96, 63–78.
- Rucci, N.; Angelucci, A. Prostate cancer and bone: The elective affinities. BioMed Res. Int. 2014, 2014, 167035.
- Gurzu, S.; Jung, I.; Kádár, Z. Aberrant metastatic behavior and particular features of early gastric cancer. APMIS 2015, 123, 999–1006.
- Fujita, I.; Toyokawa, T.; Makino, T.; Matsueda, K.; Omote, S.; Horii, J. Small early gastric cancer with synchronous bone metastasis: A case report. Mol. Clin. Oncol. 2020, 12, 202–207.
- Park, J.M.; Song, K.Y.; O, J.H.; Kim, W.C.; Choi, M.-G.; Park, C.H. Bone recurrence after curative resection of gastric cancer. Gastric Cancer 2013, 16, 362–369.
- Park, H.S.; Rha, S.Y.; Kim, H.S.; Hyung, W.J.; Park, J.S.; Chung, H.C.; Noh, S.H.; Jeung, H.-C. A prognostic model to predict clinical outcome in gastric cancer patients with bone metastasis. Oncology 2011, 80, 142–150.
- Miyamoto, W.; Yamamoto, S.; Uchio, Y. Metastasis of gastric cancer to the fifth metacarpal bone. Hand Surg. 2008, 13, 193–195.
- Silvestris, N.; Pantano, F.; Ibrahim, T.; Gamucci, T.; De Vita, F.; Di Palma, T.; Pedrazzoli, P.; Barni, S.; Bernardo, A.; Febbraro, A.; et al. Natural history of malignant bone disease in gastric cancer: Final results of a multicenter bone metastasis survey. PLoS ONE 2013, 8, e74402.
- Nakamura, K.; Tomioku, M.; Nabeshima, K.; Yasuda, S. Clinicopathologic features and clinical outcomes of gastric cancer patients with bone metastasis. Tokai J. Exp. Clin. Med. 2014, 39, 193–198.
- Mikami, J.; Kimura, Y.; Makari, Y.; Fujita, J.; Kishimoto, T.; Sawada, G.; Nakahira, S.; Nakata, K.; Tsujie, M.; Ohzato, H. Clinical outcomes and prognostic factors for gastric cancer patients with bone metastasis. World J. Surg. Oncol. 2017, 15, 8.
- Qiu, M.-Z.; Shi, S.-M.; Chen, Z.-H.; Yu, H.-E.; Sheng, H.; Jin, Y.; Wang, D.-S.; Wang, F.-H.; Li, Y.-H.; Xie, D.; et al. Frequency and clinicopathological features of metastasis to liver, lung, bone, and brain from gastric cancer: A SEER-based study. Cancer Med. 2018, 7, 3662–3672.
- Wen, L.; Li, Y.-Z.; Zhang, J.; Zhou, C.; Yang, H.-N.; Chen, X.-Z.; Xu, L.-W.; Kong, S.-N.; Wang, X.-W.; Zhang, H.-M. Clinical analysis of bone metastasis of gastric cancer: Incidence, clinicopathological features and survival. Future Oncol. 2019, 15, 2241–2249.
- Liang, C.; Chen, H.; Yang, Z.; Han, C.; Ren, C. Risk factors and prognosis of bone metastases in newly diagnosed gastric cancer. Future Oncol. 2020, 16, 733–748.
- Imura, Y.; Tateiwa, D.; Sugimoto, N.; Inoue, A.; Wakamatsu, T.; Outani, H.; Tanaka, T.; Tamiya, H.; Yagi, T.; Naka, N.; et al. Prognostic factors and skeletal-related events in patients with bone metastasis from gastric cancer. Mol. Clin. Oncol. 2020, 13, 31.
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D.; ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27 (Suppl. S5), v38–v49.
- National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology. Gastric Cancer, Version 1. 2020. Available online: (accessed on 15 November 2020).
