Photocatalysis is a classical solution to energy conversion and environmental pollution control problems. In photocatalysis, the development and exploration of new visible light catalysts and their synthesis and modification strategies are crucial. It is also essential to understand the mechanism of these reactions in the various reaction media. Recently, bismuth and graphene’s unique geometrical and electronic properties have attracted considerable attention in photocatalysis.
- bismuth/graphene
- nanohybrids
- photocatalysis
- reaction mechanisms
- energy
- pollution
1. Introduction
The increase in pollution due to urbanization and industrialization has become a significant challenge for the sustainability of human society. The waste generated in different industries during crude oil storage, transportation, and refinery has become a global problem [1][2]. The water and soil pollution caused by several pollutants’ discharge is a critical public health concern due to their toxicity. These pollutants can cause many health effects such as neurological toxicity, lung cancer, lethargy, fatigue, depression, headaches, nausea, dizziness, throat and eye irritation, and acute and chronic respiratory effects [3]. Toluene, benzene, xylene, ethyl benzene, and phenolic compounds some of the main compounds categorized as pollutants posing severe threats to our environment [4][5][6]. In the present situation, environmental pollution has increased several-fold due to the mismanagement of industrial waste. This can negatively affect the ecosystem and make lands unusable for agriculture and many other purposes [7]. Therefore, it is essential to remediate these toxic pollutants in our environment [8][9][10].
To eliminate organic pollutants from the environment, numerous technologies have recently been established for their degradation. Organic pollutants can be degraded by different methods, such as physical, chemical, biological treatments and advanced oxidation techniques [9][11][12][13][14][15]. Organic pollutant photodegradation is an attractive “green” chemical technology to control pollution, where photocatalysis is the most widely and potentially applied method used for demineralization and degradation of such pollutants [16][17].
Various light sources have been applied for the excitation of heterogeneous catalysts [18], but the photodegradation approach is more economical if sunlight can be used compared to ultraviolet light [16][19][20]. The evolution of the term “photocatalysis” shows the development of certain fundamental concepts of photochemistry. The point where photochemistry became a discipline was when it became differentiated from thermal chemistry. Indeed, several researchers saw irradiation as one of the many methods available to catalyze a response that makes it quicker by, for example, heating or processing it with certain chemicals until the beginning of the 20th century [21]. Ciamician, the first scientist to systematically understand the chemical effect of light, took great pains in finding out if he had “initiated heat” alone rather than “light” [22]. This was appropriately allotted the term “photochemical,” whilst the word “photocatalytic” applied to reactions caused by light, but with the same result as thermal reactions. Another step further was the identification of electronically excited states, which became a general idea in 1914 and were part of Bodenstein’s photochemical reactions along with reactivity and thermodynamics. In an early stage, more distinction was made in the thermochemistry of the process itself. This allowed for photosynthesis to occur when part of photon energy in the products rose [22][23].
Around 43% of visible-light energy is solar, so visible-light catalysts are chosen in photoelectrocatalysis and photocatalysis processes. Until now, several semiconductive products have been utilized, including metal oxides (Ag2O, TiO2, Cu2O, ZnO, Fe2O3, Ta2O5), metal selenides (CdSe and MOSe2), metal phosphides (Ni2P), metal sulfides (Bi2S3, ZnS, MoS2, and CdS), multi-structure oxides (Sr TiO3WO), metal halides and oxyhalides (AgBr, BiOBr) and metal-free materials (SiC, Si and g-C3N4), [24][25][26][27][28]. Those with a bandgap (Eg) greater than 3 eV, e.g., SrTiO3, TiO2, ZnO, KTaO3, ZnS, and SrTiO3, are called wide-bandgap photocatalysts, whereas catalysts with an Eg of less than 4 eV, e.g., Si, SiC, Ag2O, Bi2WO6, CdSe, InTaO4, Ag3VO4, CoO, Fe2O3, Cu2O, TaON, Ta3N5, CdS, Bi2S3, g-C3N4, and BiVO4, are photocatalysts that react to visible light [25][29].
Heterogeneous catalysts play a vital role in environmental pollution control [30][31][32]. Powdered semiconductor photocatalysts are commonly used in various areas, such as carbon reduction [33], selective organic transformations, environmental remediation [34], and water splitting [35]. There has been, in numerous applications, a growing interest in the use of semiconductors as photocatalysts. In 2015, around 5500 documents about photocatalytic applications were published, indicating that interest in heterogeneous photocatalysis was enormous and highly important in diverse research fields. This number has recently grown to over 13,000. A country-specific view of the increase in the number of publications on “photocatalytic degradation” is listed in Table 1. No commercially accessible material can currently meet all application requirements, such as cost-effectiveness, stability, high visible-light quantum efficiency, and security [36]. For such tasks to be completed, a highly effective architecture and system for environmental remediation and energy supply are needed to examine new visible-light semiconductor materials.
Table 1. Country-wise publications growth on the photocatalytic degradation of organic pollutants. (Data acquired from SciFinder).
| S. No. | Country | No. of Publications |
|---|---|---|
| 1 | China | 8838 |
| 2 | India | 1090 |
| 3 | Iran | 676 |
| 4 | South Korea | 384 |
| 5 | United States of America | 178 |
| 6 | Japan | 175 |
| 7 | Malaysia | 158 |
| 8 | Saudi Arabia | 103 |
| 9 | Pakistan | 84 |
| 10 | Italy | 77 |
| 11 | Australia | 73 |
| 12 | Spain | 72 |
| 13 | Brazil | 57 |
| 14 | United Kingdom | 48 |
The development of nanomaterials has progressed from the synthesis of single-particles to multicomponent assemblies or hierarchical structures, where two or more pre-synthesized nanomaterials are coupled to obtain multifunctionality. Such multicomponent assemblies are termed nanohybrids. The development and use of these nanohybrids requires interdisciplinary knowledge from the energy and environmental sectors, including the applications reported in references [37][38][39][40][41][42][43]. There are previously published review articles on some types and uses of nanohybrids, including gold-graphene oxide nanohybrids [39], organic/inorganic nanohybrids [44], polymer nanohybrids for oil recovery [45], nanohybrids of epoxy/polyamide with carbon nanotubes [46], protein-inorganic nanohybrids [47], gold-based inorganic nanohybrids [48] and polymer-inorganic supramolecular nanohybrids [49].
Graphene is the basic structure of all other carbon allotropes. It is well noted that the potential applications of graphene its derivatives are mainly driven by progressive production of different graphene materials such as graphene oxide (GO), reduced graphene oxide (rGO), functionalized graphene oxide (fGO), and functionalized reduced graphene oxide (frGO) with specific attention to precise applications and this is expected to continue for at least a couple of decades as promising applications and requirements are disclosed [50][51]. Various literature reports on the synthesis, modification and application of photocatalysts based on graphene for energy and environment solutions have already been published [52]. Graphene, graphene and its derivatives [53][54], graphene in photocatalysis [55], graphene doping [56], graphene and graphene oxide sponge [57], nitrogen-doped graphene [58], structure of graphene and its disorders [59], strain engineering of graphene [60], mechanics of graphene nanocomposites [61], chemical vapor deposition of graphene [62], functional modification of graphene/graphene oxide [63], graphene-based fibers [64], and graphene-based electrochemical micro-supercapacitors [65] are some of the subjects that have been reviewed.
Considering the stability, reactivity, reusability, and light-responsive effect of bismuth (Bi) it has been widely used as a photocatalyst. Several state-of-the-art review articles on topics including barium potassium bismuth oxide [66], bismuth-based composite oxides [67], bismuth ferrite nanoparticles [68], bismuth vanadate-based materials [69], bismuth tungstate photocatalysts [70], and bismuth oxyhalides [71] have been published. Annual numbers of publications on graphene photocatalysts in the last ten years are shown in Figure 1a. Similarly, bismuth-containing compounds are significant photocatalysts that react to visible light and fascinating research has been published in the field of bismuth photocatalysis over the last ten years (Figure 1b).
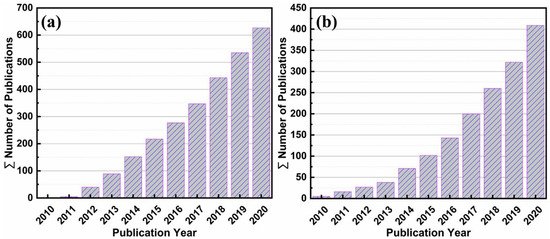
Figure 1. Annual numbers of published items in the last 10 years identified in SciFinder using the keywords: “Graphene-based photocatalysts” (a) and “Bismuth-based photocatalysts” (b).
This review, therefore, summarizes and discusses recent Bi-graphene photocatalysts and their energy and environmental sector applications. The choice of bismuth with graphene is due to the vast available literature, as shown in Figure 1. Furthermore, most bismuth-based photocatalysts are stable, reusable, photoactive, cheaper, and more environmentally friendly that other alternatives. Besides, due to some shortcomings of the pristine photocatalysts, such as charge carrier recombination, slow migration of charge carriers, and low visible light absorption [72][73].
2. Bismuth-Graphene Based Photocatalytic Materials
2.1. Bi2O3 and Bi2S3/Graphene Composites
A significant and the simplest bismuth compound is bismuth trioxide (Bi2O3). It can be used in various ceramics, fuel cells, and gas sensors [74][75]. It has also been used as a photocatalyst in organic pollutant decomposition and water splitting [76]. Bi2O3 is a visible-light-responding photocatalyst when acting as a semiconductor, and its bandgap ranges between 2.1 eV and 2.8 eV. Doping with noble materials and combination with other components have been used to increase graphene’s activity in photocatalytic (PC) form [77][78].
In recent times, the PC activity of some Bi-based semiconductors, e.g., BiVO4 [79], Bi2MoO6 [80][81], BiOX (X = Cl, Br, I), Bi2Sn2O7 [82], Bi2O3 [83], and BiSbO4 [84] in the degradation of pollutants has been described. Bismuth oxide was shown to be a strong candidate among the various Bi-based semiconductors because of its good PC and appropriate bandgap properties. Bi2O3’s PC activity is however restricted by quick recombination of the photogenerated carriers and by its susceptibility to photocorrosion. Because of the short distance between the conduction band (CB) of Bi2O3 and the valence band (VB), graphene can be designed for the sharing of Bi2O3 and graphene [85]. Under such conditions, electrons generated in the CB of Bi2O3 would quickly be coupled with graphene VB holes [86]. Therefore, the photogenerated electrons accumulated on the CB of graphene display strong reduction ability, and the photogenerated holes on the VB of Bi2O3, exhibit excellent oxidation ability [87][88]. The Z-Scheme PC activities are more effective than one component in terms of reduction and oxidation and advanced photocatalytic performance in the traditional photocatalysts [89][90]. Cui has reported a novel Z-scheme Bi2O3/graphene photocatalyst. Bi2S3 has a 1.7 eV bandgap and is a perfect photocatalytic material for light-harvesting due to its near-IR and visible light activation [91]. A number of Bi2S3 nanocrystal forms ranging from 1D nanorods and 2D nanosheets have been created with hot injection and standard non-oxidation techniques [92][93], while a solvothermal method produces 3D sea-urchin-like spheres [94].
Bismuth sulfide (Bi2S3) is a priviledged nontoxic inorganic semiconductor with excellent photocatalytic activity and chemical stability because of its good visible light response. It has been exploited and investigated mostly for optoelectronic applications. The photogenerated holes and hydroxyl radicals (-OH) in the VB of Bi2S3 (1.62 eV) are mostly utilized in dye pollutant decomposition [92]. In combination with many other photocatalysts such as CdS [95], TiO2 [28][96], and Bi2WO6 [97], the recombination rate of electron-hole pairs could be lowered. An increase in visible light absorption enhances the photocatalytic activity.
A graphene/Bi2S3 nanocomposite with narrow bandwidth was recently synthesized. Compared with the individual components, the PC of this nanocomposite was much higher. Zhou et al. stated that the well-matched bandgap of graphene/Bi2S3 heterojunction could be tailored to increase the transfer and separation efficiency of photoinduced carriers and the visible light response. These graphene/Bi2S3 composites are effective photocatalysts for the photocatalytic degradation of environmental pollutants [74].
2.2. Bi2MO6 (M = Cr, Mo, W)/Graphene Composites
Bi2MO6 (M = Mo, Cr, W) is considered the most common member of the Aurivillius family, Bi2An−1BnOn+3 (A = Sr, Ca, Ba, Bi, Pb, K, Na; B = Nb, Ti, Ta, Fe, W, Mo) is the general formula for Bi2MO6. The Bi2MO6 electronic structure is theoretically based on density functional theory (DFT) [98], while the Bi2MO6 crystal structure falls under orthorhombic space group Pca2(1). It was seen that both VB and CB of Bi2MO6 are composed of hybridized orbitals Bi6p, O2p, and Mnd (n = 3, 4, and 5) for Bi2CrO6, Bi2MoO6, and Bi2MO6, respectively [99]. Bi2MO6 compounds are suitable as visible-light-activated photocatalysts. Among all Bi2MO6 species Bi2CrO6 has a narrower bandgap, thus, it easily undergoes recombination of photogenerated holes and electrons and is thus not considered suitable as a photocatalyst and consequently few Bi2CrO6 studies are available in the field of photocatalysis. For the preparation of Bi2MoO6 samples with a wider special surface area, smaller particles, and higher photocatalytic function, the solvothermal and hydrothermal methods are effective. Several Bi2MoO6 morphologies have been described, including floral hollow spheres (solvothermal process) and nanoplates (hydrothermal method). Moreover, microwave heating was applied to synthesize Bi2MoO6 samples with high photocatalytic activity in short periods [100][101]. Major applications of Bi2MO6 (and Bi2MoO6 and Bi2WO6) photocatalysts involve the removal of organic pollutants from polluted air and water. The key pollutants that have been tested in different studies include phenol [102], dyes [103], CHCl3 and CH3CHO in wastewater [104], and NO in air [105]. Microorganisms, e.g., E. coli, were also destroyed by the addition of Bi2WO6 [106] and Bi2MoO6 [107] under visible light irradiation.
Current studies reveal the combined effect of plasmonic metals and graphene. The photocatalytic activity of semiconductors, e.g., TiO2 and ZnO, can be efficiently improved by increasing their photo-absorption ability and suppressing photogenerated electron-hole recombination. Compared to Bi2MoO6, Bi2MoO6-graphene binary composites have been developed and show improved photocatalytic performance. Graphene-based nanocomposites display desirable photocatalytic properties that their individual components do not have, therefore, improved Bi2MoO6 photocatalytic activity resulting from a combination of noble metals and graphene is expected. Bi et al. developed a rGO-Bi2MoO6/Au composite that displayed high catalytic activity for the photodegradation of rhodamine B [20]. Wang and Tian reported composites of GO-Bi2MoO6 and rGO-Bi2MoO6 [108][109]. These composites showed advanced phenol and rhodamine B degradation properties, respectively, compared to Bi2MoO6 alone [74].
2.3. BiVO4/Graphene Nanocomposites
Bismuth vanadate (BiVO4) presents interesting physicochemical properties, including ionic conductivity and ferroelasticity. A theoretical bandgap of 2.047 eV was calculated by DFT for visible-light-driven photocatalysis [110]. Both O2 p- and V3 d-orbitals are included in the BiVO4 valence band. There are three forms of BiVO4, namely monoclinic fergusonite, tetragonal zircon, and tetragonal scheelite. Reversible monoclinic fergusonite and tetragonal scheelite phase transitions occur at 255 °C. A wide range of methods have been reported for BiVO4 preparation. Monoclinic BiVO4 is obtained by both high temperature melting reactions and by solid-state reactions (SSR) [111]. Tetragonal BiVO4 has been synthesized at room temperature by a precipitation method [112]. The bandgap for the monoclinic form is 2.4 eV, while the bandgap for BiVO4 is 2.9 eV. This selective monoclinic BiVO4 preparation is advantageous for assembling effective photocatalysts with visible light shifts. There has been a report of an additional method for synthesizing monoclinic and tetragonal BiVO4 crystals in a simple water-based process [113]. A hydrothermal method has been used successfully in recent times for monoclinic BiVO4 preparation [114]. There are numerous advantages to this hydrothermal approach to selectively produce BiVO4 structures, i.e., mild experimental conditions, controllable conditions and simple experimental setups.
Photocatalytic degradation under visible light is commonly used to decompose organic pollutants (e.g., phenol and RhB) [115], and increased removal efficiency has been demonstrated [116]. BiVO4 was also used for the scission of water [117][118]. BiVO4 was shown to be an active photocatalyst for O2 evolution under visible light radiation since its conduction strip potential isn’t high enough to produce H2 by H2O reduction [119]. Booshehri et al., found BiVO4 to be a mild candidate for photocatalytic inactivation of bacteria in water under visible light irradiation [120]. For photocatalytic bactericidal activity, surface redox reactions are essential for reactive species generation [121]. In addition, the interface for charge separation and transfer in hybrid catalysts is to be considered for two components [122]. The BiVO4/Ag/graphene photocatalyst showed improved activity for photocatalytic degradation of organic pollutants [123][124] or oxidation of nitrogen monoxide and water [125]. The probability of photocatalytic wastewater or disinfection of water by the Z-scheme BiVO4/graphene is however still unknown to the best of our knowledge. Moreover, at the molecular level the photocatalysis consistency is clearly not yet investigated [74].
2.4. BiOX (X = F, Cl, Br, I)/Graphene Composites
Bismuth oxyhalides’ (BioXoptical)’s properties can work as a photocatalyst. The structure of BiOX crystals is comprised of layer structure slabs [Bi2O2] which are inserted in two halogen atoms [126][127]. Biox contain X np (n = 2–5 for Cl, F, I, and Br respectively), O 2p, and Bi 6 p-orbitals both in the valence band (VB) and conduction band (CB). In theoretical terms, the bandgaps of BiOI, BiOF, BiOBr, and BiOCl are calculated to be 1.38 eV, 2.79 eV, 1.99 eV, or 2.34 eV, while experimentally, their bandgaps are estimated to be 1.77 eV, 3.64 eV [128], 2.64 eV, and 3.22 eV [129]. There are restrictions within the GGA method that cause these differences between the experimental and calculated bandgap results. However, both indicate the general decreasing tendency of the bandgaps as the atomic number increases. BIOF was used as a photocatalyst only under UV light, while BiOI was photocatalytically active both under near-IR and visible light. Because of their appropriate bandgaps, both BiOCl and BiOBr are therefore commonly tested. For BiOX synthesis with different morphologies, several methods can be effectively applied. In addition to direct precipitation techniques, the primary methods used to synthesize the BiOX with controlled nanostructures such as nanosheets, microsphere, and nanofibers include hydrolysis, solvothermal and hydrothermal methods [130]. By adjusting the precursor pH, controlling hydrothermal treatment duration time and temperature, and by adding a template structure that can be selectively controlled, one can directly affect the photocatalytic performance. An extensive review of BiOX nanostructures was previously published [131].
Significant efforts have been carried out to design innovative photocatalysts [132][133]. Because of their excellent catalytic activity under visible light, the sequence of ternary bismuth oxyhalides (BiOX, X = Cl, Br, or I) has been commonly studied [134]. The charge separation and atomic polarization efficiency of the layered BiOX structures can be improved. BiOBr, with its crystalline PbFCl layer structure has been a big consideration among BiOX photocatalysts because of its excellent photocatalytic activity, appropriate bandgap, and high stability. The binary component and multi-component counterparts showed improved photocatalytic activity compared to single-component semiconductors. Multi-component synergies may overcome the single-component shortcomings, e.g., insufficient charge separation ability and wide-bandgap. Consequently, the BiOBr photocatalytic activity [135][136] with an indirect-transition bandgap (2.75 eV) may be efficiently enhanced by incorporating other materials.
Graphenes are currently used as a promising support platform for anchoring host NPs as well as acceptors for charge separation and superb electron transfer mediation with peculiar characteristics such as low density, high conductivity, and large surface areas [137][138][139]. The hydrothermal method has been used for the synthesis of Au/BiOBr/graphene composites [140][141].
A practical approach to shrink the bandgap, increase the catalytic activity and visible-light absorption was taken using black BiOCl material with the formation of oxygen vacancies. Although the black BiOCl is still subject to recombination of fast photocatalytic charge carriers, its photocatalytic activity is still not satisfactory. A simple and effective approach to resolve the above-related problems has been taken as the construction hetero-structures of BiOCls with the other appropriate photocatalysts. Thanks to their high electron mobility and a large surface area, the above issues could be well addressed by functional graphene-based semiconductor photocatalysts. A new BiOCl-Bi-Bi2O3/rGO heterojunction with oxygen vacancies has been developed, which provided a solid-solid, close-fit interface and strong interaction between BiOCl, Bi, rGO, and Bi2O3. BiOCl-BI2O3/rGO heterojunctions showed high photocatalytic performance due to the synergistic effect caused by effective charge separation among Bi2O3, BiOCl, rGO, and Bi-bridges. The BiOCl-Bi-Bi2O3/rGO heterojunction displayed high efficiency for photocatalytic degradation of 2-nitrophenol in industrial wastewater treatment. The significant task is to demonstrate the superior long-term photostability of the BiOCl-Bi-Bi2O3/rGO heterojunctions. In addition, a promising BiOCl-Bi-Bi-Bi2O3/rGO photocatalytic mechanism was proposed to describe primary phenomena taking place during the process, depending on multiple charge transfer channels [141].
2.5. BiPO4/Graphene Composites
BiPO4 with high photocatalytic activity for organic pollutant degradation was fabricated for the first time by a hydrothermal approach [142]. A faster hydrothermal way of synthesizing BiPO4 has also been reported [143]. The bandgap in BiPO4 prepared by hydrothermal methods is about 3.85 eV, higher than that of TiO2 (3.2 eV). BiPO4 nanocrystals synthesized with standard oxygen-free procedures have a bandwidth of around 4.6 eV. Only UV light can be used as a light source for large bandgap semiconductors. Although its bandgap is broader than that of TiO2, BiPO4 still has high photocatalytic degradation kinetics. This is because the VB of BiPO4 is 3 eV, higher than that of TiO2, and it generates more oxidative holes in its VB compared to TiO2. Photocatalytic conversion of the gas-phase benzene into CO2 by BiPO4 has also been reported in addition to the degradation of the organic pollutant in an aqueous phase. A photocatalytic gas-phase transformation of benzene to CO2 was also reported during an aqueous phase organic pollutant degradation study [144][145]. BiPO4 photocatalysts still have several drawbacks as photocatalysts however, such as low photocatalytic activity, and comparatively rapid recombination of charge carriers, wide bandgaps, low adsorption ability, and large size, which would decrease the photocatalytic activity of BiPO4 and subsequently limit its industrial-scale applications [146][147]. Consequently, it is urgent to create and design photocatalytic materials based on BiPO4, with required and useful photocatalytic performance properties. To date, numerous efforts have been made to improve the photocatalytic activity of the BiPO4 photocatalyst by doping with non-metals or metals, surface hybridization, reducing the crystal size, forming heterostructures, or combinations of μ-structure materials [148][149]. BiPO4/rGO nanocomposites exposed the importance of graphene as the support of separating electron-hole pairs, which leads to a high photocurrent. Thus, the development of BiPO4/rGO hybrids is an efficient way to improve the visible light catalytic performance of BiPO4. Extensive research has established a trend towards research in carbon-nanomaterials by doping with heteroatoms as they can adapt their fundamental properties successfully [150][151].
2.6. (BiO)2CO3/Graphene C omposites
Bismuth subcarbonate is a known solid carbonate in the BI2O3-CO2-H2O system ((BiO)2CO3 or Bi2CO5) [152]. The bandgap of (BiO)2CO3 is 3.4 eV, so wavelengths under 365 nm can therefore stimulate the bandgap [153][154]. The CB of (BiO)2CO3 generally includes hybridized p-orbitals (O2 p and Bi 6p), while its VB consists of p-orbitals (O 2p, Bi 6p, and C 2p). A hydrothermal, template-free method has been used to efficiently synthesize (BiO)2CO3 with hollow microsphere orders whose structure is dependent on Ostwald’s growing properties. The compound showed photocatalytic activity for pollutant oxidation or disinfection of air and wastewater contamination [155][156]. Several articles have described p-n heterojunctions that exhibited enhanced photocatalytic activity [74][157].
An innovative multi-component TiO2-Bi2O3/(BiO)2CO3-rGO nanocomposite has been synthesized and experimentally used for bisphenol A (BPA) photodegradation. The Bi2O3 was intended to be a visible light photosensitizer. The appropriate VB and CB’s positions TiO2 and (BiO)2CO3 were used as selective sinks for photogenerated holes and electrons, and rGO acted as a channel for charge carrier transport that extended the lifetime of the catalysts. BPA is an endocrine disruptive compound commonly used for the production of many common packaging materials [158]. These materials typically end up in waste dumps, leading to the slow leaching of BPA into water bodies. Accordingly, BPA has been chosen as the model for the photocatalytic activity of the designed photocatalysts based on environmental issues [158][159].
2.7. M(BiO3)n/Graphene Composites
Pentavalent bismuthates (M(BiO3)n (where n = 1, M = Li, Na, K, Ag; n = 2, M = Mg, Zn, Sr, Ba, and Pb) can be bought directly from commercial companies to synthesize additional Bi-based compounds, such as BiOX, as a Bi source [160]. The bandgaps of these compounds are MgBi2O6 1.61 eV, ZnBi2O6 1.53 eV, SrBi2O6 1.93 eV, SrBi2O6 1.93 eV, BaBi2O6 1.93 eV, PbBi2O6 1.92 eV [161], LiBiO3 1.8 eV, KBiO3 2.1 eV [162], NaBiO3 2.6 eV [163], and AgBiO3 2.5 eV, respectively [164]. The valency of Bi-based composites is +3 while the value of (BiO3)n is +5. The Bi3+ cation consists of two orbitals (10 d and 6 s). This indicates that the electronic structure of pentavalent bismuthates is different. Takei et al. tested nine bismuthates for degrading phenol and methylene blue [161]. High photo-catalytical activity under visible light irradiation was shown by NaBiO3, LiBiO3, BaBi2O6, and SrBi2O6. The d-electrons from Zn, Pb and Ag produce a large conduction range as well as consequently poor photocatalytic performance. Electronic systems greatly affect catalytic performance. Excellent visible-light photocatalytic activity recommends pentavalent bismuthates for different photocatalytic applications. M(BiO3)n could be used for efficient visible light photocatalytic degradation of organic pollutants [161][165].
This entry is adapted from the peer-reviewed paper 10.3390/en14082281
References
- Srivastva, N.; Shukla, A.K.; Singh, R.S.; Upadhyay, S.N.; Dubey, S.K. Characterization of bacterial isolates from rubber dump site and their use in biodegradation of isoprene in batch and continuous bioreactors. Bioresour. Technol. 2015, 188, 84–91.
- Shahimin, M.F.M.; Foght, J.M.; Siddique, T. Preferential methanogenic biodegradation of short-chain n-alkanes by microbial communities from two different oil sands tailings ponds. Sci. Total Environ. 2016, 553, 250–257.
- Hu, Y.; Wang, Z.; Wen, J.; Li, Y. Stochastic fuzzy environmental risk characterization of uncertainty and variability in risk assessments: A case study of polycyclic aromatic hydrocarbons in soil at a petroleum-contaminated site in China. J. Hazard. Mater. 2016, 316, 143–150.
- Yeh, C.-H.; Lin, C.-W.; Wu, C.-H. A permeable reactive barrier for the bioremediation of BTEX-contaminated groundwater: Microbial community distribution and removal efficiencies. J. Hazard. Mater. 2010, 178, 74–80.
- Chen, L.; Liu, Y.; Liu, F.; Jin, S. Treatment of co-mingled benzene, toluene and TCE in groundwater. J. Hazard. Mater. 2014, 275, 116–120.
- Zhou, Y.; Gao, F.; Zhao, Y.; Lu, J. Study on the extraction kinetics of phenolic compounds from petroleum refinery waste lye. J. Saudi Chem. Soc. 2014, 18, 589–592.
- Ali, S.M.; Pervaiz, A.; Afzal, B.; Hamid, N.; Yasmin, A. Open dumping of municipal solid waste and its hazardous impacts on soil and vegetation diversity at waste dumping sites of Islamabad city. J. King Saud Univ. Sci. 2014, 26, 59–65.
- Hu, G.; Li, J.; Zeng, G. Recent development in the treatment of oily sludge from petroleum industry: A review. J. Hazard. Mater. 2013, 261, 470–490.
- Ashraf, M.; Khan, I.; Usman, M.; Khan, A.; Shah, S.S.; Khan, A.Z.; Saeed, K.; Yaseen, M.; Ehsan, M.F.; Tahir, M.N.; et al. Hematite and Magnetite Nanostructures for Green and Sustainable Energy Harnessing and Environmental Pollution Control: A Review. Chem. Res. Toxicol. 2020, 33, 1292–1311.
- Shah, S.S.; Qasem, M.A.A.; Berni, R.; Del Casino, C.; Cai, G.; Contal, S.; Ahmad, I.; Siddiqui, K.S.; Gatti, E.; Predieri, S.; et al. Physico-chemical properties and toxicological effects on plant and algal models of carbon nanosheets from a nettle fibre clone. Sci. Rep. 2021, 11, 6945.
- Mymrin, V.; Pedroso, A.M.; Ponte, H.A.; Ponte, M.J.; Alekseev, K.; Evaniki, D.; Pan, R.C. Thermal engineering method application for hazardous spent petrochemical catalyst neutralization. Appl. Therm. Eng. 2017, 110, 1428–1436.
- Sun, J.; Watson, S.S.; Allsopp, D.A.; Stanley, D.; Skrtic, D. Tuning photo-catalytic activities of TiO2 nanoparticles using dimethacrylate resins. Dent. Mater. 2016, 32, 363–372.
- Ehsan, M.F.; Fazal, A.; Hamid, S.; Arfan, M.; Khan, I.; Usman, M.; Shafiee, A.; Ashiq, M.N. CoFe2O4 decorated g-C3N4 nanosheets: New insights into superoxide anion mediated photomineralization of methylene blue. J. Environ. Chem. Eng. 2020, 8, 104556.
- Khan, I.; Khan, I.; Usman, M.; Imran, M.; Saeed, K. Nanoclay-mediated photocatalytic activity enhancement of copper oxide nanoparticles for enhanced methyl orange photodegradation. J. Mater. Sci. Mater. Electron. 2020, 31, 8971–8985.
- Ehsan, M.F.; Shafiq, M.; Hamid, S.; Shafiee, A.; Usman, M.; Khan, I.; Ashiq, M.N.; Arfan, M. Reactive oxygen species: New insights into photocatalytic pollutant degradation over g-C3N4/ZnSe nanocomposite. Appl. Surf. Sci. 2020, 532, 147418.
- Singh, P.; Borthakur, A. A review on biodegradation and photocatalytic degradation of organic pollutants: A bibliometric and comparative analysis. J. Clean. Prod. 2018, 196, 1669–1680.
- Wang, Z.; Yang, Y.; Dai, Y.; Xie, S. Anaerobic biodegradation of nonylphenol in river sediment under nitrate-or sulfate-reducing conditions and associated bacterial community. J. Hazard. Mater. 2015, 286, 306–314.
- Luna, A.L.; Valenzuela, M.A.; Colbeau-Justin, C.; Vázquez, P.; Rodriguez, J.L.; Avendaño, J.R.; Alfaro, S.; Tirado, S.; Garduño, A.; José, M. Photocatalytic degradation of gallic acid over CuO–TiO2 composites under UV/Vis LEDs irradiation. Appl. Catal. A Gen. 2016, 521, 140–148.
- Pan, C.; Zhu, Y. A review of BiPO 4, a highly efficient oxyacid-type photocatalyst, used for environmental applications. Catal. Sci. Technol. 2015, 5, 3071–3083.
- Bi, J.; Fang, W.; Li, L.; Li, X.; Liu, M.; Liang, S.; Zhang, Z.; He, Y.; Lin, H.; Wu, L. Ternary reduced-graphene-oxide/Bi2MoO6/Au nanocomposites with enhanced photocatalytic activity under visible light. J. Alloys Compd. 2015, 649, 28–34.
- Rostamnia, S.; Doustkhah, E.; Golchin-Hosseini, H.; Zeynizadeh, B.; Xin, H.; Luque, R. Efficient tandem aqueous room temperature oxidative amidations catalysed by supported Pd nanoparticles on graphene oxide. Catal. Sci. Technol. 2016, 6, 4124–4133.
- Ravelli, D.; Dondi, D.; Fagnoni, M.; Albini, A. Photocatalysis. A multi-faceted concept for green chemistry. Chem. Soc. Rev. 2009, 38, 1999–2011.
- Hayat, A.; Rahman, M.U.; Khan, I.; Khan, J.; Sohail, M.; Yasmeen, H.; Liu, S.-Y.; Qi, K.; Lv, W. Conjugated Electron Donor–Acceptor Hybrid Polymeric Carbon Nitride as a Photocatalyst for CO2 Reduction. Molecules 2019, 24, 1779.
- Noh, M.F.M.; Ullah, H.; Arzaee, N.A.; Ab Halim, A.; Rahim, M.A.F.A.; Mohamed, N.A.; Safaei, J.; Nasir, S.N.F.M.; Wang, G.; Teridi, M.A.M. Rapid fabrication of oxygen defective α-Fe2O3 (110) for enhanced photoelectrochemical activities. Dalton Trans. 2020, 49, 12037–12048.
- Samsudin, M.F.R.; Ullah, H.; Tahir, A.A.; Li, X.; Ng, Y.H.; Sufian, S. Superior photoelectrocatalytic performance of ternary structural BiVO4/GQD/g-C3N4 heterojunction. J. Colloid Interface Sci. 2021, 586, 785–796.
- Zhou, P.; Yu, J.; Jaroniec, M. All-solid-state Z-scheme photocatalytic systems. Adv. Mater. 2014, 26, 4920–4935.
- Humayun, M.; Sun, N.; Raziq, F.; Zhang, X.; Yan, R.; Li, Z.; Qu, Y.; Jing, L. Synthesis of ZnO/Bi-doped porous LaFeO3 nanocomposites as highly efficient nano-photocatalysts dependent on the enhanced utilization of visible-light-excited electrons. Appl. Catal. B Environ. 2018, 231, 23–33.
- Humayun, M.; Zada, A.; Li, Z.; Xie, M.; Zhang, X.; Qu, Y.; Raziq, F.; Jing, L. Enhanced visible-light activities of porous BiFeO3 by coupling with nanocrystalline TiO2 and mechanism. Appl. Catal. B Environ. 2016, 180, 219–226.
- Kandiel, T.A.; Ahmed, M.G.; Ahmed, A.Y. Physical Insights into Band Bending in Pristine and Co-Pi-Modified BiVO4 Photoanodes with Dramatically Enhanced Solar Water Splitting Efficiency. J. Phys. Chem. Lett. 2020, 11, 5015–5020.
- Zhang, H.H.; Cao, Y.M.; Usman, M.; Li, L.J.; Li, C.S. Study on the Hydrotreating Catalysts Containing Phosphorus of Coal Tar to Clean Fuels. Adv. Mater. Res. 2012, 531, 263–267.
- Kan, T.; Sun, X.; Wang, H.; Li, C.; Muhammad, U. Production of Gasoline and Diesel from Coal Tar via Its Catalytic Hydrogenation in Serial Fixed Beds. Energy Fuels 2012, 26, 3604–3611.
- Usman, M.; Li, D.; Razzaq, R.; Latif, U.; Muraza, O.; Yamani, Z.H.; Al-Maythalony, B.A.; Li, C.; Zhang, S. Poly aromatic hydrocarbon (naphthalene) conversion into value added chemical (tetralin): Activity and stability of MoP/AC catalyst. J. Environ. Chem. Eng. 2018, 6, 4525–4530.
- Li, X.; Wen, J.; Low, J.; Fang, Y.; Yu, J. Design and fabrication of semiconductor photocatalyst for photocatalytic reduction of CO2 to solar fuel. Sci. China Mater. 2014, 57, 70–100.
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027.
- Bard, A.J.; Fox, M.A. Artificial photosynthesis: Solar splitting of water to hydrogen and oxygen. Acc. Chem. Res. 1995, 28, 141–145.
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959.
- Alsaiari, N.S.; Katubi, K.M.M.; Alzahrani, F.M.; Siddeeg, S.M.; Tahoon, M.A. The Application of Nanomaterials for the Electrochemical Detection of Antibiotics: A Review. Micromachines 2021, 12, 308.
- Alwattar, J.K.; Mneimneh, A.T.; Abla, K.K.; Mehanna, M.M.; Allam, A.N. Smart Stimuli-Responsive Liposomal Nanohybrid Systems: A Critical Review of Theranostic Behavior in Cancer. Pharmaceutics 2021, 13, 355.
- Amir, M.N.I.; Halilu, A.; Julkapli, N.M.; Ma’amor, A. Gold-graphene oxide nanohybrids: A review on their chemical catalysis. J. Ind. Eng. Chem. 2020, 83, 1–13.
- Ali Tahir, A.; Ullah, H.; Sudhagar, P.; Asri Mat Teridi, M.; Devadoss, A.; Sundaram, S. The application of graphene and its derivatives to energy conversion, storage, and environmental and biosensing devices. Chem. Record 2016, 16, 1591–1634.
- Ullah, H.; Tahir, A.A.; Mallick, T.K. Polypyrrole/TiO2 composites for the application of photocatalysis. Sens. Actuators B 2017, 241, 1161–1169.
- Sabeeh, H.; Aadil, M.; Zulfiqar, S.; Rasheed, A.; Al-Khalli, N.F.; Agboola, P.O.; Haider, S.; Warsi, M.F.; Shakir, I. Hydrothermal synthesis of CuS nanochips and their nanohybrids with CNTs for electrochemical energy storage applications. Ceram. Int. 2021, 47, 13613–13621.
- Safaei, J.; Ullah, H.; Mohamed, N.A.; Mohamad Noh, M.F.; Soh, M.F.; Tahir, A.A.; Ahmad Ludin, N.; Ibrahim, M.A.; Wan Isahak, W.N.R.; Mat Teridi, M.A. Enhanced photoelectrochemical performance of Z-scheme g-C3N4/BiVO4 photocatalyst. Appl. Catal. B 2018, 234, 296–310.
- Liang, C.; Zhang, X.; Wang, Z.; Wang, W.; Yang, M.; Dong, X. Organic/inorganic nanohybrids rejuvenate photodynamic cancer therapy. J. Mater. Chem. B 2020, 8, 4748–4763.
- Corredor, L.M.; Husein, M.M.; Maini, B.B. A review of polymer nanohybrids for oil recovery. Adv. Colloid Interface Sci. 2019, 272, 102018.
- Ahmed, W.; Gul, S.; Awais, M.; Hassan, Z.U.; Jabeen, S.; Farooq, M. A review: Novel nanohybrids of epoxy/polyamide with carbon nanotube/nano-diamond. Polym. Plast. Technol. Mater. 2021, 60, 579–600.
- Freag, M.S.; Elzoghby, A.O. Protein-inorganic Nanohybrids: A Potential Symbiosis in Tissue Engineering. Curr. Drug. Targets 2018, 19, 1897–1904.
- Ding, X.; Li, D.; Jiang, J. Gold-based Inorganic Nanohybrids for Nanomedicine Applications. Theranostics 2020, 10, 8061–8079.
- Park, D.-H.; Hwang, S.-J.; Oh, J.-M.; Yang, J.-H.; Choy, J.-H. Polymer–inorganic supramolecular nanohybrids for red, white, green, and blue applications. Prog. Polym. Sci. 2013, 38, 1442–1486.
- Mohan, V.B.; Lau, K.-T.; Hui, D.; Bhattacharyya, D. Graphene-based materials and their composites: A review on production, applications and product limitations. Compos. Part B Eng. 2018, 142, 200–220.
- Abu Nayem, S.M.; Shaheen Shah, S.; Sultana, N.; Aziz, M.A.; Saleh Ahammad, A.J. Electrochemical Sensing Platforms of Dihydroxybenzene: Part 1—Carbon Nanotubes, Graphene, and their Derivatives. Chem. Rec. 2021, in press.
- Gong, Y.; Li, M.; Li, H.; Wang, Y. Graphitic carbon nitride polymers: Promising catalysts or catalyst supports for heterogeneous oxidation and hydrogenation. Green Chem. 2015, 17, 715–736.
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145.
- Lee, X.J.; Hiew, B.Y.Z.; Lai, K.C.; Lee, L.Y.; Gan, S.; Thangalazhy-Gopakumar, S.; Rigby, S. Review on graphene and its derivatives: Synthesis methods and potential industrial implementation. J. Taiwan Inst. Chem. Eng. 2019, 98, 163–180.
- Li, X.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Xie, J. Graphene in Photocatalysis: A Review. Small 2016, 12, 6640–6696.
- Guo, B.; Fang, L.; Zhang, B.; Gong, J.R. Graphene doping: A review. Insciences J. 2011, 1, 80–89.
- Chabot, V.; Higgins, D.; Yu, A.; Xiao, X.; Chen, Z.; Zhang, J. A review of graphene and graphene oxide sponge: Material synthesis and applications to energy and the environment. Energy Environ. Sci. 2014, 7, 1564–1596.
- Wang, H.; Maiyalagan, T.; Wang, X. Review on Recent Progress in Nitrogen-Doped Graphene: Synthesis, Characterization, and Its Potential Applications. ACS Catal. 2012, 2, 781–794.
- Yang, G.; Li, L.; Lee, W.B.; Ng, M.C. Structure of graphene and its disorders: A review. Sci. Technol. Adv. Mater. 2018, 19, 613–648.
- Si, C.; Sun, Z.; Liu, F. Strain engineering of graphene: A review. Nanoscale 2016, 8, 3207–3217.
- Young, R.J.; Kinloch, I.A.; Gong, L.; Novoselov, K.S. The mechanics of graphene nanocomposites: A review. Compos. Sci. Technol. 2012, 72, 1459–1476.
- Zhang, Y.; Zhang, L.; Zhou, C. Review of Chemical Vapor Deposition of Graphene and Related Applications. Acc. Chem. Res. 2013, 46, 2329–2339.
- Yu, W.; Sisi, L.; Haiyan, Y.; Jie, L. Progress in the functional modification of graphene/graphene oxide: A review. RSC Adv. 2020, 10, 15328–15345.
- Meng, F.; Lu, W.; Li, Q.; Byun, J.-H.; Oh, Y.; Chou, T.-W. Graphene-Based Fibers: A Review. Adv. Mater. 2015, 27, 5113–5131.
- Xiong, G.; Meng, C.; Reifenberger, R.G.; Irazoqui, P.P.; Fisher, T.S. A Review of Graphene-Based Electrochemical Microsupercapacitors. Electroanalysis 2014, 26, 30–51.
- Baumert, B.A. Barium potassium bismuth oxide: A review. J. Supercond. 1995, 8, 175–181.
- Fang, W.; Shangguan, W. A review on bismuth-based composite oxides for photocatalytic hydrogen generation. Int. J. Hydrog. Energy 2019, 44, 895–912.
- Gao, T.; Chen, Z.; Huang, Q.; Niu, F.; Huang, X.; Qin, L.; Huang, Y. A review: Preparation of bismuth ferrite nanoparticles and its applications in visible-light induced photocatalyses. Rev. Adv. Mater. Sci. 2015, 40, 97–109.
- Huang, Z.-F.; Pan, L.; Zou, J.-J.; Zhang, X.; Wang, L. Nanostructured bismuth vanadate-based materials for solar-energy-driven water oxidation: A review on recent progress. Nanoscale 2014, 6, 14044–14063.
- Zhang, L.; Zhu, Y. A review of controllable synthesis and enhancement of performances of bismuth tungstate visible-light-driven photocatalysts. Catal. Sci. Technol. 2012, 2, 694–706.
- Sharma, K.; Dutta, V.; Sharma, S.; Raizada, P.; Hosseini-Bandegharaei, A.; Thakur, P.; Singh, P. Recent advances in enhanced photocatalytic activity of bismuth oxyhalides for efficient photocatalysis of organic pollutants in water: A review. J. Ind. Eng. Chem. 2019, 78, 1–20.
- Kumar, R.; Raizada, P.; Verma, N.; Hosseini-Bandegharaei, A.; Thakur, V.K.; Le, Q.V.; Nguyen, V.-H.; Selvasembian, R.; Singh, P. Recent advances on water disinfection using bismuth based modified photocatalysts: Strategies and challenges. J. Clean. Prod. 2021, 297, 126617.
- Li, X.; Zhang, W.; Cui, W.; Sun, Y.; Jiang, G.; Zhang, Y.; Huang, H.; Dong, F. Bismuth spheres assembled on graphene oxide: Directional charge transfer enhances plasmonic photocatalysis and in situ DRIFTS studies. Appl. Catal. B Environ. 2018, 221, 482–489.
- Meng, X.; Zhang, Z. Bismuth-based photocatalytic semiconductors: Introduction, challenges and possible approaches. J. Mol. Catal. A Chem. 2016, 423, 533–549.
- Wang, Y.; Wen, Y.; Ding, H.; Shan, Y. Improved structural stability of titanium-doped β-Bi2O3 during visible-light-activated photocatalytic processes. J. Mater. Sci. 2010, 45, 1385–1392.
- Zhu, G.; Que, W.; Zhang, J. Synthesis and photocatalytic performance of Ag-loaded β-Bi2O3 microspheres under visible light irradiation. J. Alloys Compd. 2011, 509, 9479–9486.
- Ma, S.; Zhan, S.; Jia, Y.; Shi, Q.; Zhou, Q. Enhanced disinfection application of Ag-modified g-C3N4 composite under visible light. Appl. Catal. B Environ. 2016, 186, 77–87.
- Samsudin, M.F.R.; Ullah, H.; Bashiri, R.; Mohamed, N.M.; Sufian, S.; Ng, Y.H. Experimental and DFT insights on microflower g-C3N4/BiVO4 photocatalyst for enhanced photoelectrochemical hydrogen generation from lake water. ACS Sustain. Chem. Eng. 2020, 8, 9393–9403.
- Ullah, H.; Tahir, A.A.; Mallick, T.K. Structural and electronic properties of oxygen defective and Se-doped p-type BiVO4 (001) thin film for the applications of photocatalysis. Appl. Catal. B 2018, 224, 895–903.
- Lin, X.; Xu, D.; Zhao, R.; Xi, Y.; Zhao, L.; Song, M.; Zhai, H.; Che, G.; Chang, L. Highly efficient photocatalytic activity of g-C3N4 quantum dots (CNQDs)/Ag/Bi2MoO6 nanoheterostructure under visible light. Sep. Purif. Technol. 2017, 178, 163–168.
- Lin, X.; Xi, Y.; Zhao, R.; Shi, J.; Yan, N. Construction of C60-decorated SWCNTs (C60-CNTs)/bismuth-based oxide ternary heterostructures with enhanced photocatalytic activity. RSC Adv. 2017, 7, 53847–53854.
- Tian, Q.; Zhuang, J.; Wang, J.; Liu, P. Novel photocatalyst, Bi2Sn2O7, for photooxidation of As (III) under visible-light irradiation. Appl. Catal. A Gen. 2012, 425, 74–78.
- Zhang, L.; Wang, W.; Yang, J.; Chen, Z.; Zhang, W.; Zhou, L.; Liu, S. Sonochemical synthesis of nanocrystallite Bi2O3 as a visible-light-driven photocatalyst. Appl. Catal. A Gen. 2006, 308, 105–110.
- Lin, X.P.; Huang, F.Q.; Wang, W.D.; Zhang, K.L. A novel photocatalyst BiSbO4 for degradation of methylene blue. Appl. Catal. A Gen. 2006, 307, 257–262.
- Zhang, Y.; Yu, H.; Li, S.; Wang, L.; Huang, F.; Guan, R.; Li, J.; Jiao, Y.; Sun, J. Rapidly degradation of di-(2-ethylhexyl) phthalate by Z-scheme Bi2O3/TiO2@ reduced graphene oxide driven by simulated solar radiation. Chemosphere 2021, 272, 129631.
- Ren, G.; Ren, X.; Ju, W.; Jiang, Y.; Han, M.; Dong, Z.; Yang, X.; Dou, K.; Xue, B.; Li, F. Controlled vertical growing of Bi2O3 nano sheets on diatomite disks and its high visible-light photocatalytic performance. J. Photochem. Photobiol. A Chem. 2020, 392, 112367.
- Zhang, J.; Hu, Y.; Jiang, X.; Chen, S.; Meng, S.; Fu, X. Design of a direct Z-scheme photocatalyst: Preparation and characterization of Bi2O3/g-C3N4 with high visible light activity. J. Hazard. Mater. 2014, 280, 713–722.
- Mohamed, N.A.; Ullah, H.; Safaei, J.; Ismail, A.F.; Mohamad Noh, M.F.; Soh, M.F.; Ibrahim, M.A.; Ludin, N.A.; Mat Teridi, M.A. Efficient Photoelectrochemical Performance of γ Irradiated g-C3N4 and Its g-C3N4@BiVO4 Heterojunction for Solar Water Splitting. J. Phys. Chem. C 2019, 123, 9013–9026.
- Wang, X.; Li, S.; Ma, Y.; Yu, H.; Yu, J. H2WO4 · H2O/Ag/AgCl composite nanoplates: A plasmonic Z-scheme visible-light photocatalyst. J. Phys. Chem. C 2011, 115, 14648–14655.
- Xie, X.; Wang, S.; Zhang, Y.; Ding, J.; Liu, Y.; Yan, Q.; Lu, S.; Li, B.; Liu, Y.; Cai, Q. Facile construction for new core-shell Z-scheme photocatalyst GO/AgI/Bi2O3 with enhanced visible-light photocatalytic activity. J. Colloid Interface Sci. 2021, 581, 148–158.
- Cui, Y.; Zhang, X.; Guo, R.; Zhang, H.; Li, B.; Xie, M.; Cheng, Q.; Cheng, X. Construction of Bi2O3/g-C3N4 composite photocatalyst and its enhanced visible light photocatalytic performance and mechanism. Sep. Purif. Technol. 2018, 203, 301–309.
- Wu, T.; Zhou, X.; Zhang, H.; Zhong, X. Bi2S3 nanostructures: A new photocatalyst. Nano Res. 2010, 3, 379–386.
- Zhang, H.; Huang, J.; Zhou, X.; Zhong, X. Single-crystal Bi2S3 nanosheets growing via attachment–recrystallization of nanorods. Inorg. Chem. 2011, 50, 7729–7734.
- Chen, J.; Qin, S.; Song, G.; Xiang, T.; Xin, F.; Yin, X. Shape-controlled solvothermal synthesis of Bi2S3 for photocatalytic reduction of CO2 to methyl formate in methanol. Dalton Trans. 2013, 42, 15133–15138.
- Kobasa, I.; Tarasenko, G. Photocatalysis of Reduction of the Dye Methylene Blue by Bi2S3/CdS Nanocomposites. Theor. Exp. Chem. 2002, 38, 255–258.
- Bessekhouad, Y.; Robert, D.; Weber, J. Bi2S3/TiO2 and CdS/TiO2 heterojunctions as an available configuration for photocatalytic degradation of organic pollutant. J. Photochem. Photobiol. A Chem. 2004, 163, 569–580.
- Zhang, Z.; Wang, W.; Wang, L.; Sun, S. Enhancement of visible-light photocatalysis by coupling with narrow-band-gap semiconductor: A case study on Bi2S3/Bi2WO6. ACS Appl. Mater. Interfaces 2012, 4, 593–597.
- Lai, K.; Wei, W.; Dai, Y.; Zhang, R.; Huang, B. DFT calculations on structural and electronic properties of Bi2MO6 (M= Cr, Mo, W). Rare Met. 2011, 30, 166–172.
- Chawla, H.; Chandra, A.; Ingole, P.P.; Garg, S. Recent advancements in enhancement of photocatalytic activity using bismuth-based metal oxides Bi2MO6 (M= W, Mo, Cr) for environmental remediation and clean energy production. J. Ind. Eng. Chem. 2021, 95, 1–15.
- Xie, H.; Shen, D.; Wang, X.; Shen, G. Microwave hydrothermal synthesis and visible-light photocatalytic activity of γ-Bi2MoO6 nanoplates. Mater. Chem. Phys. 2008, 110, 332–336.
- Stelo, F.; Kublik, N.; Ullah, S.; Wender, H. Recent advances in Bi2MoO6 based Z-scheme heterojunctions for photocatalytic degradation of pollutants. J. Alloys Compd. 2020, 829, 154591.
- Murcia-López, S.; Hidalgo, M.C.; Navío, J.A. Degradation of rhodamine B/phenol mixtures in water by sun-like excitation of a Bi2WO6–TiO2 photocatalyst. Photochem. Photobiol. 2013, 89, 832–840.
- Fu, H.; Zhang, S.; Xu, T.; Zhu, Y.; Chen, J. Photocatalytic degradation of RhB by fluorinated Bi2WO6 and distributions of the intermediate products. Environ. Sci. Technol. 2008, 42, 2085–2091.
- Tang, J.; Zou, Z.; Ye, J. Efficient photocatalytic decomposition of organic contaminants over CaBi2O4 under visible-light irradiation. Angew. Chem. Int. Ed. 2004, 43, 4463–4466.
- Huang, Y.; Ai, Z.; Ho, W.; Chen, M.; Lee, S. Ultrasonic spray pyrolysis synthesis of porous Bi2WO6 microspheres and their visible-light-induced photocatalytic removal of NO. J. Phys. Chem. C 2010, 114, 6342–6349.
- Zhang, L.-S.; Wong, K.-H.; Yip, H.-Y.; Hu, C.; Yu, J.C.; Chan, C.-Y.; Wong, P.-K. Effective photocatalytic disinfection of E. coli K-12 using AgBr− Ag− Bi2WO6 nanojunction system irradiated by visible light: The role of diffusing hydroxyl radicals. Environ. Sci. Technol. 2010, 44, 1392–1398.
- Zhang, Y.; Zhu, Y.; Yu, J.; Yang, D.; Ng, T.W.; Wong, P.K.; Jimmy, C.Y. Enhanced photocatalytic water disinfection properties of Bi2MoO6–RGO nanocomposites under visible light irradiation. Nanoscale 2013, 5, 6307–6310.
- Wang, P.; Ao, Y.; Wang, C.; Hou, J.; Qian, J. A one-pot method for the preparation of graphene–Bi2MoO6 hybrid photocatalysts that are responsive to visible-light and have excellent photocatalytic activity in the degradation of organic pollutants. Carbon 2012, 50, 5256–5264.
- Tian, G.; Chen, Y.; Zhou, J.; Tian, C.; Li, R.; Wang, C.; Fu, H. In situ growth of Bi2MoO6 on reduced graphene oxide nanosheets for improved visible-light photocatalytic activity. CrystEngComm 2014, 16, 842–849.
- Zhao, Z.; Luo, W.; Li, Z.; Zou, Z. Density functional theory study of doping effects in monoclinic clinobisvanite BiVO4. Phys. Lett. A 2010, 374, 4919–4927.
- Gotić, M.; Musić, S.; Ivanda, M.; Šoufek, M.; Popović, S. Synthesis and characterisation of bismuth (III) vanadate. J. Mol. Struct. 2005, 744, 535–540.
- Kudo, A.; Omori, K.; Kato, H. A novel aqueous process for preparation of crystal form-controlled and highly crystalline BiVO4 powder from layered vanadates at room temperature and its photocatalytic and photophysical properties. J. Am. Chem. Soc. 1999, 121, 11459–11467.
- Ullah, H.; Tahir, A.A.; Bibi, S.; Mallick, T.K.; Karazhanov, S.Z. Electronic properties of β-TaON and its surfaces for solar water splitting. Appl. Catal. B 2018, 229, 24–31.
- Liu, J.; Wang, H.; Wang, S.; Yan, H. Hydrothermal preparation of BiVO4 powders. Mater. Sci. Eng. B 2003, 104, 36–39.
- Zhang, Z.; Wang, W.; Shang, M.; Yin, W. Photocatalytic degradation of rhodamine B and phenol by solution combustion synthesized BiVO4 photocatalyst. Catal. Commun. 2010, 11, 982–986.
- Shang, M.; Wang, W.; Ren, J.; Sun, S.; Zhang, L. A novel BiVO4 hierarchical nanostructure: Controllable synthesis, growth mechanism, and application in photocatalysis. CrystEngComm 2010, 12, 1754–1758.
- Ng, Y.H.; Iwase, A.; Kudo, A.; Amal, R. Reducing graphene oxide on a visible-light BiVO4 photocatalyst for an enhanced photoelectrochemical water splitting. J. Phys. Chem. Lett. 2010, 1, 2607–2612.
- Su, J.; Guo, L.; Bao, N.; Grimes, C.A. Nanostructured WO3/BiVO4 heterojunction films for efficient photoelectrochemical water splitting. Nano Lett. 2011, 11, 1928–1933.
- Yang, J.; Wang, D.; Zhou, X.; Li, C. A theoretical study on the mechanism of photocatalytic oxygen evolution on BiVO4 in aqueous solution. Chem. A Eur. J. 2013, 19, 1320–1326.
- Booshehri, A.Y.; Goh, S.C.-K.; Hong, J.; Jiang, R.; Xu, R. Effect of depositing silver nanoparticles on BiVO4 in enhancing visible light photocatalytic inactivation of bacteria in water. J. Mater. Chem. A 2014, 2, 6209–6217.
- Wei, C.; Lin, W.Y.; Zainal, Z.; Williams, N.E.; Zhu, K.; Kruzic, A.P.; Smith, R.L.; Rajeshwar, K. Bactericidal activity of TiO2 photocatalyst in aqueous media: Toward a solar-assisted water disinfection system. Environ. Sci. Technol. 1994, 28, 934–938.
- Bai, S.; Jiang, W.; Li, Z.; Xiong, Y. Surface and interface engineering in photocatalysis. ChemNanoMat 2015, 1, 223–239.
- Chen, F.; Yang, Q.; Wang, Y.; Zhao, J.; Wang, D.; Li, X.; Guo, Z.; Wang, H.; Deng, Y.; Niu, C. Novel ternary heterojunction photcocatalyst of Ag nanoparticles and g-C3N4 nanosheets co-modified BiVO4 for wider spectrum visible-light photocatalytic degradation of refractory pollutant. Appl. Catal. B Environ. 2017, 205, 133–147.
- Lin, X.; Xu, D.; Xi, Y.; Zhao, R.; Zhao, L.; Song, M.; Zhai, H.; Che, G.; Chang, L. Construction of leaf-like g-C3N4/Ag/BiVO4 nanoheterostructures with enhanced photocatalysis performance under visible-light irradiation. Colloids Surf. A Physicochem. Eng. Asp. 2017, 513, 117–124.
- Ou, M.; Wan, S.; Zhong, Q.; Zhang, S.; Song, Y.; Guo, L.; Cai, W.; Xu, Y. Hierarchical Z-scheme photocatalyst of g-C3N4@ Ag/BiVO4 (040) with enhanced visible-light-induced photocatalytic oxidation performance. Appl. Catal. B Environ. 2018, 221, 97–107.
- Huang, W.L.; Zhu, Q. Electronic structures of relaxed BiOX (X= F, Cl, Br, I) photocatalysts. Comput. Mater. Sci. 2008, 43, 1101–1108.
- Li, H.; Long, B.; Ye, K.-H.; Cai, Y.; He, X.; Lan, Y.; Yang, Z.; Ji, H. A recyclable photocatalytic tea-bag-like device model based on ultrathin Bi/C/BiOX (X = Cl, Br) nanosheets. Appl. Surf. Sci. 2020, 515, 145967.
- Su, W.; Wang, J.; Huang, Y.; Wang, W.; Wu, L.; Wang, X.; Liu, P. Synthesis and catalytic performances of a novel photocatalyst BiOF. Scripta Mater. 2010, 62, 345–348.
- Zhang, X.; Ai, Z.; Jia, F.; Zhang, L. Generalized one-pot synthesis, characterization, and photocatalytic activity of hierarchical BiOX (X= Cl, Br, I) nanoplate microspheres. J. Phys. Chem. C. 2008, 112, 747–753.
- Zhang, M.; Yin, H.-F.; Yao, J.-C.; Arif, M.; Qiu, B.; Li, P.-F.; Liu, X.-H. All-solid-state Z-scheme BiOX (Cl, Br)-Au-CdS heterostructure: Photocatalytic activity and degradation pathway. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 124778.
- Cheng, H.; Huang, B.; Dai, Y. Engineering BiOX (X = Cl, Br, I) nanostructures for highly efficient photocatalytic applications. Nanoscale 2014, 6, 2009–2026.
- GUI, M.-S.; WANG, P.-F.; YUAN, D.; Yang, Y.-K. Synthesis and visible-light photocatalytic activity of Bi2WO6/g-C3N4 composite photocatalysts. Chin. J. Inorg. Chem. 2013, 29, 2057–2064.
- Jian, Z.; Yan, Z.; Yu-Hua, S.; Cun, L.; An-Jian, X. Flower-like Bi2WO6 porous microspheres: Assembly and photocatalytic performance. Chin. J. Inorg. Chem. 2012, 28, 739–744.
- Zhang, X.; Chang, X.; Gondal, M.; Zhang, B.; Liu, Y.; Ji, G. Synthesis and photocatalytic activity of graphene/BiOBr composites under visible light. Appl. Surf. Sci. 2012, 258, 7826–7832.
- Ma, M.; Yang, Y.; Chen, Y.; Ma, Y.; Lyu, P.; Cui, A.; Huang, W.; Zhang, Z.; Li, Y.; Si, F. Photocatalytic degradation of MB dye by the magnetically separable 3D flower-like Fe3O4/SiO2/MnO2/BiOBr-Bi photocatalyst. J. Alloys Compd. 2021, 861, 158256.
- Liu, T.; Zhang, Y.; Shi, Z.; Cao, W.; Zhang, L.; Liu, J.; Chen, Z. BiOBr/Ag/AgBr heterojunctions decorated carbon fiber cloth with broad-spectral photoresponse as filter-membrane-shaped photocatalyst for the efficient purification of flowing wastewater. J. Colloid Interface Sci. 2021, 587, 633–643.
- Jianwei, C.; Jianwen, S.; Xu, W.; Haojie, C.; Minglai, F. Recent progress in the preparation and application of semiconductor/graphene composite photocatalysts. Chin. J. Catal. 2013, 34, 621–640.
- Liu, W.; Cai, J.; Li, Z. Self-assembly of semiconductor nanoparticles/reduced graphene oxide (RGO) composite aerogels for enhanced photocatalytic performance and facile recycling in aqueous photocatalysis. ACS Sustain. Chem. Eng. 2015, 3, 277–282.
- Ibrahim, M.; Saeed, T.; Chu, Y.-M.; Ali, H.M.; Cheraghian, G.; Kalbasi, R. Comprehensive study concerned graphene nano-sheets dispersed in ethylene glycol: Experimental study and theoretical prediction of thermal conductivity. Powder Technol. 2021, 386, 51–59.
- Xue, Y.; Liang, W.; Feng, L.-J.; Li, C.-H. Preparation of Au/BiOBr/Graphene composite and its photocatalytic performance in phenol degradation under visible light. J. Fuel Chem. Technol. 2016, 44, 937–942.
- Deng, F.; Zhang, Q.; Yang, L.; Luo, X.; Wang, A.; Luo, S.; Dionysiou, D.D. Visible-light-responsive graphene-functionalized Bi-bridge Z-scheme black BiOCl/Bi2O3 heterojunction with oxygen vacancy and multiple charge transfer channels for efficient photocatalytic degradation of 2-nitrophenol and industrial wastewater treatment. Appl. Catal. B Environ. 2018, 238, 61–69.
- Pan, C.; Zhu, Y. New type of BiPO4 oxy-acid salt photocatalyst with high photocatalytic activity on degradation of dye. Environ. Sci. Technol. 2010, 44, 5570–5574.
- Li, G.; Ding, Y.; Zhang, Y.; Lu, Z.; Sun, H.; Chen, R. Microwave synthesis of BiPO4 nanostructures and their morphology-dependent photocatalytic performances. J. Colloid Interface Sci. 2011, 363, 497–503.
- Long, B.; Huang, J.; Wang, X. Photocatalytic degradation of benzene in gas phase by nanostructured BiPO4 catalysts. Prog. Nat. Sci. Mater. Int. 2012, 22, 644–653.
- Ola, O.; Ullah, H.; Chen, Y.; Thummavichai, K.; Wang, N.; Zhu, Y. DFT and Experimental Studies of Iron Oxide-based Nanocomposites for Efficient Electrocatalysis. J. Mater. Chem. C 2021.
- Cao, J.; Xu, B.; Lin, H.; Chen, S. Highly improved visible light photocatalytic activity of BiPO4 through fabricating a novel p–n heterojunction BiOI/BiPO4 nanocomposite. Chem. Eng. J. 2013, 228, 482–488.
- An, W.; Cui, W.; Liang, Y.; Hu, J.; Liu, L. Surface decoration of BiPO4 with BiOBr nanoflakes to build heterostructure photocatalysts with enhanced photocatalytic activity. Appl. Surf. Sci. 2015, 351, 1131–1139.
- Gao, E.; Wang, W. Role of graphene on the surface chemical reactions of BiPO4–rGO with low OH-related defects. Nanoscale 2013, 5, 11248–11256.
- Zou, X.; Dong, Y.; Zhang, X.; Cui, Y.; Ou, X.; Qi, X. The highly enhanced visible light photocatalytic degradation of gaseous o-dichlorobenzene through fabricating like-flowers BiPO4/BiOBr pn heterojunction composites. Appl. Surf. Sci. 2017, 391, 525–534.
- Shah, S.S.; Alfasane, M.A.; Bakare, I.A.; Aziz, M.A.; Yamani, Z.H. Polyaniline and heteroatoms–enriched carbon derived from Pithophora polymorpha composite for high performance supercapacitor. J. Energy Storage 2020, 30, 101562.
- Shah, S.S.; Cevik, E.; Aziz, M.A.; Qahtan, T.F.; Bozkurt, A.; Yamani, Z.H. Jute Sticks Derived and Commercially Available Activated Carbons for Symmetric Supercapacitors with Bio-electrolyte: A Comparative Study. Synth. Met. 2021, 77, 116765.
- Taylor, P.; Sunder, S.; Lopata, V.J. Structure, spectra, and stability of solid bismuth carbonates. Can. J. Chem. 1984, 62, 2863–2873.
- Dong, F.; Zheng, A.; Sun, Y.; Fu, M.; Jiang, B.; Ho, W.-K.; Lee, S.; Wu, Z. One-pot template-free synthesis, growth mechanism and enhanced photocatalytic activity of monodisperse (BiO)2CO3 hierarchical hollow microspheres self-assembled with single-crystalline nanosheets. CrystEngComm 2012, 14, 3534–3544.
- Lin, K.; Qian, J.; ZhaO, Z.; Wu, G.; Wu, H. Synthesis of a carbon-loaded Bi2O2CO3/TiO2 photocatalyst with improved photocatalytic degradation of methyl orange dye. J. Nanosci. Nanotechnol. 2020, 20, 7653–7658.
- Chen, R.; So, M.H.; Yang, J.; Deng, F.; Che, C.-M.; Sun, H. Fabrication of bismuth subcarbonate nanotube arrays from bismuth citrate. Chem. Commun. 2006, 21, 2265–2267.
- Dong, F.; Sun, Y.; Fu, M.; Ho, W.-K.; Lee, S.C.; Wu, Z. Novel in situ N-doped (BiO)2CO3 hierarchical microspheres self-assembled by nanosheets as efficient and durable visible light driven photocatalyst. Langmuir 2011, 28, 766–773.
- Bin Mohd Yusoff, A.R.; Mahata, A.; Vasilopoulou, M.; Ullah, H.; Hu, B.; da Silva, W.J.; Schneider, F.K.; Gao, P.; Ievlev, A.V.; Liu, Y. Observation of large Rashba spin–orbit coupling at room temperature in compositionally engineered perovskite single crystals and application in high performance photodetectors. Mater. Today 2021, in press.
- Hasan, M.M.; Islam, T.; Imran, A.; Alqahtani, B.; Shah, S.S.; Mahfoz, W.; Karim, M.R.; Alharbi, H.F.; Aziz, M.A.; Ahammad, A.J.S. Mechanistic Insights of the Oxidation of Bisphenol A at Ultrasonication Assisted Polyaniline-Au Nanoparticles Composite for Highly Sensitive Electrochemical Sensor. Electrochim. Acta 2021, 374, 137968.
- Žerjav, G.; Djinović, P.; Pintar, A. TiO2-Bi2O3/(BiO)2CO3-reduced graphene oxide composite as an effective visible light photocatalyst for degradation of aqueous bisphenol A solutions. Catal. Today 2018, 315, 237–246.
- Chang, X.; Huang, J.; Cheng, C.; Sui, Q.; Sha, W.; Ji, G.; Deng, S.; Yu, G. BiOX (X = Cl, Br, I) photocatalysts prepared using NaBiO3 as the Bi source: Characterization and catalytic performance. Catal. Commun. 2010, 11, 460–464.
- Takei, T.; Haramoto, R.; Dong, Q.; Kumada, N.; Yonesaki, Y.; Kinomura, N.; Mano, T.; Nishimoto, S.; Kameshima, Y.; Miyake, M. Photocatalytic activities of various pentavalent bismuthates under visible light irradiation. J. Solid State Chem. 2011, 184, 2017–2022.
- Ramachandran, R.; Sathiya, M.; Ramesha, K.; Prakash, A.; Madras, G.; Shukla, A. Photocatalytic properties of KBiO3 and LiBiO 3 with tunnel structures. J. Chem. Sci. 2011, 123, 517–524.
- Kako, T.; Zou, Z.; Katagiri, M.; Ye, J. Decomposition of organic compounds over NaBiO3 under visible light irradiation. Chem. Mater. 2007, 19, 198–202.
- Yu, X.; Zhou, J.; Wang, Z.; Cai, W. Preparation of visible light-responsive AgBiO3 bactericide and its control effect on the Microcystis aeruginosa. J. Photochem. Photobiol. B Biol. 2010, 101, 265–270.
- Chang, X.; Huang, J.; Cheng, C.; Sha, W.; Li, X.; Ji, G.; Deng, S.; Yu, G. Photocatalytic decomposition of 4-t-octylphenol over NaBiO3 driven by visible light: Catalytic kinetics and corrosion products characterization. J. Hazard. Mater. 2010, 173, 765–772.
