Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Plant Sciences
The plant hormone abscisic acid (ABA) rapidly accumulates in plants in response to environmental stress and plays a pivotal role in the reaction to various stimuli. Increasing evidence demonstrates a significant role of ubiquitination and subsequent selective degradation in controlling ABA signaling. We present updated information on the components of the ABA signaling pathway modified by different E3 ligases.
- abscisic acid
- ubiquitination
- E3 ubiquitin ligases
1. Introduction
Plants are often subjected to various detrimental environmental stressors. Despite their sessile lifestyle, plants are able to maintain their internal equilibrium when exposed to external challenges. This balance, called homeostasis, can be achieved by internal mechanisms that alter cell metabolism, thanks to which the whole organism can maintain relatively constant conditions. Such rapid and appropriate genetic and metabolic acclimation, which is a crucial issue for plants’ survival, is maintained due to the coordinated action of plant hormones and cellular degradation mechanisms influencing proteostasis.
Protein homeostasis (or proteostasis) integrates cellular pathways that mediate biogenesis, folding, trafficking, and the degradation of polypeptides to maintain the required concentrations of all proteins that compose the proteome. This field has been intensively investigated by numerous research groups [1]. Proteins are often tagged for removal by ubiquitination. Polyubiquitinated proteins and protein aggregates are degraded via the ubiquitin–proteasome system (UPS) pathway and via the autophagy–vacuolar route. The UPS pathway is restricted in its ability to degrade aggregated proteins, which are too large to pass through the narrow proteasome entrance channel [2]. Thus, autophagy represents a major mechanism in plants for degrading macromolecular ubiquitinated protein aggregates as a response to stressors [3,4].
Phytohormones are not only involved in protein degradation mechanisms, but also play a pivotal role in plant responses to various stimuli. Although the relationships between them are poorly understood, it is generally accepted that extensive crosstalk and multiple regulatory loops are responsible for maintaining the balance of growth and stress responses [9].
Phytohormones are master regulators of plant growth, development, and stress response. Of them, abscisic acid (ABA) is rapidly accumulated in plants in response to abiotic stress [10]. Similarly to most other hormones, ABA is involved in developmental processes, including seed maturation, seed dormancy and germination, primary root growth, and flowering time control, and in the response to adverse environmental stressors [10,11]. Plants exposed to abiotic stress quickly activate the ABA signaling cascade, resulting in the activation of ABA-responsive transcription factors and the induction of ABA-responding genes. However, after achieving stress tolerance, it is necessary to terminate (or attenuate) the ABA pathway. The balance is maintained by the selective degradation of specific components of the ABA signaling pathway upon their tagging for decay by ubiquitination. Conventional degradation of the tagged components takes place via the proteasome; however, increasing evidence demonstrates a significant role of autophagy in controlling ABA signaling [9,12,13,14,15,16]. In addition to this interplay with degradation systems, an additional layer of complexity is provided by extensive crosstalk between ABA and other phytohormones [17,18,19,20].
2. Principles of ABA Perception and Signaling and Their Control by Selective Degradation Systems
Reversible protein phosphorylation is a post-translational modification catalyzed by protein kinases and phosphatases. It acts as a molecular switch for almost all key events of cell metabolism and signaling in eukaryotes. Additionally, in the field of ABA signal transduction, protein kinases and phosphatases play pivotal roles, forming a reversible protein phosphorylation cascade, including ABA receptors (pyrabactin resistance/regulatory components of ABA receptor (PYR/RCAR)), type 2C protein phosphatases (PP2Cs), protein kinases (Snf1 (sucrose-non-fermentation 1)-related kinases subfamily 2, SnRK2s), and downstream targets. The key function of RCARs is to indirectly control the activity of SnRK2s, which phosphorylate numerous stress-activated targets in the response to ABA, including ABA-responsive transcription factors (TFs) [21,22]. This is accomplished through a negative regulatory pathway, involving RCARs, PP2C, and SnRK2 proteins and E3 ubiquitin ligases (Figure 1).
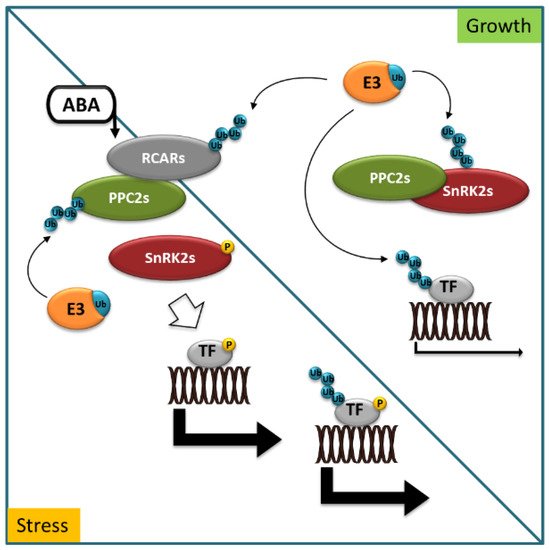
Figure 1. Abscisic acid (ABA) signaling in stress and growth. In growth-promoting conditions, ABA receptors (RCARs), protein kinases (SnRK2s) dephosphorylated by type 2C protein phosphatases (PP2Cs), and ABA-responsive transcription factors (TF) are marked for degradation with ubiquitin (Ub) by specific E3 ligases (E3). In stress conditions, ABA accumulates and binds to RCARs, allowing for interaction with PP2Cs to inhibit phosphatase activity. SnRK2s are released to phosphorylate and control the activity of downstream targets to trigger physiological responses. Blue circles indicate ubiquitination, yellow circles indicate phosphorylation events.
When ABA accumulates in cells in response to environmental stress or developmental cues, ABA binds to RCARs and triggers a conformational change that allows the binary complex (receptor–ABA) to physically interact with PP2Cs and inhibit its phosphatase activity. The various RCAR–PP2C heterodimers preclude binding of SnRK2s to PP2Cs, thus stimulating SnRK2s kinase activity. Consequently, SnRK2s are released to phosphorylate and control the activity of downstream factors to trigger physiological responses [23].
3. Role of Ubiquitination and Selective Degradation in ABA Perception and Signaling
Ubiquitination is (next to phosphorylation) another reversible, post-translational modification, originally discovered as a critical element in highly regulated proteolysis and regarded as essential for many other cellular processes. Ubiquitin (Ub) serves as a reusable tag that decides the cellular fate of the proteins and is recognized by numerous Ub-binding proteins directing the target proteins for selective turnover [24,25,26]. Ub, often in the form of polymeric chains, is covalently attached to the target proteins through a three-step E1–E2–E3 conjugation cascade (Figure 2).
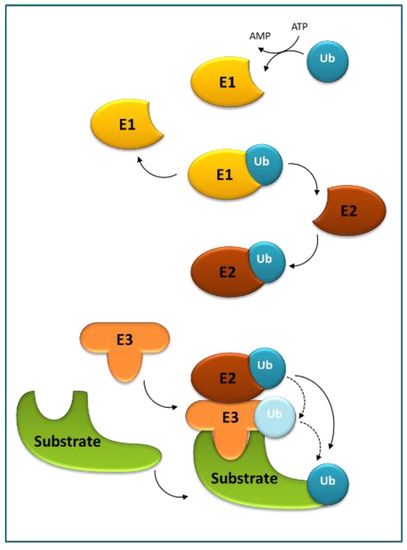
Figure 2. The ubiquitination cascade. Ubiquitin-activating enzyme (E1) activates ubiquitin (Ub) in an ATP-dependent manner, and next the thioester-linked Ub is transferred to Ub-conjugating enzyme (E2). Subsequently, E2 enzymes catalyze the attachment of Ub to a substrate using Ub ligase (E3). E3s, responsible for substrate recognition, transfer Ub either directly from E2 or form an E3–Ub intermediate prior to the transfer.
The process starts with E1 (or Ub-activating enzyme) in which Ub is activated in an ATP-dependent manner, through a thioester bond between the C-terminus of Ub and a cysteine residue of E1, and next the thioester-linked Ub is transferred onto a cysteine residue of E2 (or Ub-conjugating enzyme) by transesterification to form an unstable E2–Ub intermediate. Next, E2 enzymes catalyze the attachment of Ub to lysine (Lys) residues of target proteins using an E3 (or Ub ligase). E3 enzymes are responsible for substrate recognition and either directly transfer the Ub to the substrates from E2 or form an E3–Ub intermediate prior to the transfer. In successive rounds, additional Ub moieties can be attached to one of the seven lysine residues (Lys6, Lys11, Lys27, Lys29, Lys33, Lys48, and Lys63) or the N-terminal methionine (Met1) of Ub to produce poly-Ub chains. The end-product is a Ub–protein conjugate that contains an isopeptide bond between the C-terminal glycine residue of Ub and one of the Lys residues in the substrate. These structurally distinct ubiquitylation patterns are recognized by various effector proteins harboring a ubiquitin-binding domain (UBD) to result in diverse downstream signals. For example, poly-Ub chains are linked by Lys48 target substrates to the 26S proteasome for degradation, whereas poly-Ub chains are linked by Lys63 direct substrates to autophagosomes, both with the concomitant release of Ub moieties for reuse [27].
The E3 Ub ligases can be classified into single- and multi-subunit groups [28]. The single-subunit group includes several subfamilies based upon their mechanisms of action and the presence of specific domains: HECT (homology to E6–AP C terminus), RING (really interesting new gene), and U-box type E3s. The multi-subunit group, cullin-RING box1-ligases (CRLs), are further divided into four subfamilies: SCF (S phase kinase-associated protein 1–cullin 1–F-box), BTB (Bric-a-brac–Tramtrack–Broad complex), DDB (DNA damage-binding domain-containing), and APC (anaphase-promoting complex).
Three E2 enzymes, UBC32, UBC33, and UBC34 play a negative role in drought stress response and ABA signaling [29], and numerous E3 ligases are involved in the inhibition of ABA signaling in optimal conditions, ABA induction upon environmental stress, and ABA attenuation after achieving stress tolerance [12]. The currently known components of ABA perception and signaling and E3 ligases involved in the process are listed in Table 1.
Table 1. ABA pathway components targeted by E3 ligases.
| Pathway Component | Specific Target | E3 Ligase [References] (Remarks) |
|---|---|---|
| ABA Receptors | RCAR1/PYL9 | DDA1 [30]; REA1 [31]; PUB22 [32]; PUB23 [32] |
| RCAR3/PYL8 | RIFP1 [33]; DDA1 [30] | |
| RCAR10/PYL4 | DDA1 [30]; RSL1 [34]; UBC26 (E2)-RFA4 (E3) [35]; RFA1 [35] | |
| RCAR11/PYR1 | RSL1 [34]; RFA1 [35]; RFA4 [35] | |
| Type 2C protein phosphatases (PP2C) | ABI1 | PUB12 [36]; PUB13 [36]; BPM3 [37]; BPM5 [37]; UBC27/AIRP3 [38]; COP1 [39] |
| ABI2 | RGLG1 [40]; RGLG5 [40] | |
| HAB1 | BPM3 [37]; BPM5 [37] | |
| HAB2/NHL29 | RGLG1 [40]; RGLG5 [40] | |
| AHG1 | PIR1.2 [41]; PIR2 [41] | |
| AHG3/PP2CA | RGLG1 [40]; RGLG5 [40]; PIR1.2 [41]; PIR2 [41]; COP1 [39]; BPM3 [37]; BPM5 [37] | |
| HAI1/SAG113 | PIR1.2 [41]; PIR2 [41] | |
| HAI3 | PIR1.2 [41]; PIR2 [41] | |
| SnRK2 kinases | SnRK2.3/SRK2I | PP2-B11 [42] |
| SnRK2.6/OST1 | HOS15 [43] | |
| ABA-responsive transcription factors | ABI5 | DWA1 [44]; DWA2 [44]; KEG [45]; ABD1 [46]; COP9 [47] |
| ABI3 | AIP2 [48,49] | |
| ABI4 | COP1 [50] | |
| ABF1 | KEG [51,52] | |
| ABF2/AREB1 | KEG [53] | |
| ABF3/AREB2 | KEG [51,52] | |
| DREB2 | DRIP1 [54]; DRIP2 [54]; BPM2 [55] | |
| HB6 | BTB1-6/BPM1-6 [56] | |
| MYB96 | MIEL1 [57] (Myb96 activates ABI4) | |
| MYB30 | RHA2b [58]; MIEL1 [57,59] | |
| RAV1 | BPM1 [60,61] | |
| Other regulatory and signaling factors and ABA-responding genes | ADA2B | SKIP24/At1g08710 [62] |
| ATP1/SDIRIP1 | AIRIP2 [63,64]; AIRP1 [63,64]; SDIR1 [65]; (SDIRIP1 activates ABI5) | |
| ACD11 | XBAT35.2 [66]; (ACD11 is one of ABA-responding genes) | |
| RD21 | AIRP3/LOG2 [67]; (positive role in ABA responses; RD21 is Cys protease) | |
| CIPK26 | KEG [68,69] | |
| PP2A | CHIP [70] |
This entry is adapted from the peer-reviewed paper 10.3390/ijms22094638
This entry is offline, you can click here to edit this entry!
