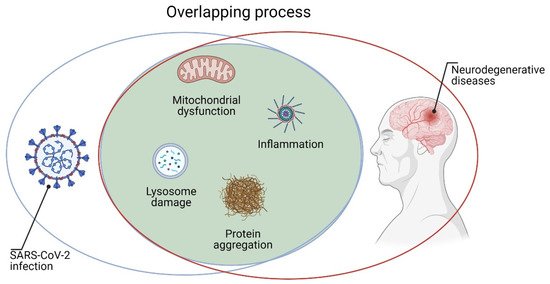
The emergency caused by SARS-CoV-2 had, and still has, devastating socio-economic aspects. Assessing the impact of COVID-19 on vulnerable groups of people is crucial for the adaptation of governments’ responses. Growing scientific evidence suggests that it is essential to keep the attention on people after acute SARS-CoV-2 infection; indeed, some clinical manifestations are frequently present even after recovery. There is consensus on the need to define which symptoms persist after the infection and which disabilities may arise after COVID-19. Recent reviews, case reports, and original contributions suggest that various organs may be affected, and neurological symptoms are present in about one third of patients with COVID-19. Neurological complications after severe COVID-19 infection might include delirium, brain inflammation, stroke, and nerve damage. In the recent pandemic, neurologists and neurobiologists have a chance to study key features of infection neurology.
- COVID-19
- SARS-CoV-2
- neurology
- brain damage
- post-scute COVID-19 neurological syndrome
1. Neurobiology of SARS-CoV-2 Infection
2. Potential Mechanisms for the Penetration of SARS-Cov2 in CNS
2.1. Direct Route
2.2. Indirect Route via Bloodstream
3. SARS-CoV-2: Cellular Mechanism
4. Neuroimmunology of COVID-19 Infection
5. Clinic Manifestation of Neuropathology of COVID-19 Infection
- Nuzzo, D.; Picone, P. Potential neurological effects of severe COVID-19 infection. Res. 2020, 158, 1–5. [CrossRef] [PubMed]
- Li, Z.; Liu, T.; Yang, N.; Han, D.; Mi, X.; Li, Y.; Liu, K.; Vuylsteke, A.; Xiang, H.; Guo, X. Neurological manifestations of patients with COVID-19: Potential routes of SARS-CoV-2 neuroinvasion from the periphery to the brain. Med. 2020, 14, 533–541. [CrossRef] [PubMed]
- Li, K.; Wohlford-Lenane, C.; Perlman, S.; Zhao, J.; Jewell, A.K.; Reznikov, L.R.; Gibson-Corley, K.N.; Meyerholz, D.K.; McCray, P.B., Jr. Middle east respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. Infect. Dis. 2016, 213, 712–722. [CrossRef] [PubMed]
- Lochhead, J.J.; Kellohen, K.L.; Ronaldson, P.T.; Davis, T.P. Distribution of insulin in trigeminal nerve and brain after intranasal administration. Rep. 2019, 9, 1–9. [CrossRef] [PubMed] S
- Song, E.; Zhang, C.; Israelow, B.; Lu-Culligan, A.; Prado, A.V.; Skriabine, S.; Lu, P.; Weizman, O.-E.; Liu, F.; Dai, Y.; et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. Exp. Med. 2021, 218, e20202135. [CrossRef]
- Fotuhi, M.; Mian, A.; Meysami, S.; Raji, C.A. Neurobiology of COVID-19. Alzheimer’s Dis. 2020, 76, 3–19. [CrossRef] [PubMed]
- Magrone, T.; Magrone, M.; Jirillo, E. Focus on receptors for coronaviruses with special reference to angiotensin-converting enzyme 2 as a potential drug target—A perspective. Metab. Immune Disord. Drug Targets 2020, 20, 807–811. [CrossRef]
- Karpinski,T.M.;Ozarowski,M.;Seremak-Mrozikiewicz,A.;Wolski,H.;Wlodkowic,D.The2020racetowardsSARS-CoV-2 specific vaccines. Theranostics 2021, 11, 1690–1702. [CrossRef]
- Rhea, E.M.; Logsdon, A.F.; Hansen, K.M.; Williams, L.M.; Reed, M.J.; Baumann, K.K.; Holden, S.J.; Raber, J.; Banks, W.A.; Erickson, M.A. The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice. Neurosci. 2021, 24, 368–378. [CrossRef]
- Lippi, A.; Domingues, R.; Setz, C.; Outeiro, T.F.; Krisko, A. SARS-CoV -2: At the crossroad between aging and neurodegeneration. Disord. 2020, 35, 716–720. [CrossRef] [PubMed]
- Di Carlo, M.; Giacomazza, D.; Picone, P.; Nuzzo, D.; San Biagio, P.L. Are oxidative stress and mitochondrial dysfunction the key players in the neurodegenerative diseases? Free Radic. Res. 2012, 46, 1327–1338. [CrossRef]
- Picone, P.; Nuzzo, D.; Caruana, L.; Scafidi, V.; Di Carlo, M. Mitochondrial dysfunction: Different routes to Alzheimer’s disease Oxid. Med. Cell. Longev. 2014, 2014, 1–11. [CrossRef] [PubMed]
- Nuzzo, D.; Picone, P.; Caruana, L.; Vasto, S.; Barera, A.; Caruso, C.; Di Carlo, M. Inflammatory mediators as biomarkers in brain disorders. Inflammation 2013, 37, 639–648. [CrossRef]
- Nath, A. Neurologic complications of coronavirus infections. Neurology 2020, 94, 809–810. [CrossRef] [PubMed]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Rev. Immunol. 2020, 20, 355–362. [CrossRef] [PubMed]
- Ahmadpoor, P.; Rostaing, L. Why the immune system fails to mount an adaptive immune response to a COVID-19 infection. Int. 2020, 33, 824–825. [CrossRef] [PubMed]
- Li, Y.; Fu, L.; Gonzales, D.M.; Lavi, E. Coronavirus neurovirulence correlates with the ability of the virus to induce proinflammatory cytokine signals from astrocytes and microglia. Virol. 2004, 78, 3398–3406. [CrossRef] [PubMed]
- Koralnik, I.J.; Tyler, K.L. COVID -19: A Global Threat to the Nervous System. Neurol. 2020, 88, 1–11. [CrossRef]
- Connors, J.M.; Levy, J.H. Thromboinflammation and the hypercoagulability of COVID-19. Thromb. Haemost. 2020, 18, 1559–1561. [CrossRef]
- Mohamud, A.Y.; Griffith, B.; Rehman, M.; Miller, D.; Chelb, A.; Chebl, A.; Patel, S.C.; Howell, B.; Kole, M.; Marin, H. Intraluminal carotid artery thrombus in COVID-19: Another danger of cytokine storm? J. Neuroradiol. 2020, 41, 1677–1682. [CrossRef]
- Wijeratne, T.; Gillard-Crewther, S.; Sales, C.; Karimi, L. COVID-19 pathophysiology predicts that ischemic stroke occurrence is an expectation, not an exception—A systematic review. Frontiers in Neurology. Neurol. 2021, 11, 607221. [CrossRef]
- Al-Dalahmah, O.; Thakur, K.T.; Nordvig, A.S.; Prust, M.L.; Roth, W.; Lignelli, A.; Uhlemann, A.-C.; Miller, E.H.; Kunnath- Velayudhan, S.; del Portillo, A.; et al. Neuronophagia and microglial nodules in a SARS-CoV-2 patient with cerebellar hemorrhage. Neuropathol. Commun. 2020, 8, 147. [CrossRef]
- Ellul, M.A.; Benjamin, L.; Singh, B.; Lant, S.; Michael, B.D.; Easton, A.; Kneen, R.; Defres, S.; Sejvar, J.; Solomon, T. Neurological associations of COVID-19. Lancet Neurol. 2020, 19, 767–783. [CrossRef]
- Sadeghi, A.; Tahmasebi, S.; Mahmood, A.; Kuznetsova, M.; Valizadeh, H.; Taghizadieh, A.; Nazemiyeh, M.; Aghebati-Maleki, L.; Jadidi-Niaragh, F.; Abbaspour-Aghdam, S.; et al. Th17 and Treg cells function in SARS-CoV2 patients compared with healthy controls. Cell. Physiol. 2021, 236, 2829–2839. [CrossRef]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L.; et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Arch. Oto Rhino Laryngol. 2020, 277, 2251–2261. [CrossRef]
- Bénézit, F.; Le Turnier, P.; Declerck, C.; Paillé, C.; Revest, M.; Dubée, V.; Tattevin, P.; Arvieux, C.; Baldeyrou, M.; Chapplain, J.-M.; et al. Utility of hyposmia and hypogeusia for the diagnosis of COVID-19. Lancet Infect. Dis. 2020, 20, 1014–1015. [CrossRef]
- Varatharaj, A.; Thomas, N.; A Ellul, M.; Davies, N.W.S.; A Pollak, T.; Tenorio, E.L.; Sultan, M.; Easton, A.; Breen, G.; Zandi, M.; et al. UK-wide surveillance of neurological and neuropsychiatric complications of COVID-19: The first 153patients. Lancet Psychiatry 2020, 7, 875–882. [CrossRef]
- Lindan, C.E.; Mankad, K.; Ram, D.; Kociolek, L.K.; Silvera, V.M.; Boddaert, N.; Stivaros, S.M.; Palasis, S. Neuroimaging manifestations in children with SARS-CoV-2 infection: A multinational, multicentre collaborative study. Lancet Child Adolesc. Health 2021, 5, 167–177. [CrossRef]
- Rogers, J.P.; Chesney, E.; Oliver, D.; A Pollak, T.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020, 7, 611–627. [CrossRef]
This entry is adapted from the peer-reviewed paper 10.3390/jcm10091947
