Epilepsy is a common brain disorder characterized by recurrent epileptic seizures with neuronal hyperexcitability. Apart from the classical imbalance between excitatory glutamatergic transmission and inhibitory γ-aminobutyric acidergic transmission, cumulative evidence suggest that cholinergic signaling is crucially involved in the modulation of neural excitability and epilepsy.
- cholinergic
- muscarinic
- nicotinic
- excitability
- epilepsy
1. Introduction
More than 70 million people have epilepsy worldwide, accounting for about 1% of the population, which makes it one of the most common neurological conditions [1]. Epilepsy is usually characterized by recurrent seizures resulting from the hypersynchronous discharge of large populations of neurons in the brain [2]. The exact mechanism of epilepsy is still not well understood. Classically, it caused by an imbalance of “excitation–inhibition” that usually is closely related to the excitatory glutamatergic transmission and inhibitory γ-aminobutyric acidergic (GABAergic) transmission. Based on this classical theory, most current anti-epileptic drugs (AEDs) are mainly used to control seizures via blocking the activity of excitatory glutamatergic transmission or enhancing the activity of inhibitory GABAergic transmission. However, there are still 30% of patients who become drug-resistant to epilepsy [3]. Thus, the other alternative mechanisms underlying epilepsy and some new therapies are still being intensively sought.
The cholinergic system in the brain modulates neuronal excitability, synaptic transmission, and synaptic plasticity, playing a significant role in many physiological functions [4]. The evidence of cholinergic mechanisms involved in epilepsy emerged at the beginning of the 19th century [5]. Numerous studies have shown that the systemic administration of cholinergic agonists carbachol or pilocarpine have long been known to induce seizure activity [6]. Then, findings from both experimental and clinical research indicated dysfunctional cholinergic signaling in epilepsy. However, the causal role of cholinergic neurons in the generation, propagation, and control of seizures has limited data and many inconsistent reports recently. Notably, we recently found that the selective activation of cholinergic neurons in the medial septum (MS) by using optogenetics could produce obvious anti-seizure effects [7]. These data collectively support the notion that cholinergic signaling may play a critical but heterogeneous role in epilepsy. As the different functions of acetylcholine (ACh) hinge on the site of release, receptor subtype, and target neuronal population or neural circuit, gaining an in-depth understanding of cholinergic system potential mechanisms in epilepsy are necessary stepping stones to uncover their wide-ranging applications in the clinical arena for the treatment of epilepsy.
2. Cholinergic Signaling in Epilepsy
Given the outstanding roles of ACh in the modulation of neural excitability by binding different subtypes of AChRs, it is not surprisingly that cholinergic dysfunction is closely correlated with epilepsy, which is mainly caused by the imbalance of excitation and inhibition. Then, we will mainly focus our discussion on the relationship between cholinergic signaling and epilepsy.
2.1. mAChRs and Epilepsy
A study suggested that there was a reduction of ChAT activity and mAChRs binding in the piriform cortex, amygdala, and nucleus basalis, a decrease of AChE activity in the piriform cortex, and a loss of Na+-dependent high-affinity choline uptake in the piriform cortex and amygdala in a kainic acid-induced epilepsy model [8]. Furthermore, clinical data demonstrated that the binding of mAChRs antagonist I-iododexetimide was decreased in the anterior hippocampus in partial seizures patients [9]. These reflect the dysfunction of cholinergic signaling in epilepsy.
Muscarinic excitation contributes to increasing susceptibility to epileptogenesis and rewiring hippocampal circuitry [10]. Importantly, pilocarpine, which is an mAChRs agonist, is one of the most common used chemicals to induce seizure models. Of the mAChRs, M1 is among the most heavily expressed in the forebrain and midbrain regions and has been proposed to play key roles in the regulation of epilepsy. A study using mice with deletion of the five muscarinic receptor subtypes made several observations; only M1 KO mice did not display seizures and survived after pilocarpine administration, while M2-M5 KO mice all displayed clonic seizures and died within 60 min after pilocarpine administration [11]. It is noteworthy that the mAChRs agonist pilocarpine does not evoke seizure activity in both homozygous and heterozygous M1 mutant mice [12], which suggests that the role of the M1 subtype mediates pilocarpine-induced seizures. Moreover, M1 mAChRs are vital to γ generation, which is thought to be generated by PV cells and precedes seizure onset [13]. Molecular pharmacology studies indicate that the M1 mAChRs selectivity antagonist VU0255035 is efficacious in reducing pilocarpine-induced seizures in mice [14] and holding over the process of status epilepticus after organophosphates (OPs) such as paraoxon or soman exposure [15]. Together, those data suggested that M1 mAChRs may be the important subtype in the regulation of epileptic seizures.
M2 receptors always increased in various epilepsy models including febrile seizure, hippocampal sclerosis, and other neocortical pathologies [16]. Recent studies have shown that the increase of M2 mAChRs in the brainstem in pentylenetetrazole (PTZ)-kindled epileptic rats [17]. In vitro receptor binding studies have further shown an enhancement of M2 receptors binding in the lateral amygdala nuclei of TLE patients, while binding to M3 receptors was reduced [18]. Intriguingly, there is no obvious seizure phenotype in global M2 knockout mice [11]. Additionally, sparteine, an anticonvulsant drug, increasing the hippocampal M4 receptor expression on PTZ-induced seizures, indicating that the M4 receptor may be also critical for seizures [19].
As previously indicated, limbic and brainstem systems are two important anatomical systems involved in epileptic seizures, leading to the limbic seizure and the brainstem seizure. Intrahippocampal and intracerebroventricular administration of mAChRs agonists in rats produced sustained limbic seizures and brain damage. Intrahippocampal pilocarpine or bethanechol administration-induced limbic seizures are initiated via mAChRs and further mediated excitotoxicity via NMDARs [20]; EEG in CA3 showed spiking activity of high frequency, with rapid propagation to the lateral septum, amygdala, and neocortex along with the hippocampus [21]. Intraamygdaloid administration of kainic acid elicits epileptiform electroencephalographic activity; subsequently, neuronal loss and gliosis were marked at the various hippocampal fields, the midline thalamic nuclei, lateral septum, and cortical areas [22]. Furthermore, ACh may tonically enhance the excitability of cerebral cortical neurons, which might account for an increase in the effectiveness of other excitatory inputs and facilitate the development of epileptogenesis [23]. These results suggest that the overstimulation of mAChRs leads to limbic seizures.
Tonic-clonic seizure is considered to be mediated by brainstem structures. A microinjection of carbachol into the nucleus reticularis pontis oralis, one of the brainstem structures, inhibited the maximal electroshock seizure (MES) in rats [24]. A microinjection of carbachol into the periaqueductal gray (PAG) region of rats induced seizure behavior accompanied by epileptiform electrocorticogram afterdischarge recorded from the parietal cortex. Interestingly, limbic seizure activity, similar to amygdala-kindled seizures was also induced in two animals. In addition, a PAG microinjection of bicuculline induced clonic seizures, myoclonic activity, or limbic seizures [25]. These reports indicate that the mAChRs signaling in the brainstem system may regulate the limbic seizure and brainstem seizure activity collectively.
Despite there already being various studies about the role of mAChRs in epileptic seizures (Table 1), how mAChRs are involved in the different stages of epilepsy still needs to be investigated.
Table 1. Summaries of findings reporting the role of mAChRs in epilepsy.
| Epilepsy Model | Time Point | Observations | References |
|---|---|---|---|
| Temporal lobes with complex partial seizures | Interictal period | The binding of mAChRs antagonist I-iododexetimide was decreased in the anterior hippocampus. | [9] |
| Patients with drug-resistant focal temporal lobe epilepsy | Interictal period | M2 receptors always increased in various seizures including febrile seizure, hippocampal sclerosis, and other neocortical pathologies. | [16] |
| Patients with intractable temporal lobe epilepsy | Interictal period | An enhancement of M2 receptors binding in the lateral amygdala nuclei of TLE patients, while binding to M3 receptors was reduced. | [18] |
| Kainic acid | 3 days after injection of kainic acid | 1. The reduction of ChAT activity in the piriform cortex, amygdala, and nucleus basalis. 2. The reduction of AChE activity in the piriform cortex. 3. The decrease of mAChRs binding in the piriform cortex, amygdala, and nucleus basalis. 4. The decrease of Na+-dependent high-affinity choline uptake in the piriform cortex and amygdala. |
[8] |
| Pilocarpine | 30 min after administration of pilocarpine | 1. M1 KO mice did not display seizures and survived after pilocarpine administration. 2. M2-M5 KO mice all had a seizure (clonic seizures) and died within 1 h after pilocarpine administration. |
[11] |
| Pilocarpine | 45 min after administration of pilocarpine | The inability of pilocarpine to evoke seizures in both homozygous and heterozygous M1 mutant mice. | [12] |
| Pilocarpine | 45 min after administration of pilocarpine | 1. VU0255035 suppresses the potentiation of NMDAR currents induced by carbachol in hippocampal pyramidal cells. 2. VU0255035 inhibits pilocarpine-induced seizures. |
[14] |
| OPs | 60 min after administration of OPs | VU0255035 retarded the process of status epilepticus after OPs exposure. | [15] |
| PTZ kindling model | 30 min after administration of PTZ | The increase of M2 receptors was observed in PTZ-kindled in the brainstem. | [17] |
| PTZ kindling model | 180 and 240 min after administration of PTZ | Sparteine increases the hippocampal M4 receptor expression. | [19] |
2.2. nAChRs and Epilepsy
The systemic or central administration of α7 antagonist MLA is known to block nicotine-induced seizures in mice [26][27], and choline dose-dependently ameliorated seizure severity in PTZ-kindled mice [28]. A previous study tested the anti-seizure activity of various novel amino-alkyl-cyclohexane derivatives, among which nAChRs antagonists have shown an overlap potency between channel blocking at nAChRs and NMDARs. nAChRs preferring antagonists strongly relieved MES and nicotine-induced seizure in mice. The effect of anticonvulsant in the MES was reduced by an additional injection of a subconvulsant dose of nicotine. However, such efficacious anticonvulsants were not observed in kindled rats [29]. These indicated that nAChRs antagonists might be a promising therapeutic approach to treat generalized seizures rather than complex partial seizures. Furthermore, α7 nAChRs currently were found to regulate the hyperfunction of glutamatergic synaptic transmission in the hippocampus samples obtained from patients with mesial temporal lobe epilepsy with hippocampal sclerosis [30].
As described for a variety of nocturnal epilepsy syndromes, autosomal dominant sleep-related hyper motor epilepsy (ADSHE) predominantly related to sleep, and approximately 12% of the ADSHE families carry mutations on genes coding for subunits of the neuronal nAChRs (major subtypes: homomeric α7 and heteromeric α4β2). To date, ADSHE mutations are mainly in CHRNA2 (α2I279N), CHRNA4 (α4S248F, α4S252L, α4T265I, α4776ins3), and CHRNB2 (β2V287M, β2V287L, β2I312M, β2L301V, β2V308A). A previous study suggested that the modulation of α4β2 nicotinic receptors plays a role not only in ADSHE but also in other genetic epileptic syndromes such as idiopathic generalized epilepsy and could serve as a biomarker of epilepsy syndromes with a genetic background. The mutant in β2V287L presynaptic nAChRs triggering neuronal firing, serving as an enhancement of neurotransmitter release or the abnormal mutant in postsynaptic nAChRs that may cause hyperexcitability [31]; β2V287L also causes spontaneous seizures during periods of increased δ wave activity [32]. Interestingly, the previous study suggested that the treatment of carbamazepine (CBZ) in ADSHE is mainly through nAChRs, which is supported by the evidence that 100 μM CBZ inhibits ACh-evoked currents at the human α4β2 nicotinic receptors and the ADSHE α4S248F or α4L-776ins3 mutant receptors with 3-fold increase in sensitivity to CBZ [33]. Additionally, an increase of midbrain nAChRs density could be involved in the pathological of ADSHE through the brainstem cholinergic signaling in the ascending arousal system [34]. A study of ADSHE variants in CHRNB2 and CHRNA4 closely relevant to patients with insular epilepsy recently, CHRNB2 and CHRNA4 increased nicotinic currents in whole-cell recording [35]. In addition, clinical data demonstrated that mutations in CHRNA4 may be a novel gene causing genetic or focal epilepsy with febrile seizures [36] and familial partial epilepsy with variable foci [37], it aims to broaden the genotypic-phenotypic spectrum of combined epileptic in CHRNA4.
In addition, an experimental study has demonstrated that cholinergic systems closely linking to the pathogenesis of Rett syndrome (RTT), and RTT patients suffer from epilepsy up to 80% [38]. Mutations in the X-linked gene encoding the transcriptional regulator Mecp2 cause RTT. Conditional deletion of Mecp2 in cholinergic neurons resulted in the alteration of epilepsy susceptibility, which could be relieved by re-expressing Mecp2 in the BF cholinergic neurons of Chat-Mecp2−/y mice, which implicated the relationship of BF cholinergic system and epilepsy. Chat-Mecp2−/y mice displayed frequent hyperexcitability discharges. Furthermore, the administration of α7 nAChRs agonist PNU282987 in the CA1 of the hippocampus increased the seizure onset time [39]. These findings collectively proved that the dysfunction of cholinergic neurons can contribute to epilepsy through nAChRs (Table 2).
Table 2. Summaries of findings reporting the role of nAChRs in epilepsy.
| Epilepsy Model | Time Point | Observations | References |
|---|---|---|---|
| Patients with mesial temporal lobe epilepsy with hippocampal sclerosis | Interictal period | α7 nAChRs were found to regulate hyperfunction of glutamatergic synaptic transmission in the hippocampus. | [30] |
| HEK293 cells co-expressing the human α4 nAChRs and the wild-type and the V287L mutant patient | - | 1. The mutant in β2V287L presynaptic nAChRs triggering neuronal firing, serving as an enhancement of neurotransmitter release. 2. The abnormal mutant in postsynaptic nAChRs may cause hyperexcitability. |
[31] |
| Reconstituted in Xenopus oocytes | - | 100 μM CBZ inhibits ACh-evoked currents at the human α4β2 nicotinic receptors, and the ADSHE α4S248F or α4L-776ins3 mutant receptors, with a roughly 3 fold increase in sensitivity to CBZ. | [33] |
| ADSHE patients | Interictal period | An increase of midbrain nAChRs density in the ADSHE. | [34] |
| Patients with insular epilepsy | Interictal period | Mutant nACh receptors increased nicotinic currents in whole-cell recording. | [35] |
| Genetic or focal epilepsy with febrile seizures (GEFS+) patients | Interictal period | CHRNA4 was the pathogenic gene of GEFS+. | [36] |
| Familial partial epilepsy with variable foci (FPEVF) patients | Interictal period | cHRNA4 was the pathogenic gene of FPEVF. | [37] |
| Nicotine | Intraperitoneally injected 15 min before the nicotine treatment. | Nicotine elicits convulsive seizures by activating amygdalar neurons mainly via α7 nACh receptors. | [26] |
| PTZ kindling | Exposed to PTZ injections on day 3, 6, and 9 of treatment to assess seizure severity score. | The amelioration of epilepsy by α7 nAChRs agonist choline chloride in PTZ-kindled mice model. | [28] |
| MES and nicotine-induced seizure test in mice;Amygdala-kindling in rats. | 1.Nicotine-induced seizure starting immediately after nicotine injection and up to 5 min afterwards. 2. MES and kindling assesed interictal period. |
1. Various novel amino-alkyl-cyclohexane derivatives, among which nAChRs antagonists have shown an overlap potency between channel blocking at nAChRs and NMDARs. 2. nAChRs preferring antagonists were strongly relived MES and nicotine-induced seizure in mice. 3. The effect of anticonvulsant in the MES was all reduced by an additional injection of a subconvulsant dose of nicotine. 4. Such efficacious anticonvulsants were not affected in kindled rats |
[29] |
| Pilocarpine | EEG activities recorded 7 days post-surgical recovery | 1. Chat-Mecp2−/y mice displayed frequent hyperexcitability discharges. 2. Administration of pilocarpine produces status epilepticus in Chat-Mecp2−/y mice. 3. Administration of α7 nAChRs agonist PNU282987 in the CA1 of the hippocampus increased the seizures onset time. |
[39] |
Similar as mAChRs, nAChRs are also implicated in the pathogenesis of the different type of epilepsy. Nicotine induced seizures by activating hippocampus and amygdalar neurons mainly via α7 nAChRs [27]. In humans, nAChR mutations associated with ADSHE seizures occur in the frontal lobe [32]. These results suggest that the overstimulation of nAChRs leads to limbic seizures, while intra-inferior colliculus microinjection of different doses in nAChRs antagonists has a different role in the modulation of spontaneous seizures [40]. It indicated the nAChRs may also contribute to brainstem seizures. The role of the cholinergic system in epilepsy has long been studied, but most researchers have focused on mAChRs rather than nAChRs, and the role of nAChRs is yet to be further invested in limbic seizure and the brainstem seizure.
2.3. Cholinergic Neurons Circuit in Epilepsy
Currently, studies of clinical and experimental models of epilepsy suggest a more precise conceptual mechanism that seems to underlie epilepsy: a change in the excitation–inhibition (E-I) balance in circuit-level dysfunction. Therefore, exploring and understanding the precise circuit-level cholinergic mechanism of epileptic seizures is essential for precise circuit therapy and regulation. The question arises as to how cholinergic neurons modulate the circuit-level dysfunction of an epileptic seizure. Epilepsy originates from the limbic system, especially from amygdaloid and hippocampal regions. Numerous studies have attempted to explain how the BF cholinergic system projects to the cortex [41], hippocampus, and amygdala [42][43], which are critical regions for the seizure generation and spread (Figure 2). For example, the changes in the amygdaloidal cholinergic connections from the BF may contribute to epilepsy-related hyperexcitability [28]. Nicotine elicits convulsive seizures by activating amygdala neurons mainly via α7 nACh receptors. Intracerebrally, physostigmine, a reversible cholinergic medication, in limbic structures has been reported to prolong seizure by increased sensitivity to kindling stimulations [44]. However, intraventricular administration of 192 Immunoglobulin G -saporin, which inhibits cholinergic projection to the hippocampus and cortex respectively, facilitates seizure induced by amygdala kindling [45]. These data suggested that cholinergic neurons may play a critical but heterogeneous role in epilepsy at the circuit level.
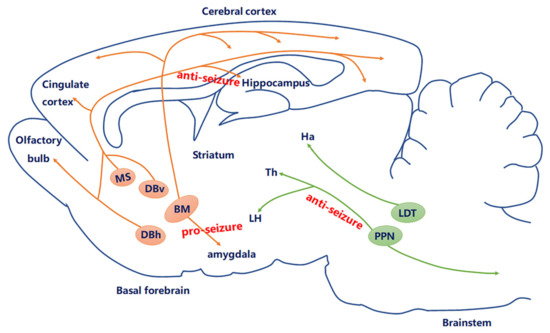
Figure 2. Current knowledge of the role of the cholinergic circuit in epilepsy. The central cholinergic afferents mostly originate from the basal forebrain (BF), including medial septum (MS), DBv, DBh, and nucleus basalis (NB). Another derives from the brain stem, including the laterodorsal tegmental nucleus (LDT) and pedunculopontine tegmental nucleus (PPN). Currently, studies have proven that MS–hippocampal cholinergic neurons produced anti-seizure effects; optogenetic stimulation of PPN cholinergic neurons may be a new way to regulate cortical dysfunction during epileptic seizures through subcortical arousal networks; amygdala cholinergic connections to the BF may contribute to epilepsy.
The hippocampus that receives cholinergic projection from the BF has been a particular focus in the study of TLE. In TLE, ACh regulates the spread of excitatory activity within hippocampal and cortical circuits during the seizure; seizures are initiated in the hippocampus or entorhinal cortex (EC) due to the dysfunction of cholinergic tone. The cholinergic tone modulates ongoing hippocampal activities by enhancing excitatory and depressing inhibitory transmissions, thus increasing excitatory output to the EC to further promote θ generation in the EC–hippocampal network [46]. Recently, the selective activation of MS–hippocampal cholinergic neurons enhanced θ rhythm and suppressed peri-θ frequency bands, creating sharp-wave ripples [47]. We found that MS cholinergic neurons ceased firing during hippocampal seizures. Optogenetic stimulation of the MS–hippocampal cholinergic circuit reduces hippocampal seizures. This anti-seizure effect was mediated by the hippocampal somatostatin neuron, as the chemogenetic inhibition of hippocampal somatostatin-positive (rather than parvalbumin-positive) subtype of GABAergic neurons reversed the antiseizure effect of the MS–hippocampus cholinergic circuit [7]. Collectively, these data suggested that the BF–hippocampal cholinergic circuit has been implicated in the pathophysiology of epilepsy and may be a promising anti-seizure target. However, it is still unclear whether the activation of cholinergic projections to other ictogenic regions of the temporal lobe suppresses seizures. Importantly, we previous showed that the hippocampal subiculum is an important gate for seizure generalization and drug-resistant states [48][49][50][51], but whether cholinergic input within the subiculum is involved in seizure modulation need further study. It is possible that regional differences in the role of ACh could begin to explain the discrepancy between the anticonvulsant effects in our study and the proconvulsant effects reported in other models.
Additionally, the brainstem PPN contains cholinergic neurons and provides the bulk of the cholinergic input to the thalamus, particularly to its relay and reticular nuclei, which is centrally involved in attention or arousal [52]. Prior work demonstrates a decrease in the levels of choline of both the thalamus and cortex for reduced subcortical arousal during partial seizures. Moreover, the hyperpolarization of PPN and BF cholinergic neurons and reduction of excitatory synaptic input and firing are accompanied by a decrease in EPSP-like activity during focal limbic seizures [53][54]; limbic seizures also caused cortical low-frequency oscillations by inhibiting cholinergic arousal systems in the forebrain. All these data support the possible cellular mechanism of decreased subcortical cholinergic arousal in focal seizures by improving cognition. Interestingly, there is an enhancement in cortical γ activity and a depression in δ activity in response to the selective activation of cholinergic brainstem neurons in the PPN during focal hippocampal seizures [55], which implicated that optogenetic stimulation of subcortical arousal networks may be a new means to moderate cortical dysfunction during epileptic seizures.
The prefrontal cortex (PFC) is known to play an essential role in epileptic activity. Intracerebral microinjection of carbachol into the medial PFC of rats induced a high amplitude spiking representative of seizures, which is accompanied by an atypical form of seizures [56][57]. The PFC cholinergic projections can boost the γ rhythms local networks and regulate the early activity within PFC–hippocampal circuits [58]. As previously suggested, limbic thalamic nuclei amplified seizures from the temporal hippocampal formation to the PFC [59][60], whereas it remains unknown how the cholinergic projection of PFC–hippocampal circuits interact with seizure modulation.
The inferior colliculus is the initiation site for acoustically evoked seizures (or audiogenic seizures, AGS). Previously, studies demonstrated that an intracellular microinjection of intermediate doses of nAChRs antagonists decreased the threshold current of seizure initiation, while higher doses of the nAChRs antagonists caused spontaneous seizures [40]. Additionally, a study indicated that microinjections of carbachol into the IC elicited myoclonic seizures, and microinjections of the gallamine into the IC induced AGS susceptibility [61]. Nevertheless, the correlation between cholinergic circuit in the IC and epilepsy is still unclear.
Likewise, the giant cholinergic interneurons of the striatum regulate several aspects of basal ganglia function, which mainly affects motor function. Relative to other brain areas, the striatum contains higher levels of ACh, as well as both mAChRs and nAChRs that mediate its presynaptic and postsynaptic effects [62]. However, the relationship between cholinergic neurons of the striatum and seizure is still largely unknown.
This entry is adapted from the peer-reviewed paper 10.3390/molecules26082258
References
- Thijs, R.D.; Surges, R.; O’Brien, T.J.; Sander, J.W. Epilepsy in adults. Lancet 2019, 393, 689–701.
- Sontheimer, H. Seizure Disorders and Epilepsy. Dis. Nerv. Syst. 2015, 61–95.
- Wang, Y.; Chen, Z. An update for epilepsy research and antiepileptic drug development: Toward precise circuit therapy. Pharmacol. Ther. 2019, 201, 77–93.
- Picciotto, M.R.; Higley, M.J.; Mineur, Y.S. Acetylcholine as a neuromodulator: Cholinergic signaling shapes nervous system function and behavior. Neuron 2012, 76, 116–129.
- Langley, J.N. Observations on the physiological action of extracts of the supra-renal bodies. J. Physiol. 1901, 27, 237–256.
- Curia, G.; Longo, D.; Biagini, G.; Jones, R.S.; Avoli, M. The pilocarpine model of temporal lobe epilepsy. J. Neurosci. Methods 2008, 172, 143–157.
- Wang, Y.; Wang, Y.; Xu, C.; Wang, S.; Tan, N.; Chen, C.; Chen, L.; Wu, X.; Fei, F.; Cheng, H.; et al. Direct Septum-Hippocampus Cholinergic Circuit Attenuates Seizure through Driving Somatostatin Inhibition. Biol. Psychiatry 2020, 87, 843–856.
- Schliebs, R.; Zivin, M.; Steinbach, J.; Rothe, T. Changes in cholinergic but not in GABAergic markers in amygdala, piriform cortex, and nucleus basalis of the rat brain following systemic administration of kainic acid. J. Neurochem. 1989, 53, 212–218.
- Müller-Gärtner, H.W.; Mayberg, H.S.; Fisher, R.S.; Lesser, R.P.; Wilson, A.A.; Ravert, H.T.; Dannals, R.F.; Wagner, H.N., Jr.; Uematsu, S.; Frost, J.J. Decreased hippocampal muscarinic cholinergic receptor binding measured by 123I-iododexetimide and single-photon emission computed tomography in epilepsy. Ann. Neurol. 1993, 34, 235–238.
- Yi, F.; DeCan, E.; Stoll, K.; Marceau, E.; Deisseroth, K.; Lawrence, J.J. Muscarinic excitation of parvalbumin-positive interneurons contributes to the severity of pilocarpine-induced seizures. Epilepsia 2015, 56, 297–309.
- Bymaster, F.P.; Carter, P.A.; Yamada, M.; Gomeza, J.; Wess, J.; Hamilton, S.E.; Nathanson, N.M.; McKinzie, D.L.; Felder, C.C. Role of specific muscarinic receptor subtypes in cholinergic parasympathomimetic responses, in vivo phosphoinositide hydrolysis, and pilocarpine-induced seizure activity. Eur. J. Neurosci. 2003, 17, 1403–1410.
- Hamilton, S.E.; Loose, M.D.; Qi, M.; Levey, A.I.; Hille, B.; McKnight, G.S.; Idzerda, R.L.; Nathanson, N.M. Disruption of the m1 receptor gene ablates muscarinic receptor-dependent M current regulation and seizure activity in mice. Proc. Natl. Acad. Sci. USA 1997, 94, 13311–13316.
- Fisahn, A.; Yamada, M.; Duttaroy, A.; Gan, J.W.; Deng, C.X.; McBain, C.J.; Wess, J. Muscarinic induction of hippocampal gamma oscillations requires coupling of the M1 receptor to two mixed cation currents. Neuron 2002, 33, 615–624.
- Sheffler, D.J.; Williams, R.; Bridges, T.M.; Xiang, Z.; Kane, A.S.; Byun, N.E.; Jadhav, S.; Mock, M.M.; Zheng, F.; Lewis, L.M.; et al. A novel selective muscarinic acetylcholine receptor subtype 1 antagonist reduces seizures without impairing hippocampus-dependent learning. Mol. Pharmacol. 2009, 76, 356–368.
- Miller, S.L.; Aroniadou-Anderjaska, V.; Pidoplichko, V.I.; Figueiredo, T.H.; Apland, J.P.; Krishnan, J.K.; Braga, M.F. The M1 Muscarinic Receptor Antagonist VU0255035 Delays the Development of Status Epilepticus after Organophosphate Exposure and Prevents Hyperexcitability in the Basolateral Amygdala. J. Pharmacol. Exp. Ther. 2017, 360, 23–32.
- Palomero-Gallagher, N.; Schleicher, A.; Bidmon, H.-J.; Pannek, H.-W.; Hans, V.; Gorji, A.; Speckmann, E.-J.; Zilles, K. Multireceptor analysis in human neocortex reveals complex alterations of receptor ligand binding in focal epilepsies. Epilepsia 2012, 53, 1987–1997.
- Akyuz, E.; Doganyigit, Z.; Paudel, Y.N.; Kaymak, E.; Yilmaz, S.; Uner, A.; Shaikh, M.F. Increased ACh-Associated Immunoreactivity in Autonomic Centers in PTZ Kindling Model of Epilepsy. Biomedicines 2020, 8, 113.
- Graebenitz, S.; Kedo, O.; Speckmann, E.J.; Gorji, A.; Panneck, H.; Hans, V.; Palomero-Gallagher, N.; Schleicher, A.; Zilles, K.; Pape, H.C. Interictal-like network activity and receptor expression in the epileptic human lateral amygdala. Brain 2011, 134, 2929–2947.
- Villalpando-Vargas, F.; Medina-Ceja, L.; Santerre, A.; Enciso-Madero, E.A. The anticonvulsant effect of sparteine on pentylenetetrazole-induced seizures in rats: A behavioral, electroencephalographic, morphological and molecular study. J. Mol. Histol. 2020, 51, 503–518.
- Smolders, I.; Khan, G.M.; Manil, J.; Ebinger, G.; Michotte, Y. NMDA receptor-mediated pilocarpine-induced seizures characterization in freely moving rats by microdialysis. Br. J. Pharmacol. 1997, 121, 1171–1179.
- Turski, W.A.; Cavalheiro, E.A.; Turski, L.; Kleinrok, Z. Intrahippocampal bethanechol in rats behavioural, electroencephalographic and neuropathological correlates. Behav. Brain Res. 1983, 7, 361–370.
- Ben-Ari, Y.; Tremblay, E.; Ottersen, O.P. Injections of kainic acid into the amygdaloid complex of the rat an electrographic, clinical and histological study in relation to the pathology of epilepsy. Neuroscience 1980, 5, 515–528.
- Krnjević, K.; Pumain, R.; Renaud, L. The mechanism of excitation by acetylcholine in the cerebral cortex. J. Physiol. 1971, 215, 247–268.
- Peterson, S.L.; Armstrong, J.J. Muscarinic receptors mediate carbachol-induced inhibition of maximal electroshock seizures in the nucleus reticularis pontis oralis. Epilepsia 1999, 40, 20–25.
- Peterson, S.L.; Armstrong, J.J.; Walker, M.K. Focal microinjection of carbachol into the periaqueductal gray induces seizures in the forebrain of the rat. Epilepsy Res. 2000, 42, 169–181.
- Damaj, M.I.; Glassco, W.; Dukat, M.; Martin, B.R. Pharmacological characterization of nicotine-induced seizures in mice. J. Pharmacol. Exp. Ther. 1999, 291, 1284–1291.
- Iha, H.A.; Kunisawa, N.; Shimizu, S.; Tokudome, K.; Mukai, T.; Kinboshi, M.; Ikeda, A.; Ito, H.; Serikawa, T.; Ohno, Y. Nicotine Elicits Convulsive Seizures by Activating Amygdalar Neurons. Front. Pharmacol. 2017, 8.
- Sharma, N.K.; Kaur, S.; Goel, R.K. Exploring the ameliorative role of α7 neuronal nicotinic acetylcholine receptor modulation in epilepsy and associated comorbidities in post-PTZ-kindled mice. Epilepsy Behav. 2020, 103.
- Löscher, W.; Potschka, H.; Wlaź, P.; Danysz, W.; Parsons, C.G. Are neuronal nicotinic receptors a target for antiepileptic drug development? Studies in different seizure models in mice and rats. Eur. J. Pharmacol. 2003, 466, 99–111.
- Banerjee, J.; Dey, S.; Dixit, A.B.; Tripathi, M.; Doddamani, R.; Sharma, M.C.; Chandra, P.S. α7 nicotinic receptors contributes to glutamatergic activity in the hippocampus of patients with mesial temporal lobe epilepsy with hippocampal sclerosis (MTLE-HS). J. Neural Transm. 2020, 127, 1441–1446.
- De Fusco, M.; Becchetti, A.; Patrignani, A.; Annesi, G.; Gambardella, A.; Quattrone, A.; Ballabio, A.; Wanke, E.; Casari, G. The nicotinic receptor beta 2 subunit is mutant in nocturnal frontal lobe epilepsy. Nat. Genet. 2000, 26, 275–276.
- Becchetti, A.; Aracri, P.; Meneghini, S.; Brusco, S.; Amadeo, A. The role of nicotinic acetylcholine receptors in autosomal dominant nocturnal frontal lobe epilepsy. Front. Physiol. 2015, 6, 22.
- Picard, F.; Bertrand, S.; Steinlein, O.K.; Bertrand, D. Mutated nicotinic receptors responsible for autosomal dominant nocturnal frontal lobe epilepsy are more sensitive to carbamazepine. Epilepsia 1999, 40, 1198–1209.
- Picard, F.; Bruel, D.; Servent, D.; Saba, W.; Fruchart-Gaillard, C.; Schollhorn-Peyronneau, M.A.; Roumenov, D.; Brodtkorb, E.; Zuberi, S.; Gambardella, A.; et al. Alteration of the in vivo nicotinic receptor density in ADNFLE patients: A PET study. Brain 2006, 129, 2047–2060.
- Cadieux-Dion, M.; Meneghini, S.; Villa, C.; Toffa, D.H.; Wickstrom, R.; Bouthillier, A.; Sandvik, U.; Gustavsson, B.; Mohamed, I.; Cossette, P.; et al. Variants in CHRNB2 and CHRNA4 Identified in Patients with Insular Epilepsy. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 2020, 47, 800–809.
- Jiang, Y.L.; Yuan, F.; Yang, Y.; Sun, X.L.; Song, L.; Jiang, W. CHRNA4 variant causes paroxysmal kinesigenic dyskinesia and genetic epilepsy with febrile seizures plus? Seizure 2018, 56, 88–91.
- Wang, N.; Huang, H.L.; Zhou, H. Study of candidate gene cHRNA4 for familial epilepsy syndrome. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1765–1769.
- Jian, L.; Nagarajan, L.; de Klerk, N.; Ravine, D.; Bower, C.; Anderson, A.; Williamson, S.; Christodoulou, J.; Leonard, H. Predictors of seizure onset in Rett syndrome. J. Pediatr. 2006, 149, 542–547.e543.
- Zhang, Y.; Cao, S.X.; Sun, P.; He, H.Y.; Yang, C.H.; Chen, X.J.; Shen, C.J.; Wang, X.D.; Chen, Z.; Berg, D.K.; et al. Loss of MeCP2 in cholinergic neurons causes part of RTT-like phenotypes via alpha7 receptor in hippocampus. Cell Res. 2016, 26, 728–742.
- McCown, T.J.; Breese, G.R. Effects of apamin and nicotinic acetylcholine receptor antagonists on inferior collicular seizures. Eur. J. Pharmacol. 1990, 187, 49–58.
- Jones, B.E. Activity, modulation and role of basal forebrain cholinergic neurons innervating the cerebral cortex. Prog. Brain Res. 2004, 145, 157–169.
- Rosal Lustosa, I.; Soares, J.I.; Biagini, G.; Lukoyanov, N.V. Neuroplasticity in Cholinergic Projections from the Basal Forebrain to the Basolateral Nucleus of the Amygdala in the Kainic Acid Model of Temporal Lobe Epilepsy. Int. J. Mol. Sci. 2019, 20, 5688.
- Unal, C.T.; Pare, D.; Zaborszky, L. Impact of basal forebrain cholinergic inputs on basolateral amygdala neurons. J. Neurosci. 2015, 35, 853–863.
- Girgis, M. Participation of muscarinic cholinergic receptors may be an important requirement of the kindling process. Exp. Neurol. 1980, 70, 458–461.
- Ferencz, I.; Leanza, G.; Nanobashvili, A.; Kokaia, M.; Lindvall, O. Basal forebrain neurons suppress amygdala kindling via cortical but not hippocampal cholinergic projections in rats. Eur. J. Neurosci. 2000, 12, 2107–2116.
- Friedman, A.; Behrens, C.J.; Heinemann, U. Cholinergic dysfunction in temporal lobe epilepsy. Epilepsia 2007, 48 (Suppl. 5), 126–130.
- Vandecasteele, M.; Varga, V.; Berenyi, A.; Papp, E.; Bartho, P.; Venance, L.; Freund, T.F.; Buzsaki, G. Optogenetic activation of septal cholinergic neurons suppresses sharp wave ripples and enhances theta oscillations in the hippocampus. Proc. Natl. Acad. Sci. USA 2014, 111, 13535–13540.
- Wang, Y.; Xu, C.; Xu, Z.; Ji, C.; Liang, J.; Wang, Y.; Chen, B.; Wu, X.; Gao, F.; Wang, S.; et al. Depolarized GABAergic Signaling in Subicular Microcircuits Mediates Generalized Seizure in Temporal Lobe Epilepsy. Neuron 2017, 95, 92–105.e105.
- Xu, C.; Wang, Y.; Zhang, S.; Nao, J.; Liu, Y.; Wang, Y.; Ding, F.; Zhong, K.; Chen, L.; Ying, X.; et al. Subicular pyramidal neurons gate drug resistance in temporal lobe epilepsy. Ann. Neurol. 2019, 86, 626–640.
- Ruan, Y.; Xu, C.; Lan, J.; Nao, J.; Zhang, S.; Fan, F.; Wang, Y.; Chen, Z. Low-frequency Stimulation at the Subiculum is Anti-convulsant and Anti-drug-resistant in a Mouse Model of Lamotrigine-resistant Temporal Lobe Epilepsy. Neurosci. Bull. 2020, 36, 654–658.
- Fei, F.; Wang, X.; Wang, Y.; Chen, Z. Dissecting the role of subiculum in epilepsy: Research update and translational potential. Prog. Neurobiol. 2021, 102029.
- Dautan, D.; Huerta-Ocampo, I.; Witten, I.B.; Deisseroth, K.; Bolam, J.P.; Gerdjikov, T.; Mena-Segovia, J. A major external source of cholinergic innervation of the striatum and nucleus accumbens originates in the brainstem. J. Neurosci. 2014, 34, 4509–4518.
- Andrews, J.P.; Yue, Z.; Ryu, J.H.; Neske, G.; McCormick, D.A.; Blumenfeld, H. Mechanisms of decreased cholinergic arousal in focal seizures: In vivo whole-cell recordings from the pedunculopontine tegmental nucleus. Exp. Neurol. 2019, 314, 74–81.
- Motelow, J.E.; Li, W.; Zhan, Q.; Mishra, A.M.; Sachdev, R.N.; Liu, G.; Gummadavelli, A.; Zayyad, Z.; Lee, H.S.; Chu, V.; et al. Decreased subcortical cholinergic arousal in focal seizures. Neuron 2015, 85, 561–572.
- Furman, M.; Zhan, Q.; McCafferty, C.; Lerner, B.A.; Motelow, J.E.; Meng, J.; Ma, C.; Buchanan, G.F.; Witten, I.B.; Deisseroth, K.; et al. Optogenetic stimulation of cholinergic brainstem neurons during focal limbic seizures: Effects on cortical physiology. Epilepsia 2015, 56, e198–e202.
- Crawley, J.N.; Stivers, J.A.; Martin, J.V.; Mendelson, W.B. Cholinergic induction of seizures in the rat prefrontal cortex. Life Sci. 1986, 38, 2347–2354.
- Stivers, J.A.; Skirboll, L.R.; Long, R.; Crawley, J.N. Anatomical analysis of frontal cortex sites at which carbachol induces motor seizures in the rat. Pharmacol. Biochem. Behav. 1988, 30, 129–136.
- Janiesch, P.C.; Kruger, H.S.; Poschel, B.; Hanganu-Opatz, I.L. Cholinergic control in developing prefrontal-hippocampal networks. J. Neurosci. 2011, 31, 17955–17970.
- Sloan, D.M.; Bertram, E.H., 3rd. Changes in midline thalamic recruiting responses in the prefrontal cortex of the rat during the development of chronic limbic seizures. Epilepsia 2009, 50, 556–565.
- Sloan, D.M.; Zhang, D.; Bertram, E.H., 3rd. Increased GABAergic inhibition in the midline thalamus affects signaling and seizure spread in the hippocampus-prefrontal cortex pathway. Epilepsia 2011, 52, 523–530.
- Bagri, A.; Di Scala, G.; Sandner, G. Myoclonic and tonic seizures elicited by microinjection of cholinergic drugs into the inferior colliculus. Therapie 1999, 54, 589–594.
- Lim, S.A.; Kang, U.J.; McGehee, D.S. Striatal cholinergic interneuron regulation and circuit effects. Front. Synaptic. Neurosci. 2014, 6, 22.
