Piper betle (L) is a popular medicinal plant in Asia. Plant leaves have been used as a traditional medicine to treat various health conditions. It is highly abundant and inexpensive, therefore promoting further research and industrialization development, including in the food and pharmaceutical industries.
- antibacterial
- antifungal
- betel leaves
- Piper betle
1. Introduction
Piper betle (L) commonly known as betel vine belongs to the family Piperaceae. It is a popular medicinal plant in Asia. The leaf is the most widely used and studied part of the betel vine. There are chewing habit practices of betel leaves in many countries which are believed beneficial for avoiding bad breath, strengthening the gum, preserving the teeth, and stimulating the digestive system [1][2]. In traditional medicine practices, betel leaves are used for vaginal douching in Indonesia [3], as a gargle mouthwash in India and Thailand [4], and as a treatment for dental problems, headaches, arthritis, and joint pain in Malaysia [1]. In Srilanka, the betel leaf juice is used to treat skin ailments [5]. Additionally, its boiled leaves could be used as cough medicine, tonic, or astringent [2]. Traditional applications of betel leaves are related to their antibacterial and antifungal properties.
Over the past decades, antibacterial resistance has been threatening humans and has caused a global health crisis. Some bacterial strains are resistant to antibiotics such as vancomycin intermediate Staphylococcus aureus (VISA), vancomycin-resistant Enterococcus (VRE), methicillin-resistant S. aureus (MRSA), and extended spectrum β-lactamase (ESβL) enzyme producing Gram-negative bacteria, Pseudomonas aeruginosa, Streptococcus pneumoniae, S. aureus, and Mycobacterium tuberculosis, Enterococcus faecium, Klebsiella pneumoniae, Acinetobacter baumannii, and Enterobacter spp. [6][7]. Besides bacteria, fungi can also lead to infectious diseases. Approximately 300 fungal species on Earth are known to cause illnesses such as Candida spp. and dermatophytes [8][9]. Moreover, in the food industry, bacteria and fungi cause problems during product processing and storage. Food spoilage due to pathogen contamination is not only harmful to consumers but also brings heavy economic losses to manufacturers [10]. Therefore, research in this area continues to develop new safe and effective antimicrobial agents that could be applied in many related fields.
2. Phytochemicals in Betel Leaves
2.1. Betel Leaves Extract (BLE)
Piper betle contains numerous phytochemicals depending on its botanical origin and the solvent used for extraction. A preliminary phytochemical analysis of betel leaves from Malaysia showed that alkaloids, tannins, glycosides, reducing sugars, and saponins were found in the water extract of betel leaves [11]. Moreover, a study determined the total content of phenol, flavonoid, and tannin in water, ethanol, ethyl acetate, acetone, and dichloromethane extracts of betel leaves from Mauritius [12]. The highest total phenol, flavonoid, and tannin were found in the acetone, dichloromethane, and ethanol extracts, respectively. The sample of betel leaves collected from Tamilnadu, India is known to contain steroids, tannins, proteins, amino acids, flavonoids, terpenoids, mucilage, volatile oil, saponin, carbohydrates, and fixed oil, but an absence of alkaloids [13]. Furthermore, some studies have effectively isolated bioactive compounds from BLE (Figure 1) such as phytol, acyclic diterpene alcohol, 4-chromanol, hydroxychavicol or 4-allylpyrocatechol, and allylpyrocatechols 1 [14][15][16][17].
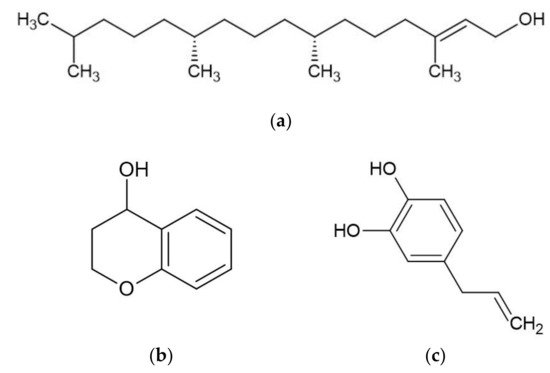
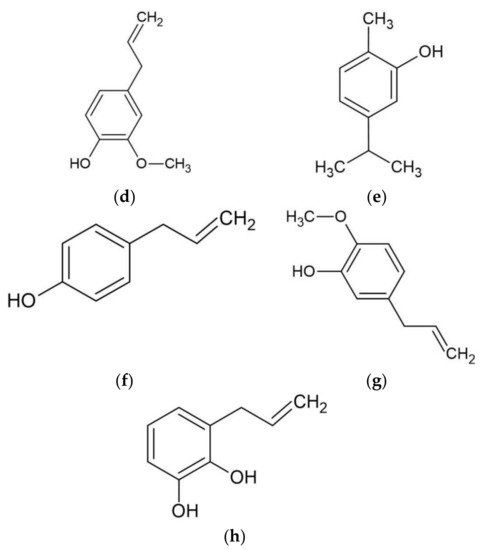
Figure 1. Major bioactive compounds in betel leaves extracts and essential oil. (a) phytol; (b) 4-chromanol; (c) hydroxychavicol; (d) eugenol; (e) carvacrol; (f) chavicol; (g) chavibetol; (h) allylpyrocatechols 1.
2.2. Betel Leaves Essential Oil (BLEO)
Betel leaves contain 0.15% to 0.2% essential oil which are classified as monoterpenes, sesquiterpenes, phenylpropanoids, and aldehydes (Table 1). The constituents of BLEO are strongly dependent on its botanical origin, age of the plant, and harvesting time. Various compounds of BLEO may affect its aroma, taste, and bioactivity [18]. GC-MS analysis of BLEO from different places in India showed that phenylpropanoid groups such as acetyl eugenol, eugenol, chavicol, and safrole were the major components [19]. Interestingly, Indian BLEO obtained from the Sagar Bangla cultivar contained chavicol, but not from the Magahi cultivar. The study also revealed that BLEO contained eugenol (40%) and a combination of carvacrol and chavicol (up to 40%) with chavibetol as a marker compound as depicted in Figure 1. Meanwhile, another study found additional main compounds including estragole, linalool, α-copaene, anethole, and caryophyllene α-terpinene, p-cymene, 1,8-cineole, β-caryophyllene, α-humulene, allyl pyrocatechol, allylcatechol, methyl eugenol, estragol (methyl chavicol), chavibetol, chavibetol acetate, safrol, 4-allyl-2-methoxy-phenolacetate, and 3-allyl-6-methoxyphenol [18][20][21].
3. Antibacterial Property of Betel Leaves
The extract, essential oil, preparation, and isolated compounds of betel leaves are effective against numerous Gram-negative and Gram-positive bacteria . The bacteria tested included foodborne pathogens and other bacteria, including multidrug-resistant (MDR) bacteria that cause severe infectious diseases in humans. Most of the published research investigated the antibacterial activity of BLEs resulting from solvents with different polarities such as water, ethanol, ethyl acetate, acetone, and dichloromethane. Each extract contained diverse bioactive compounds which may affect their antibacterial activity [12][22]. The antibacterial tests of betel leaves were varied in methods and results, complicating the comparison between studies. Furthermore, the current review showed that the study of antibacterial activity of BLE was greater than that of BLEO.
4. Antifungal Properties of Betel Leaves
Numerous methods have been applied to test the antifungal properties of betel leaves including solid dilution, broth dilution, micro-dilution, well diffusion, and solid diffusion assays, resulting in minimum inhibitory concentration (MIC), minimum fungicidal concentration (MFC), and inhibition zones (Table 4). Similar to antibacterial activity, recalculation of MIC and MFC, and measurement of MFC/MIC ratio to determine fungicidal and fungistatic effects, were also conducted. Candica albicans was the most screened fungal species with MIC ranging from 0.01% to 0.07% [2][23][24][25][26][27] The fungicidal effects of BLE and BLEO against various fungal species including Aspergillus flavus, Aspergillus fumigatus, Aspergillus niger, Aspergillus parasiticus, C. albicans, Candida glabrata, Candida krusei, Candida neoformans, Candida parapsilosis, Candida tropicalis, Epidermophyton floccosum, Trichophyton mentagrophytes, Trichophyton rubrum, Microsporum canis, and Microsporum gypseum [23][24][26][28]. Meanwhile, the fungistatic effect was only recorded from hexane and ethyl acetate extract of betel leaves against C. albicans [24], and its isolate, hyroxychavicol, against C. krusei [26]. A few of these species can contaminate food and spread aflatoxin, which is harmful to humans [18][29]. Other fungal species are clinically significant human pathogens that cause dental disorders and dermatophyte infections [2][14][25][28].
5. Safety Profiles of Betel Leaves
An acute toxicity study in both male and female ICR mice showed the safety of the methanol extract of betel leaves orally. The median lethal dose (LD50) of the extract was higher than 5000 mg/kg body weight [30]. There was also an evaluation of oral acute and sub-acute toxicity (28 days) and genotoxicity of an herbal formulation containing betel leaves alcoholic extract in rats and cellular models. This study revealed the absence of major adverse reactions [31]. Moreover, betel leaves were considered safe in terms of hematotoxicity, hepatotoxicity, genotoxicity, weights of organs, gross morphology, stress, or aversive behaviors in rats [32]. Another study discovered the nontoxicity of the ethanol extract of betel leaves on normal human dermal fibroblasts (HDFn) [33].
6. Commercial Application of Betel Leaves
There are some available commercial products containing betel leaves such as dietary supplements, mouthwash, medicinal products, and cosmetic and personal care goods including shampoo, soap, face cream, antiseptic lotions, toothpaste, and perfumes [34]. Current antimicrobial studies of betel leaves were focusing on oral pathogens, MDR Gram-negative and Gram-positive bacteria, and dermatophytes [17][33][24][26]. Thus, future development of medicinal products from betel leaves could be useful for preventing oral diseases, curing dermatophyte infections, and for the treatment and management of other infectious diseases. Additionally, a study has developed a simple, safe, cost-effective, and eco-friendly preparation of silver nanoparticles with polyaniline coating using water extract of betel leaves. The nanoparticles showed potential antibacterial properties and could be further studied in various applications such as medical devices and pharmaceutical and biomedical industries [35].
In the food industry, essential oil is a promising food additive to protect and enhance the shelf life of products during processing and storage. BLEO is an ideal food preservative agent due to its antifungal and antioxidant properties [18]. Many experiments have investigated the antimicrobial properties of BLEO against foodborne pathogens [18][36][29]. Moreover, BLEO is not only beneficial to prevent spoilage of food products but also guarantees their safety for consumer health especially due to the ability of BLEO to suppress aflatoxin production. Aflatoxin, a mycotoxin from A. flavus, is an example of fungal contamination in food products. The toxin is known to be hepatocarcinogenic, teratogenic, mutagenic, and immunosuppressive. An investigation revealed that BLEO in apple juice could deactivate spores or inhibit spore germination which is required to limit fungal infection and mycotoxin production [29]. Further research on the overall acceptability of sensory aspects of the essential oil-treated foodstuffs is necessary to avoid market failure of the product [37].
This entry is adapted from the peer-reviewed paper 10.3390/molecules26082321
References
- Fazal, F.; Mane, P.P.; Rai, M.P.; Thilakchand, K.R.; Bhat, H.P.; Kamble, P.S.; Palatty, P.L.; Baliga, M.S. The Phytochemistry, Traditional Uses and Pharmacology of Piper Betel. Linn (Betel Leaf): A Pan-Asiatic Medicinal Plant. Chin. J. Integr. Med. 2014.
- Kaypetch, R.; Thaweboon, S. Antifungal Property of Piper Betle Leaf Oil against Oral Candida Species. Matec. Web Conf. 2018, 242, 01021.
- Joesoef, M.R.; Sumampouw, H.; Linnan, M.; Schmid, S.; Idajadi, A.; St Louis, M.E. Douching and Sexually Transmitted Diseases in Pregnant Women in Surabaya, Indonesia. Am. J. Obs. Gynecol. 1996, 174, 115–119.
- Chowdhury, U.; Baruah, P.K. Betelvine (Piper Betle L.): A Potential Source for Oral Care. Curr. Bot. 2020, 87–92.
- Arambewela, L.; Arawwawala, M.; Withanage, D.; Kulatunga, S. Efficacy of Betel Cream on Skin Ailments. J. Complementary Integr. Med. 2010, 7.
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340.
- Hafidh, R.R.; Abdulamir, A.S.; Vern, L.S.; Abu Bakar, F.; Abas, F.; Jahanshiri, F.; Sekawi, Z. Inhibition of Growth of Highly Resistant Bacterial and Fungal Pathogens by a Natural Product. Open Microbiol. J. 2011, 5, 96–106.
- Akpan, A.; Morgan, R. Oral Candidiasis. Postgrad. Med. J. 2002, 78, 455–459.
- Benedict, K.; Chiller, T.M.; Mody, R.K. Invasive Fungal Infections Acquired from Contaminated Food or Nutritional Supplements: A Review of the Literature. Foodborne Pathog. Dis. 2016, 13, 343–349.
- Pawar, S.; Kalyankar, V.; Dhamangaonkar, B.; Dagade, S.; Waghmode, S.; Cukkemane, A. Biochemical Profiling of Antifungal Activity of Betel Leaf (Piper Betle L.) Extract and Its Significance in Traditional Medicine. J. Adv. Res. Biotechnol. 2017, 2, 1–4.
- Kaveti, B.; Tan, L.; Sarnnia; Kuan, T.S.; Baig, M. Antibacterial Activity Of Piper Betel Leaves. Int. J. Pharm. Teach. Pract. 2011, 2, 129–132.
- Taukoorah, U.; Lall, N.; Mahomoodally, F. Piper Betle L. (Betel Quid) Shows Bacteriostatic, Additive, and Synergistic Antimicrobial Action When Combined with Conventional Antibiotics. S. Afr. J. Bot. 2016, 105, 133–140.
- Periyanayagam, K.; Jagadeesan, M.; Kavimani, S.; Vetriselvan, T. Pharmacognostical and Phyto-Physicochemical Profile of the Leaves of Piper Betle L. Var Pachaikodi (Piperaceae)—Valuable Assessment of Its Quality—ScienceDirect. Available online: (accessed on 22 February 2021).
- Ali, A.; Lim, X.Y.; Wahida, P.F. The Fundamental Study of Antimicrobial Activity of Piper Betle Extract in Commercial Toothpastes. J. Herb. Med. 2018, 14, 29–34.
- Kurnia, D.; Hutabarat, G.S.; Windaryanti, D.; Herlina, T.; Herdiyati, Y.; Satari, M.H. Potential Allylpyrocatechol Derivatives as Antibacterial Agent Against Oral Pathogen of S. Sanguinis ATCC 10,556 and as Inhibitor of MurA Enzymes: In Vitro and in Silico Study. Drug Des. Devel. 2020, 14, 2977–2985.
- Srinivasan, R.; Devi, K.R.; Kannappan, A.; Pandian, S.K.; Ravi, A.V. Piper Betle and Its Bioactive Metabolite Phytol Mitigates Quorum Sensing Mediated Virulence Factors and Biofilm of Nosocomial Pathogen Serratia Marcescens in Vitro. J. Ethnopharmacol. 2016, 193, 592–603.
- Teanpaisan, R.; Kawsud, P.; Pahumunto, N.; Puripattanavong, J. Screening for Antibacterial and Antibiofilm Activity in Thai Medicinal Plant Extracts against Oral Microorganisms. J. Tradit. Complementary Med. 2017, 7, 172–177.
- Prakash, B.; Shukla, R.; Singh, P.; Kumar, A.; Mishra, P.K.; Dubey, N.K. Efficacy of Chemically Characterized Piper betle L. Essential Oil against Fungal and Aflatoxin Contamination of Some Edible Commodities and Its Antioxidant Activity. Int. J. Food Microbiol. 2010, 142, 114–119.
- Karak, S.; Acharya, J.; Begum, S.; Mazumdar, I.; Kundu, R.; De, B. Essential Oil of Piper Betle L. Leaves: Chemical Composition, Anti-Acetylcholinesterase, Anti-β-Glucuronidase and Cytotoxic Properties. J. Appl. Res. Med. Aromat. Plants 2018, 10, 85–92.
- Salehi, B.; Zakaria, Z.A.; Gyawali, R.; Ibrahim, S.A.; Rajkovic, J.; Shinwari, Z.K.; Khan, T.; Sharifi-Rad, J.; Ozleyen, A.; Turkdonmez, E.; et al. Piper Species: A Comprehensive Review on Their Phytochemistry, Biological Activities and Applications. Molecules 2019, 24, 1364.
- Madhumita, M.; Guha, P.; Nag, A. Extraction of Betel Leaves (Piper Betle L.) Essential Oil and Its Bio-Actives Identification: Process Optimization, GC-MS Analysis and Anti-Microbial Activity. Ind. Crop. Prod. 2019, 138, 111578.
- Tan, Y.P.; Chan, E.W.C. Antioxidant, Antityrosinase and Antibacterial Properties of Fresh and Processed Leaves of Anacardium Occidentale and Piper Betle. Food Biosci. 2014, 6, 17–23.
- Aumeeruddy-Elalfi, Z.; Gurib-Fakim, A.; Mahomoodally, F. Antimicrobial, Antibiotic Potentiating Activity and Phytochemical Profile of Essential Oils from Exotic and Endemic Medicinal Plants of Mauritius. Ind. Crop. Prod. 2015, 71, 197–204.
- Phumat, P.; Khongkhunthian, S.; Wanachantararak, P.; Okonogi, S. Potential of Piper Betle Extracts on Inhibition of Oral Pathogens. Drug Discov. 2017, 11, 307–315.
- Phumat, P.; Khongkhunthian, S.; Wanachantararak, P.; Okonogi, S. Comparative Inhibitory Effects of 4-Allylpyrocatechol Isolated from Piper Betle on Streptococcus Intermedius, Streptococcus Mutans, and Candida Albicans. Arch. Oral Biol. 2020, 113, 104690.
- Ali, I.; Khan, F.G.; Suri, K.A.; Gupta, B.D.; Satti, N.K.; Dutt, P.; Afrin, F.; Qazi, G.N.; Khan, I.A. In Vitro Antifungal Activity of Hydroxychavicol Isolated from Piper Betle L. Ann. Clin. Microbiol. Antimicrob. 2010, 9, 7.
- Sivareddy, B.; Reginald, B.A.; Sireesha, D.; Samatha, M.; Reddy, K.H.; Subrahamanyam, G. Antifungal Activity of Solvent Extracts of Piper Betle and Ocimum Sanctum Linn on Candida Albicans: An in Vitro Comparative Study. J. Oral Maxillofac. Pathol. 2019, 23, 333–337.
- Aiemsaard, J.; Punareewattana, K. Antifungal Activities of Essential Oils of Syzygium Aromaticum, Piper Betle, and Ocimum Sanctum against Clinical Isolates of Canine Dermatophytes. Sci. Asia 2017, 43, 223.
- Basak, S.; Guha, P. Use of Predictive Model to Describe Sporicidal and Cell Viability Efficacy of Betel Leaf (Piper Betle L.) Essential Oil on Aspergillus Flavus and Penicillium Expansum and Its Antifungal Activity in Raw Apple Juice. LWT 2017, 80, 510–516.
- Al-Adhroey, A.H.; Nor, Z.M.; Al-Mekhlafi, H.M.; Amran, A.A.; Mahmud, R. Antimalarial Activity of Methanolic Leaf Extract of Piper Betle L. Molecules 2010, 16, 107–118.
- Sengupta, K.; Mishra, A.T.; Rao, M.K.; Sarma, K.V.; Krishnaraju, A.V.; Trimurtulu, G. Efficacy of an Herbal Formulation LI10903F Containing Dolichos Biflorus and Piper Betle Extracts on Weight Management. Lipids Health Dis. 2012, 11, 176.
- Arambewela, L.S.R.; Arawwawala, L.D.A.M.; Kumaratunga, K.G.; Dissanayake, D.S.; Ratnasooriya, W.D.; Kumarasingha, S.P. Investigations on Piper Betle Grown in Sri Lanka. Pharm. Rev. 2011, 5, 159–163.
- Valle, D.L.; Cabrera, E.C.; Puzon, J.J.M.; Rivera, W.L. Antimicrobial Activities of Methanol, Ethanol and Supercritical CO2 Extracts of Philippine Piper Betle L. on Clinical Isolates of Gram Positive and Gram Negative Bacteria with Transferable Multiple Drug Resistance. PLoS ONE 2016, 11, e0146349.
- Madhumita, M.; Guha, P.; Nag, A. Bio-Actives of Betel Leaf (Piper Betle L.): A Comprehensive Review on Extraction, Isolation, Characterization, and Biological Activity. Phytother. Res. 2020, 34, 2609–2627.
- Rashida, M.; Islam, I.; Haque, A.; Rahman, A.; Hossain, T.; Hamid, A. Antibacterial Activity of Polyaniline Coated Silver Nanoparticles Synthesized from Piper Betle Leaves Extract. Iran. J. Pharm. Res. 2016, 15, 591–597.
- Roy, A.; Guha, P. Formulation and Characterization of Betel Leaf (Piper Betle L.) Essential Oil Based Nanoemulsion and Its in Vitro Antibacterial Efficacy against Selected Food Pathogens. J. Food Process. Preserv. 2018, 42, e13617.
- Basak, S. The Use of Fuzzy Logic to Determine the Concentration of Betel Leaf Essential Oil and Its Potency as a Juice Preservative. Food Chem. 2018, 240, 1113–1120.
