Bioabsorbable materials made from polymeric compounds have been used in many fields of regenerative medicine to promote tissue regeneration. These materials replace autologous tissue and, due to their growth potential, make excellent substitutes for cardiovascular applications in the treatment of congenital heart disease.
- tissue engineering
- large animal model
- pulmonary valve
- aortic patch
- pulmonary vein
1. Introduction
Congenital heart disease (CHD) accounts for approximately one-third of all congenital anomalies worldwide affecting approximately 40,000 newborns in the United States each year [1,2]. Despite advances in management, CHD remains a leading cause of death in newborns and can lead to lifelong morbidity in survivors [1]. A significant portion of cardiac surgery morbidity and mortality is attributed to the synthetic conduits and patches frequently used for cardiac repair. Made from materials such as polytetrafluoroethylene (PTFE or Gore-Tex), these artificial materials are susceptible to thrombogenicity, stenosis, iatrogenic calcification, immune rejection, and infection [3,4]. Grafts constructed from these materials also lack growth potential, contributing to one of the greatest causes of morbidity in pediatric patients—somatic overgrowth, or the process by which patients outgrow their grafts. Alternative material choices have largely revolved around allografts, xenografts, and autologous tissues such as pericardium or saphenous vein; however, each of these have varying degrees of complications and still do not address the need for growth potential [5].
Regenerative medicine provides a potential solution to the use of these various materials, allowing for the added benefit of avoiding issues such as immune rejection, somatic overgrowth, infection, and calcification [6,7]. This is made possible through the strategy of implanting a bioabsorbable scaffold that degrades over time which is replaced with autologous vascular tissue that can repair, reform, and even grow with the patient [8]. Many groups have sought to create different surgical materials [9,10], but have been met with numerous obstacles that must be addressed in order for these materials to be acceptable for clinical practice. For this reason, very few cardiovascular materials utilizing regenerative medicine principles have made it to the clinical trial stage [11]. It is our sense that there is an unintended disconnect between the materials being studied by materials science experts/engineers and those found most useful by surgeons implanting them.
2. Tissue-Engineered Pulmonary Valve
The pulmonary valve (PV) is the most commonly affected valve in CHD. Separating the right ventricle (RV) and pulmonary trunk, the PV is a tri-leaflet structure leading to the lower pressure pulmonary system. It serves to facilitate forward blood flow during systole and prevent retrograde blood flow during diastole. The regulation of flow is a function of its geometric shape, cellular composition, and extracellular matrix (ECM). Geometrically, leaflet alignment via the commissures and annulus determines appropriate coaptation of the PV and thus prevention of regurgitation. Valve leaflets are complex structures composed of various cells types that create and maintain an intricate ECM based on environmental stimuli [14]. As such, conditions (congenital or iatrogenic) affecting these valvular components place the patient at increased risk of significant morbidity and mortality, often times necessitating PV replacement (PVR) [15,16,17,18,19].
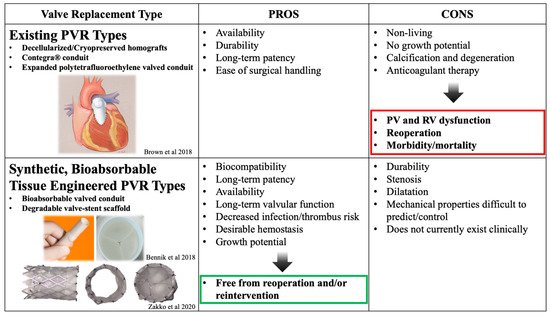
Isolated PVR in a CHD patient is much less common than a PVR that is a result of sequelae from right ventricular outflow tract (RVOT) modification during initial repair of the CHD lesion [20]. In fact, the majority of two-chamber endocardial repairs for CHD involve repair of a dysfunctional RVOT using a valved conduit or bioprosthetic pulmonary valve (Table 1). Some of the most commonly used conduits are decellularized homografts, conduits with valves made of e-PTFE, or the glutaraldehyde-fixed, bovine-jugular vein-derived Contegra® valved conduit. Long-term complications related to modification of the RVOT during endocardial repair are the leading cause of catheterization and/or reoperation in the distant postoperative period for these patients [21]. These complications include potential for infection due to non-autologous tissue, deterioration of valve function from calcification, associated pulmonary valve stenosis, and right heart failure due to pulmonary valve regurgitation. Ideally, the goal of the initial RVOT reconstruction and conduit placement in CHD patients would be avoidance of repeat surgical or transcatheterization intervention. However, this is limited by current treatment options and materials. To prevent right heart failure from PV regurgitation, it is important to perform either surgical or transcatheter PVR before the right ventricular function is irreversibly compromised.
| Classification of Diseases | Examples of Diagnoses | |
|---|---|---|
| RVOT Congenital Defect | Stenosis | Tetralogy of Fallot +/− absent pulmonary valve syndrome |
| Isolated pulmonary stenosis | ||
| Atresia | Pulmonary atresia +/− VSD | |
| Truncus Arteriosus | ||
| RVOT Iatrogenic Defect | Rastelli Procedure | DORV + VSD + sub-pulmonary stenosis |
| TGA or Corrected TGA + sub-pulmonary stenosis |
||
| Ross Procedure | Aortic Stenosis (congenital or acquired) | |
| Aortic Regurgitation (congenital or acquired) | ||
| Secondary Pulmonary Regurgitation |
PR after repair of Tetralogy of Fallot | |
| PR after pulmonary valvuloplasty | ||
PVR traditionally involves either surgical or transcatheter approaches using an array of different mechanical, allo-/xenogenic, or synthetic valve substitutes each with their own varying levels of success. For surgical PVR, bioprosthetic (mainly decellularized) homograft valves have been a widely utilized valve choice due to their decreased thrombogenicity and the lack of required lifelong anticoagulation associated with mechanical valves. However, their apparent short lifespan and tendency to calcify/degenerate has been a known issue for many years, requiring multiple reinterventions throughout a pediatric patient’s lifetime [23,24]. The more recently accepted approach for PVR emerged in 2000 as Bonhoeffer performed the first transcatheter valve replacement. A native, valved bovine jugular vein sewn onto a stent was implanted into the conduit of a 12-year-old boy with repaired pulmonary atresia and a ventricular septal defect [25]. Since then, the field of transcatheter PVR has grown substantially with the two most common transcatheter PV being the Melody® Transcatheter Pulmonary Valve (Medtronic, Minneapolis, MN, USA) [26,27] and the Sapien Transcatheter Heart Valve (Edwards Lifesciences, Irvine, CA, USA) [28]. The Melody® valve is constructed from a bovine jugular vein, whereas the Sapien valve is constructed with bovine pericardium. Both valves are secured within balloon-expandable stents to facilitate deployment. The strengths and weaknesses of these valves are beyond the scope of this review; however, the selection largely depends on the patient’s anatomy [19,29]. Though effective at relieving PV insufficiency, bioprosthetic transcatheter valves (both surgical and transcatheter) have long-term complications related to somatic overgrowth, degeneration, calcification, rejection, the need for long-term anticoagulation and may lead to eventual replacement [18,30,31,32].
Tissue engineering offers a promising alternative as it has the potential to mitigate these complications due to the ability to create living, native tissue that grows with the patient [33]. Initial work using tissue-engineering strategies for PVR focused on decellularized xeno- and homografts seeded with various cell populations [34,35]. Despite relatively encouraging results, lengthy incubation periods required for in vitro cell culture rendered these tissue-engineered pulmonary valves (TEPVs) less than ideal, and has pushed groups to develop constructs without the need of cell seeding prior to implantation [36,37]. Further work in this field has sought to define an ideal PVR, with the goal of creating an “off-the-shelf” TEPV capable of self-renewal and growth within pediatric patients. While the concept of tissue engineering encompasses advantages over current technology [38], this section will focus solely on select studies regarding synthetic, bioabsorbable TEPV scaffolds.
The benefits of creating cardiac valves using tissue-engineering principles and synthetic, bioabsorbable biomaterials are remarkable [39]. Using synthetic, bioabsorbable materials alleviates the issue of limited material supply that many non-synthetic TEPVs constructs encounter. Additionally, as the body degrades the scaffolds and replaces it with its own tissue, the neo-valve has the potential to grow with the patient. This bypasses the need for additional replacement procedures secondary to PV insufficiency from somatic overgrowth. Synthetic materials also lend themselves to being more customizable as medicine continues to search for patient-specific solutions (Figure 1). Nonetheless, challenges remain in constructing a synthetic, fully resorbable TEPV limiting its widespread clinical use.
Just as current treatment options are delivered via transcatheter or surgical approaches, TEPVs have also been studied through both delivery modalities. In the transcatheter approach, the leaflets are completely degradable; however, current metal stents that house the valve are permanent. One manufacturing approach involves creating the structure ex vivo using autologous cells seeded onto a scaffold, culturing these constructs in a bioreactor to allow for ECM production, followed by decellularization and subsequent implantation. An example of this is the polyglycolic acid (PGA) and poly(4-hydroxybutyrate) PV that has undergone several changes to its design with positive large animal model results [43,44,45]. Another strategy avoided ex vivo culture altogether, using the TEPV recipient’s own immune system, along with a bioresorbable polymer bisurea-modified poly-carbonate scaffold, to assist with remodeling [46,47]. Although these valve designs have seen some exciting preclinical success, the long-term efficacy remains to be seen as animal models developed pulmonary regurgitation at earlier timepoints secondary to inappropriate valve regeneration, calcification or contracture. This is likely due to the complex structure of the native PV and the need for an equally complex TEPV in order to competently assume its role in controlling blood flow.
Regenerating such a structure involves an understanding of the delicate balance between cellular and molecular mechanisms as well as mechanical forces driving the regenerative process. Further studies aimed at elucidating the mechanisms driving the tissue regeneration process are needed before more effective clinical translation of TEPVs can occur. A mouse model of PV transplantation may assist in evaluating these mechanisms through the power of transgenic mice [48], albeit downsizing such mechanically complex structures may prove to be a separate but equally challenging hurdle. Furthermore, the role of long-term stent placement in this pediatric population is unknown. Studies evaluating stent placement in pulmonary stenosis not requiring PVR have complication rates ranging from 10 to 33%, and require frequent reinterventions [49]. The risk of damage to the valve in the event of a stent complication or to the stent in the case of a valvular intervention has yet to be fully described. Still in its beginning stages, a zinc–aluminum alloy degradable metal stent housing a synthetic, electrospun polycaprolactone valve is being used in a fetal transcatheter ovine model and may provide an alternative solution to the issue of long-term stent placement [42]. Despite zinc being an essential metal for many cellular processes, there are still only limited in vitro and in vivo data demonstrating its impact within the cardiovascular system and tissue regeneration pathway [50,51].
Although transcatheter approaches are an arguably more clinically attractive approach due to the decreased morbidity for the patient, surgically implanted synthetic TEPVs have seen the most success to date. Perhaps the most successful surgically implanted TEPV is that from Xeltis (Zurich, Switzerland). Made from a ureido-pyrimidinone supramolecular polymer, this valve-conduit structure has seen promising preclinical results with adequate hemodynamic profiles in sheep up to two years after implantation [52]. Currently, clinical trials are underway evaluating two different types of this TEPV in the Xplore-1 and Xplore-2 trials. Data presented at the International Conference of Tissue Engineered Heart Valves 2020 revealed that 11 out of 12 patients had severe pulmonary regurgitation at two years in the Xplore-1 trial. In the Xplore-2 trial, there were no signs of severe regurgitation; however, out of the 6 enrolled patients, 1 developed valve stenosis and 1 required reoperation at one year [53]. A similar issue is raised over the surgically placed biodegradable scaffolds, as severe pulmonary regurgitation would require reintervention and possibly additional PV replacement. Further work targeting the mechanisms driving regeneration would again be ideal to help guide additional modifications to scaffold design.
Regardless of the delivery method, tissue regeneration relies on a complex cell-mediated remodeling process inherent in many bioengineering approaches. The major obstacle for TEPVs is leaflet retraction and valvular insufficiency. Computational modeling offers a potential solution for this as a powerful tool that allows for consideration of many different mechanical and biological factors affecting valve design and performance. Simon Hoerstrup’s group has validated this concept, demonstrating that computational modeling could improve their PGA-based TEPV scaffold design and guide tissue remodeling for improved long-term performance and in vivo imaging results in sheep up to one year [54]. Though the logistical and technical challenges that come with validation of a computational modeling approach are many, this type of modeling is important for advancing the field of TEPVs in the search for a viable, bioabsorbable pulmonary valve replacement capable of self-repair, growth and remodeling. Nonetheless, we await continued studies in the field and are excited for the future of synthetic, bioabsorbable TEPVs.
3. Regenerative Medicine Solutions for Pulmonary Vein Stenosis
Pulmonary vein stenosis (PVS) can either be congenital or a postoperative complication of total anomalous pulmonary venous connection (TAPVC) repair (9-18%) and has a poor prognosis (Figure 3) [79,80]. The most severe form of PVS is pulmonary vein obstruction. Though the exact mechanism of PVS remains unclear, the histopathological changes related to development of PVS include intimal thickening at the surgical anastomosis sites and extension of the anastomosis into the pulmonary veins of the lung parenchyma [81]. Surgery is considered the preferred approach in most cases of congenital or acquired PVS with severe symptoms [82]. Specifically, (1) endarterectomy (resection of the stenotic ring and anastomosis of the pulmonary vein directly to the left atrial endocardium) and (2) pericardial patch venoplasty (resection of the stenotic tissue and anastomosis of the patch to enlarge the stenotic segment) are the most common traditional techniques (Figure 3A). Newer, suture-less marsupialization techniques providing direct adhesion of the pericardium surrounding the affected pulmonary vein to the left atrium (thus avoiding direct suturing of the cut end of the vessel) have been reported to help reduce the risk of restenosis by preventing suture line deformation and reducing tissue growth stimulation [80]. Overall, published surgical outcomes are moderate, with only half of cases being free of restenosis or death after several years [83,84]. Furthermore, pneumonectomy may be indicated in cases of severe or uncontrolled hemoptysis, and lung transplantation has been performed in patients with severe pulmonary hypertension resulting from PVS [85].
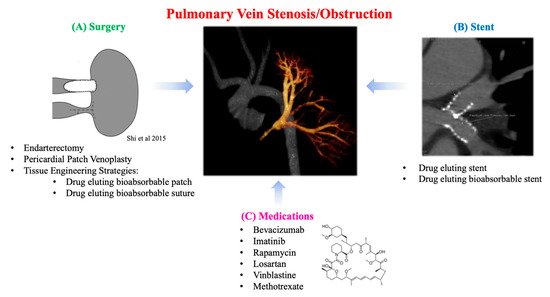
Another treatment modality for PVS is transcatheter balloon angioplasty and stent implantation (Figure 3B). This approach is challenging as it can require high-pressure balloons, and stents which can be eventually expanded to an adult size of >12 mm as the patient grows. Results from these types of interventions are suboptimal, requiring frequent re-dilation due to high rates of restenosis [86]. Early study of bare metal stents (BMS) in patients with PVS and CHD showed that freedom from occlusion or severe in-stent stenosis at 1 year was 37%. In an effort to alleviate this complication, drug-eluting stents (DES) have been increasingly utilized for the treatment of PVS [87]. In addition, DES have been shown to be more effective in treating PVS than BMS. Stented pulmonary veins are at risk for somatic overgrowth requiring reintervention which has led to further studies to avoid this process [88].
Several groups are conducting translational research to solve the mechanism of PVS in an effort to find new solutions using known drugs for this challenging disease process (Figure 3C). Saiki et al. have created an animal model of postoperative PVS in pigs and conducted several studies to elucidate the mechanism of intimal thickening in the pulmonary veins of the lung parenchyma [89]. They confirmed the proliferation of secretory smooth muscle-like cells as the main change in the pulmonary veins within the lung parenchyma. Activation of the mTOR pathway was observed in the pulmonary veins within the lung parenchyma, suggesting that this pathway may also contribute to proliferation. To further investigate this, extravascular application of a sustained-release film of rapamycin (an immunosuppressive agent) to the anastomosis demonstrated inhibition of intimal thickening at the anastomosis and mTOR activity. This suggests a possible new therapeutic strategy for PVS prevention. Zhu et al. have previously reported a piglet model of PVS with progressive diffuse obstructive intimal hyperplasia in the upstream pulmonary veins, recapitulating the clinical pathogenesis of PVS [90,91]. Specimens from the upstream zonal pulmonary veins of the pig model and human PVS patients were associated with robust expression of transforming growth factor-β (TGF-β). Systemic administration of losartan, a known TGF-β inhibitor, ameliorated the pulmonary hypertension and intimal hyperplasia associated with PVS. This indicates the medication’s potential usefulness as a prophylactic treatment for patients at high risk of developing PVS after pulmonary vein surgery. Similarly, Rehman et al. reported a study of vinblastine and methotrexate as therapies for infants and children with progressive multivessel PVS, targeting the presence of myo-fibroblasts within the lesion [92]. Quinonenz et al. also mentioned the effectiveness of chemotherapeutic agents that target neointimal proliferation being used to treat PVS [93,94]. A prospective, open-label clinical study of the use of bevacizumab and imatinib has shown an overall improvement in disease progression and patient survival in patients with multivessel PVS [95]. Taken together, these findings suggest that the prognosis of pulmonary vein stenosis may be improved by controlling excessive intimal thickening associated with post-anastomotic inflammation and rapid somatic growth in children.
Replacing materials such as conventional stents, patches, and suture with bioabsorbable materials would be very beneficial in preventing PVS. For example, the current generation of bioabsorbable drug-eluting platforms have been evaluated in coronary artery disease and have shown strong results. In the future, there may be a crossover to the treatment of pediatric PVS. Bioabsorbable stents are designed to support the body conduit only during its healing process, the mass and strength of the stent decreases with time, and the mechanical load is gradually transferred to the surrounding tissue. The bioabsorbable stent also allows for longer term delivery of drugs from the internal reservoir to the conduit wall, eliminating the need for a second surgery to remove the device [96]. The development of bioabsorbable suture may also inhibit intimal thickening of the anastomosis, similar to stents. Padmakumar has developed a biodegradable, drug-releasing suture that prevents intimal thickening [97].
Since bioabsorbable materials such as patches are associated with excessive inflammation and material thickening during the process of new tissue formation [85], the inhibition of intimal thickening by the addition of biological inhibitors may also play a significant role in resolving this phenomenon. We have created a vascular patch with sustained release of rapamycin and are currently testing its efficacy in a sheep model. The patch is still the first choice for neonatal TAPVC repair, and we hope that the development of this research will change the prognosis of pediatric patients.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines9050478
