Interindividual heterogeneity in response to treatment is a real public health problem. It is a factor that can be responsible not only for ineffectiveness or fatal toxicity but also for hospitalization due to iatrogenic effects, thus increasing the cost of patient care. Several research teams have been interested in what may be at the origin of these phenomena, particularly at the genetic level and the basal activity of organs dedicated to the inactivation and elimination of drug molecules. Today, a new branch is being set up, explaining the enigmatic part that could not be explained before. Pharmacomicrobiomics attempts to investigate the interactions between bacteria, especially those in the gut, and drug response.
- pharmacomicrobiomics
- drug response
- bacteria
- intestinal microbiota
1. Introduction
The term pharmacomicrobiomics was first used in 2010 when a new branch was defined to understand differential responses between humans to several drugs based on microbiota [1]. Starting from the beginning, the approach of individualization of treatment is not recent. Indeed, this notion has been initiated first by considering the underlying dysfunctions, mainly the dosage adjustment or even the change of the molecule carried out during renal or hepatic failure. Subsequently, clinicians have adopted the “therapeutic drug monitoring” for certain drug molecules, for which a blood concentration–therapeutic efficacy correlation is well established, associated with significantly different concentrations between patients taking the same dose of the same treatment, as is the case with ciclosporin or tacrolimus, known to have a narrow therapeutic window [2][3].
The advent of genetic techniques and their progressive availability has made it possible to explain the number of differential responses to treatments, ranging from ineffectiveness to fatal toxicities. Further, tools have been developed for use in clinical practice to define the genetic status of patients by predicting their drug response [4][5]. This was of great interest and marked a major step forward in the approach to therapeutic individualization.
However, studies of genetic diversity could not explain all the interindividual treatment responses. It is currently estimated that pharmacogenomics could only explain 20 to 95% of these events [6]. This has prompted researchers to investigate what may be the cause of a distinct response to treatment, outside genetics and known underlying dysfunctions.
Today, the intestinal microbiota attracts much interest in an attempt to explain, in addition to other previously well-known aspects, the differences in responses to treatment. Indeed, during the absorption of a given drug, the latter is confronted with a widely diversified ecosystem of bacterial species, qualified by several authors as a metabolic organ [7][8][9]. The composition of the intestinal microbiota is subject to great differences between individuals, but also within the same organism over time, as it is constantly influenced by host-related factors, such as the immune system, as well as external factors, such as diet [10].
2. Impact of Gut Microbiota on Drug Effect
The human body contains several microorganisms whose number is close to that of its whole cells, which is several trillion. The involvement of these microorganisms has been well established in the regulation of the immune system as well as the metabolism of polysaccharides and vitamins [11]. Moreover, certain species forming part of the intestinal microbiota have the enzymatic machinery necessary for the biotransformation of molecules found in the intestinal lumen, including drugs (Table 1). This biotransformation concerns, on one hand, the drug molecules ingested, before their absorption to gain the blood circulation, but also drugs, or their metabolites, which have followed a hepatic elimination via the bile. The result of this bacterial transformation of xenobiotics, which mainly consists of hydrolysis and reduction, unlike hepatic metabolism consisting of oxidation and conjugation reactions, gives metabolites that can be inactive, active or even toxic [8]. In addition, in addition to hydrolysis reactions, the intestinal microbiota is endowed with N-oxide cleavage, proteolysis, deconjugation, and others [12].
Table 1. Summary table on the different bacteria–drug interactions and their consequences.
| Drugs | Microbs | Body Site | Effects | References |
|---|---|---|---|---|
| Drug Effect Influenced by Bacteria | ||||
| Sulfasalazine | Bacteria possessing azoreductase enzymes | Gut | Cleavage into its two active metabolites, Salazopyrin and 5-amine salicylic acid | Peppercorn MA and Goldman P, 1972 |
| Warfarin | Bacteroides, Escherichia–Shigella and Klebsiella | Gut | weak response to the drug | Wang L et al., 2020 |
| Enterococcus | Gut | High response to the drug | ||
| Digoxin | Eggerthella lenta | Gut | reduction of digoxin to its inactive metabolite, dihydro-digoxin | Haiser HJ et al., 2014; Koppel N et al., 2018 |
| Levodopa | Helicobacter pylori | Stomach | decreased drug absorption | Hashim H et al., 2014 |
| Cyclophosphamide (CTX) | Enterococcus hirae, Lactobacillus johnsonii, Lactobacillus murinus | Mesenteric lymph nodes and the spleen | Enhancement of the antitumor adaptive immunological response to CTX | Viaud S et al., 2013; Daillère et al., 2016 |
| CTLA-4 checkpoint inhibitors | Bacteroides fragilis | Gut | Restore the response to the treatment | Vétizou M et al., 2015 |
| Anti PD-1 | Akkermentia muciniphila, Collinsella aerofaciens, Enterococcus faecium, Ruminococcaceae family, Bifidobacterium spp. | Gut | Enhanced response to treatment | Gopalakrishnan V et al., 2018; Matson V et al., 2018; Routy B et al., 2017 |
| Gemcitabine | Mycoplasma hyorhinis, bacteria belonging to the Gammaproteobacteria, Escherichia coli | Tumor tissue | Gemcitabine resistance | Galler et al., 2017; Lehouritis P et al., 2015 |
| Irinotecan | Opportunistic or enterohepatic bacteria possessing β-glucuronidases enzymes | Gut | Production of toxic metabolites responsible for diarrhea | Stein A et al., 2010 |
| NSAIDs | Gut | Production of toxic metabolites responsible for mucosal damage in the small intestine | Higuchi et al., 2009; Boelsterli UA et al., 2013 | |
| Bacteria abundance influenced by drugs | ||||
| Proton pump inhibitors | Clostridium difficile, Salmonella, diarrheagenic Escherichia coli and beta glucuronidase-producing bacteria | Gut | Increased bacteria | Dial et al., 2004; Bruno G et al., 2019; Blackler RW et al., 2015; Davis JA et al., 2020; Wallace JL et al., 2011 |
| Bifidobacterium spp. and Akkermentia muciniphila | Gut | Decreased bacteria | ||
| Metformin | Roseburia, butyrivibrio genera and Akkermentia muciniphila | Increased bacteria, responsible for better epithelial permeability and improvement in glucose and lipid metabolism | Forslund K et al., 2015; Shin NR et al., 2014; Wu H et al., 2017 | |
| Bidirectional effect | ||||
| Methotrexate (MTX) | Enterobacterial group, Ruminococcaceae, Bacteroidetes phyla and Bacteroides fragilis | Gut | Decreased bacteria | Ramos-Romero S et al., 2018; Zhou B et al., 2018 |
| Lachnospiraceae family | Gut | Increased bacteria | ||
| Prevotella maculosa | Gut | Enhancement of the response to the treatment | Zhang et al., 2015 | |
2.1. The Gut Microbiota Responsible for Drug Response
2.1.1. Sulfasalazine
Sulfasalazine is a good, if not the first, example of considering the intestinal microbiota as a major step in the metabolism of a drug. In fact, bacterial azo-reductases make it possible to cleave sulfasalazine into its two active metabolites, sulfapyridine and 5-amine salicylic acid, with anti-inflammatory effects, effective in the treatment of ulcerative colitis, Crohn’s disease and rheumatoid arthritis (Figure 1A) [13]. The demonstration of these bacterial azo-reductases dates back to 1930 when researchers described their involvement in activating an antibacterial drug, Prontosil, which showed no effect in vitro [14].
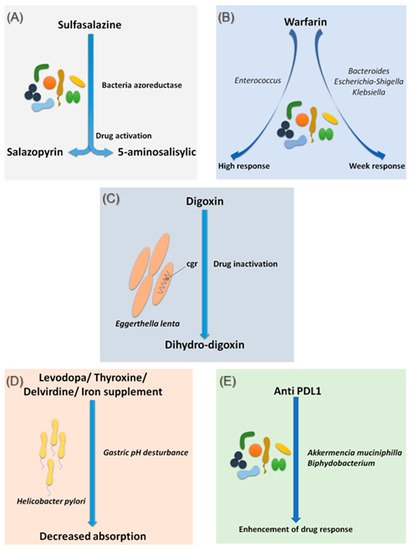
Figure 1. Effects of bacteria on drug absorption and response to treatment. (A) Effect of bacterial azo reductases on the bioactivation of sulfasalazine. (B) Effect of the different compositions of the intestinal microbiota on the response to warfarin. (C) Effect of Eggerthella lenta bacteria on the bio-inactivation of digoxin. (D) Involvement of Helicobacter pylori in gastric pH modification leading to decreased drug absorption. (E) Involvement of Akkermansia muciniphila and Bifidobacterium in improving the response to anti-PDL1 treatments. Illustrations were created partially with biorender.com.
2.1.2. Warfarin
Since 1954, warfarin has been the first oral anticoagulant to prevent and treat thromboembolic complications in humans [15]. Warfarin is characterized by a marked interindividual response variability. Several studies have examined the genetic status of patients receiving a vitamin K inhibitor and have succeeded in identifying mutations predictive of treatment response, notably the VKORC1 and CYP2C9 mutations, genes encoding enzymes involved in the metabolism of vitamin K and coumarin vitamin K inhibitors, respectively [16]. On the other hand, 35% of patients with delayed responses to warfarin remain unexplained [17]. It is only recently that researchers have begun to explore the possible influence of the intestinal microbiota in response to warfarin. This idea arose because of the known link between the gut microbiota and vitamin K metabolism. Recently, Wang et al. explored gut microbial diversity in 200 patients with a high, normal, or low response to warfarin. A significant presence of the genera Bacteroides, Escherichia–Shigella and Klebsiella, were reported in patients with a weak response, as the Escherichia–Shigella genera has the enzymatic machinery essential for synthesizing vitamin K, while that the genus Enterococcus was associated with an elevated response to warfarin (Figure 1B) [18]. This study is the first to establish a link between gut microbiota and response to warfarin, a narrow-spectrum therapeutic molecule, for which an unsuitable dosage can be associated with hemorrhage or, rather, in inefficiency. Such promising results need to be confirmed and refined by further research in the future.
2.1.3. Digoxin
Digoxin is a natural cardiotonic glycoside that has been used for more than two centuries in the treatment of heart failure and certain types of arrhythmias. Digoxin is ineffective in 10 to 15% of patients treated with conventional doses [19]. The particularity of digoxin is that it is associated with a particular intestinal bacterium, Eggerthella lenta, originally classified as Eubacterium lentum (Figure 1C) [20]. It is noteworthy that this species shows increased growth in diabetic patients [21][22]. Although the mechanism of interaction is not clearly understood, strains belonging to this bacterial species are thought to possess a two-gene cytochrome encoding operon system, designed as a two-gene cardiac glycoside reductase (cgr) operon, which is significantly upregulated in the presence of digoxin, which in turn results in the reduction of digoxin to its inactive metabolite, dihydro-digoxin, in which the lactone ring is reduced [23][24]. It has been reported that the presence of Eggerthella lenta strains was associated with decreased efficacy of digoxin [24][25]. Importantly, arginine, a semi-essential amino acid for humans, shows inhibition of cgr, which results in the prevention of digoxin inactivation by E. lenta. Therefore, it is now assumed that a diet rich in amino acids, particularly arginine, may be conducive to an adequate response to digoxin treatment [23]. A peculiarity of the cgr system is that it has pockets, which bind both digoxin and other compounds, referred to as digoxin-like, as in the case of fumarate, which has a higher affinity than digoxin [26]. This particularity suggests the future development of adjuvant drugs preventing the activation of digoxin by this mechanism.
2.1.4. Levodopa
Species of the gut microbiota, which are commensal and normally free from any adverse effects, are not the only ones to have a well-established effect on optimal drug activity. Indeed, it has been shown that the presence of Helicobacter pylori, the bacterium responsible for gastric ulcers, was significantly associated with a decrease in levodopa absorption, the benchmark drug for Parkinson’s disease (Figure 1D) [27]. Meanwhile, an increase in levodopa absorption of about 21 to 54% was obtained following the eradication of this bacterium [28]. The supposed mechanism of interaction is linked to the modification of gastric pH caused by the bacteria. Another hypothesis suggests a physical attachment between the bacterium and the drug leading to a decrease in the bioavailability [28][29]. In addition to levodopa, helicobacter pylori also appear to decrease the absorption of thyroxine, delavirdine and iron supplements [30].
2.1.5. Chemotherapy and Immunomodulator Drugs
It is not surprising that the intestinal microbiota can influence the response to immunomodulating and cytotoxic treatments, as long as it is directly related to the functioning of the immune system [31][32]. In addition, patients receiving immunomodulating and cytotoxic drugs, mainly the cancer population, already have multifactorial impairment of the intestinal microbiota by the host environment and diet, surgery, using adjuvant drugs, as well as by the effect of these drugs themselves.
The intestinal microbiota influences the action of these drug classes via xenometabolism and community structure alteration mechanisms and via immunomodulation mechanisms [33]. These interactions can occur either intraluminal or in the lymphoid organs following drug-induced bacterial translocation [34][35]. In this context, one study reported that cyclophosphamide (CTX), an alkylating drug whose function also depends on the stimulation of anticancer immunity, induced transmucosal translocation of specific bacteria, such as Enterococcus hirae and species belonging to the Lactobacillus genus (Lactobacillus johnsonii, Lactobacillus murinus), in the mesenteric lymph nodes and the spleen, which lead to T-helper 17 (Th17) cell differentiation, thereby enhancing the adaptive antitumor immune response to CTX [36]. In another study, Daillère et al. have demonstrated that oral gavage of E. hirae in antibiotic pretreated mice could restore response to CTX (Figure 2A) [37]. In addition, it has been reported that a memory Th1 immune response towards E. hirae may predict progression-free survival in patients with end-stage lung and ovarian cancer [33][38].
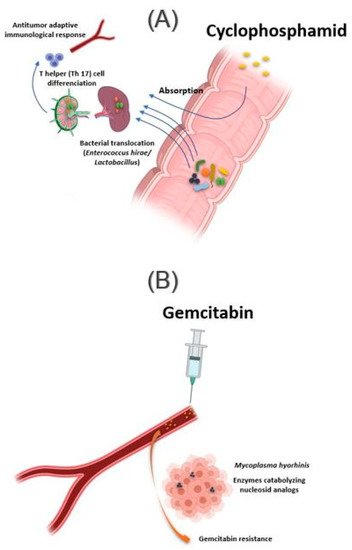
Figure 2. Influence of bacteria found in tissue on response to cancer chemotherapy treatment. (A) Oral absorption of cyclophosphamide induces transmucosal translocation of specific bacteria, such as Enterococcus hirae and species belonging to the Lactobacillus genus (Lactobacillus johnsonii, Lactobacillus murinus), in the mesenteric lymph nodes and the spleen. This leads to T-helper 17 (Th17) cell differentiation, enhancing the adaptive antitumor immune response to CTX. (B) The presence of Mycoplasma hominis in the tumor leads to resistance to gemcitabine treatment via their enzymes catabolizing nucleoside analogous. Illustrations were created partially with biorender.com.
Another association exists, this time with immune checkpoint inhibitors and Bacteroides species. Indeed, T-cell reactions to Bacteroides fragilis have been found in patients with melanomas responding to treatment with CTLA-4 checkpoint inhibitors. In vivo investigations were able to restore the response to ipilimumab in germ-free mice by administering B. fragilis or adoptive transfer B. fragilis-specific T cells [39].
Several studies have also associated the abundance of Akkermentia muciniphila, Collinsella aerofaciens Enterococcus faecium, Ruminococcaceae family and Bifidobacterium spp. with an adequate response to Anti PD-1 [40][41][42]. Moreover, fecal microbial transplantation of human responders into germ-free mice restored the antitumor effect of PD-1 blockade in the recipient mice. Another study reported an interaction between Bifidobacterium and dendritic cells, resulting in T cells activation and enhancement of the protective anticancer response of anti-PD-L1 [43].
In addition to the intestinal microbiota, bacteria can also modulate the effect of a drug while localizing in the tumor tissue. This is the case of Mycoplasma hyorhinis, which has enzymes catabolizing nucleoside analogs, making it responsible for gemcitabine resistance. Other bacteria, especially those belonging to the Gammaproteobacteria, are also responsible for gemcitabine resistance (Figure 2B). Interestingly, a significant percentage of ductal adenocarcinomas of the human pancreas, a type of tumor commonly treated with gemcitabine, contain the culprit bacteria [44]. Interestingly, the administration of ciprofloxacin to rodents could reverse the gemcitabine resistance induced by intratumoral Gammaproteobacteria. In another study, Lehouritis et al. reported that gemcitabine efficacy might also be impaired by E. coli [45].
Other cytotoxic molecules subject to the influence of the intestinal microbiota, but in a different context, will be discussed below.
2.2. The Gut Microbiota Causing Drug Toxicity
2.2.1. Irinotecan
The gut microbiota can be the cause of drug toxicity. The well-established link between the gut microbiota and drug-related toxicity, to date, is bacterial beta-glucosidase. These β-glucosidases allow the hydrolysis of the hepatic glycoside metabolites, called aglycones. Indeed, it has been reported by various studies that several drugs, or classes of drugs, could be the substrate of these enzymes giving rise to the formation of toxic metabolites. The first example is camptothecin-11 (CPT-11), also known as irinotecan, a drug used to treat colon cancer. Indeed, CPT-11 is a prodrug activated initially by hepatic carboxylesterases, giving rise to SN-38, responsible for the cytotoxic effect. In the second step, the SN-38 will be glucuronidased also at the hepatic level to obtain the SN-38G so that it is excreted via the bile to reach the intestine. At this level, bacterial β-glucuronidases will make the opposite reaction, reconverting SN-38G into SN-38, toxic for the intestinal epithelial cells, causing intense diarrhea in up to 80% of treated patients (Figure 3A) [46]. It should be noted that ciprofloxacin and low doses of amoxapine are effective in suppressing the bacterial activity of β-glucuronidase and mucositis due to the absorption of irinotecan [47][48]. Moreover, the search for specific inhibitors of these β-glucosidases seems to be an intriguing approach. Indeed, Cheng et al. reported that compound TCH-3562 showed specific inhibition of E. coli beta-glucosidases without affecting human β-glucosidases [49].
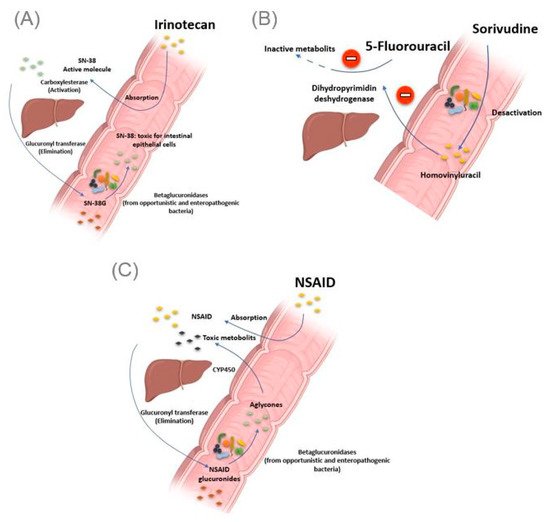
Figure 3. Intestinal bacteria involved in drug toxicity. (A) The prodrug irinotecan is activated initially by hepatic carboxylesterases, giving rise to SN-38, responsible for the cytotoxic effect. Second, the SN-38 is glucuronidased in the liver to obtain the SN-38G, which is excreted via the bile to reach the intestine. At this level, bacterial β-glucuronidases make the opposite reaction, reconverting SN-38G into SN-38, toxic for the intestinal epithelial cells, causing intense diarrhea. (B) Bacterial enzymes metabolize sorivudine into bromovinyluracil. The latter is absorbed and inactivates hepatic dihydropyrimidine dehydrogenase, an enzyme involved in the inactivation of 5 fluorouracil. This resulted in extremely high concentrations of 5 fluorouracil, inducing death. (C). NSAIDs are normally glucuronidated in the liver. NSAIDs glucuronides reach the intestine via the bile. At this level, the bacterial beta-glucuronidase hydrolyzes them into aglycones, which are again reabsorbed and taken in charge by the cytochrome P450 to give potentially cytotoxic intermediates responsible for intestinal toxicity. Illustrations were created partially with biorender.com.
Toxic effects of chemotherapy drugs can also occur because of the bacterial metabolism of another drug taken concomitantly. This is reminiscent of the story of the 16 Japanese cancer patients treated simultaneously with 5 fluorouracil and sorivudine, a potent antiviral drug, where the intestinal microbiota was the first culprit [50]. Indeed, enzymes from the Bacteroides genus, such as Bacteroides vulgatus, B. thetaiotaomicron and Bacteroides eggerthii, metabolized sorivudine into bromovinyluracil, a metabolite that inactivates hepatic dihydropyrimidine dehydrogenase, an enzyme involved in the inactivation of 5 fluorouracil [51][52]. This resulted in extremely high concentrations of 5 fluorouracil, inducing death (Figure 3B) [53].
2.2.2. Nonsteroidal Anti-Inflammatory Drugs
As with Irinotecan and through the same bacterial glucuronidases, nonsteroidal anti-inflammatory drugs (NSAIDs) are metabolized in the gut to give toxic metabolites to the intestinal mucosa. In fact, in addition to the gastric ulcers induced by this drug class via the inhibition of the synthesis of protective prostaglandins of the gastric wall, it has been described, with the advent of new gastroenterological exploration techniques, that NSAIDs caused mucosal damage in the small intestine [54]. Indeed, the NSAIDs glucuronides by the liver reach the intestine via the bile. At this level, the bacterial β-glucuronidase hydrolyze them into aglycones, which are again reabsorbed and taken in charge by the cytochrome P450 to give potentially cytotoxic intermediates, responsible for this intestinal toxicity (Figure 3C) [55]. Like TCH-3562 for irinotecan, a recent study reported that Inh1 showed a reduction in the intestinal side effects of diclofenac via specific inhibition of β-glucosidases in an animal model [56].
Although several commensal bacteria of the intestine produce β-glucuronidases of different sequences and structures, providing beneficial functions to the organism, it has been reported that de-glucuronidation of drugs, leading to toxic metabolites, is carried out mainly by opportunistic or enteropathogenic bacteria, in particular, Clostridium perfrengens and Escherichia coli. This particularity is explained by internal differences between the different types of this enzyme, differences concerning conformations, hydrophobicity and flexibility [57]. The potentiating effect of bacterial β-glucosidase-induced drug toxicity, particularly intestinal toxicity, is not limited to irinotecan and NSAIDs. Other molecules have also been shown to be substrates for these enzymes, such as Regorafenib, a tyrosine kinase inhibitor with an antitumor effect, as well as venotonic flavonoids [58][59][60].
2.2.3. Impact of Non-Antibiotic Drugs on the Gut Microbiota
In the other direction, the gut microbiota is not spared from disturbance or influence by drugs. It is assumed that 10% of the interindividual variations of the gut flora composition can be explained by drug use [7][61]. Indeed, several drugs are known to induce dysbiosis. Others push the growth of particular bacterial species resulting in a beneficial effect in humans.
2.2.4. Proton Pomp Inhibitors
Proton pump inhibitors (PPI) decrease gastric acidity and are used primarily to treat ulcers and gastritis. Through the increase in gastric pH, the bacteria present in the oral cavity find the capacity to release and maintain themselves in the stomach and gut [62][63]. In addition, by this decrease in acidity, pathogenic bacteria using the oral route also find this barrier weakened, as are the cases of Clostridium difficile responsible for pseudomembranous diarrhea as well as Salmonella and diarrheagenic Escherichia coli (Figure 4A) [64][65]. In addition, other studies have associated using PPIs with a decrease in the abundance of certain commensal bacteria in the gut, such as Bifidobacterium spp. and A. muciniphila, versus an increase in β-glucuronidase-producing bacteria [66][67][68].
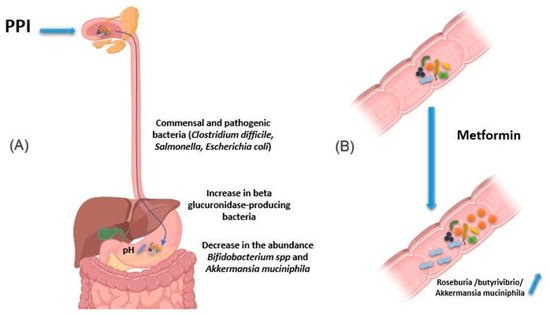
Figure 4. Effect of drugs in modifying the composition of the intestinal microbiota. (A) PPIs induce an increase in gastric pH. Thus, the bacteria present in the oral cavity find the capacity to release and maintain themselves in the stomach and gut. In addition, pathogenic bacteria using the oral route also find this barrier weakened, as are the cases of Clostridium difficile, Salmonella and diarrheagenic Escherichia. In addition, using PPIs is associated with a decrease in the abundance of certain commensal bacteria in the gut, such as Bifidobacterium spp. and A. muciniphila, versus an increase in beta glucuronidase-producing bacteria. (B) Metformin induces changes in the composition of the intestinal flora, making it rich in bacteria producing short-chain fatty acids, such as butyrates like Roseburia and butyrivibrio genera, and bacteria degrading mucin-like A. muciniphila. The use of metformin thus restores better epithelial permeability and improves glucose and lipid metabolism. Illustrations were created partially with biorender.com.
2.2.5. Metformin
Metformin is an antihyperglycemic agent used as a first-line treatment in type 2 diabetics. It is also used in obese people to reduce fat mass. Intravenous metformin administration has been shown to be associated with reduced blood-glucose-lowering relative to oral metformin [59]. Indeed, the mechanism of action of metformin is based on the decrease in hepatic synthesis and intestinal absorption of glucose and an increase in the sensitivity of muscle cells to insulin [69]. Metformin has been reported to induce changes in the composition of the intestinal flora, making it rich in bacteria like Roseburia and butyrivibrio genera producing short-chain fatty acids (SCFAs), such as butyrates. These SCFAs boost glycolysis, enhance epithelial barrier function by promoting epithelial growth and immune responses to damage [70]. Metformin also induces bacteria degrading mucin-like A. muciniphila, which could mediate the therapeutic effect of metformin by promoting intestinal stem cells-mediated epithelial development contributing thus to maintain intestinal homeostasis (Figure 4B) [71][72][73][74]. It is important to note that these species are found below normal in diabetic patients [75][76]. The use of metformin thus restores better epithelial permeability and improves glucose and lipid metabolism.
This entry is adapted from the peer-reviewed paper 10.3390/jpm11050345
References
- Rizkallah, M.R.; Saad, R.; Aziz, R.K. The Human Microbiome Project, personalized medicine and the birth of phar-macomicrobiomics. Curr. Pharm. Pers. Med. 2010, 8, 182–193.
- Birkett, D. Pharmacokinetics made easy: Therapeutic drug monitoring. Aust. Prescr. 1997, 20, 9–11.
- Tange, S.M.; Grey, V.L.; Senécal, P.E. Therapeutic Drug Monitoring in Pediatrics: A Need for Improvement. J. Clin. Pharmacol. 1994, 34, 200–214.
- Kalow, W. Pharmacogenomics: Historical Perspective and Current Status. Pharmacogenomics 2005, 311, 003–016.
- Marshall, A. Getting the right drug into the right patient. Nat. Biotechnol. 1997, 15, 1249–1252.
- Kalow, W.; Tang, B.-K.; Endrenyi, L. Hypothesis: Comparisons of inter- and intra-individual variations can substitute for twin studies in drug research. Pharmacogenetics 1998, 8, 283–289.
- Doestzada, M.; Vila, A.V.; Zhernakova, A.; Koonen, D.P.Y.; Weersma, R.K.; Touw, D.J.; Kuipers, F.; Wijmenga, C.; Fu, J. Pharmacomicrobiomics: A novel route towards personalized medicine? Protein Cell 2018, 9, 432–445.
- Sun, C.; Chen, L.; Shen, Z. Mechanisms of gastrointestinal microflora on drug metabolism in clinical practice. Saudi Pharm. J. 2019, 27, 1146–1156.
- Feng, W.; Liu, J.; Ao, H.; Yue, S.; Peng, C. Targeting gut microbiota for precision medicine: Focusing on the efficacy and toxicity of drugs. Theranostics 2020, 10, 11278–11301.
- Rodríguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.I.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C.; et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 2015, 26, 26050.
- Conlon, M.A.; Bird, A.R. The Impact of Diet and Lifestyle on Gut Microbiota and Human Health. Nutrients 2015, 7, 17–44.
- Kim, D.-H. Gut Microbiota-Mediated Drug-Antibiotic Interactions. Drug Metab. Dispos. 2015, 43, 1581–1589.
- Peppercorn, M.; Goldman, P. The role of intestinal bacteria in the metabolism of salicylazosulfapyridine. J. Pharmacol. Exp. Ther. 1972, 181, 555–562.
- Fuller, A.T. Is p-aminobenzenesulphonamide the active agent in protonsil therapy? Lancet 1937, 229, 194–198.
- Pirmohamed, M. Warfarin: Almost 60 years old and still causing problems. Br. J. Clin. Pharmacol. 2006, 62, 509–511.
- Wise, E.S.; Gadomski, S.P.; McMaster, W.G.; Wilson, R.J.; Nelms, J.K.; Hocking, K.M.; Brophy, C.M. The influence of VKORC1 and CYP2C9 mutations on warfarin response after total hip and knee arthroplasty. J. Orthop. 2015, 12, S145–S151.
- Xue, L.; Holford, N.; Ding, X.-L.; Shen, Z.-Y.; Huang, C.-R.; Zhang, H.; Zhang, J.-J.; Guo, Z.-N.; Xie, C.; Zhou, L.; et al. Theory-based pharmacokinetics and pharmacodynamics of S- and R-warfarin and effects on international normalized ratio: Influence of body size, composition and genotype in cardiac surgery patients. Br. J. Clin. Pharmacol. 2016, 83, 823–835.
- Wang, L.; Liu, L.; Liu, X.; Xiang, M.; Zhou, L.; Huang, C.; Shen, Z.; Miao, L. The gut microbes, Enterococcus and Escherichia-Shigella, affect the responses of heart valve replacement patients to the anticoagulant warfarin. Pharmacol. Res. 2020, 159, 104979.
- MacLeod-Glover, N.; Mink, M.; Yarema, M.; Chuang, R. Digoxin toxicity Case for retiring its use in elderly patients? Can. Fam. Physician 2016, 62, 223–228.
- Haiser, H.J.; Gootenberg, D.B.; Chatman, K.; Sirasani, G.; Balskus, E.P.; Turnbaugh, P.J. Predicting and Manipulating Cardiac Drug Inactivation by the Human Gut Bacterium Eggerthella lenta. Science 2013, 341, 295–298.
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60.
- Ugarte-Torres, A.; Gillrie, M.R.; Griener, T.P.; Church, D.L. Eggerthella lenta Bloodstream Infections Are Associated with Increased Mortality Following Empiric Piperacillin-Tazobactam (TZP) Monotherapy: A Population-based Cohort Study. Clin. Infect. Dis. 2018, 67, 221–228.
- Haiser, H.J.; Seim, K.L.; Balskus, E.P.; Turnbaugh, P.J. Mechanistic insight into digoxin inactivation byEggerthella lentaaugments our understanding of its pharmacokinetics. Gut Microbes 2014, 5, 233–238.
- Koppel, N.; Bisanz, J.; Pandelia, M.-E.; Turnbaugh, P.J.; Balskus, E.P. Discovery and characterization of a prevalent human gut bacterial enzyme sufficient for the inactivation of a family of plant toxins. eLife 2018, 7, e33953.
- Saha, J.R.; Butler, V.P.; Neu, H.C.; Lindenbaum, J. Digoxin-inactivating bacteria: Identification in human gut flora. Science 1983, 220, 325–327.
- Kumar, K.; Jaiswal, S.K.; Dhoke, G.V.; Srivastava, G.N.; Sharma, A.K.; Sharma, V.K. Mechanistic and structural insight into promiscuity based metabolism of cardiac drug digoxin by gut microbial enzyme. J. Cell. Biochem. 2018, 119, 5287–5296.
- Hashim, H.; Azmin, S.; Razlan, H.; Yahya, N.W.; Tan, H.J.; Manaf, M.R.; Ibrahim, N.M. Eradication of Helicobacter pylori infec-tion improves levodopa action, clinical symptoms and quality of life in patients with Parkinson’s disease. PLoS ONE 2014, 9, e112330.
- Lahner, E.; Annibale, B.; Delle Fave, G. Systematic review: Helicobacter pylori infection and impaired drug absorp-tion. Aliment. Pharmacol. Ther. 2009, 29, 379–386.
- Niehues, M.; Hensel, A. In-vitro interaction of L-dopa with bacterial adhesins of Helicobacter pylori: An explanation for clinicial differences in bioavailability? J. Pharm. Pharmacol. 2009, 61, 1303–1307.
- Çamcı, G.; Oğuz, S. Association between Parkinson’s Disease and Helicobacter Pylori. J. Clin. Neurol. 2016, 12, 147–150.
- Lazar, V.; Ditu, L.-M.; Pircalabioru, G.G.; Gheorghe, I.; Curutiu, C.; Holban, A.M.; Picu, A.; Petcu, L.; Chifiriuc, M.C. Aspects of Gut Microbiota and Immune System Interactions in Infectious Diseases, Immunopathology, and Cancer. Front. Immunol. 2018, 9, 1830.
- Wu, H.-J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14.
- Pouncey, A.L.; Scott, A.J.; Alexander, J.L.; Marchesi, J.; Kinross, J. Gut microbiota, chemotherapy and the host: The influence of the gut microbiota on cancer treatment. Ecancermedicalscience 2018, 12, 868.
- Erdman, S.E.; Poutahidis, T. Gut microbiota modulate host immune cells in cancer development and growth. Free. Radic. Biol. Med. 2017, 105, 28–34.
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal Bacteria Control Cancer Response to Therapy by Modulating the Tumor Microenvironment. Science 2013, 342, 967–970.
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillère, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The Intestinal Microbiota Modulates the Anticancer Immune Effects of Cyclophosphamide. Science 2013, 342, 971–976.
- Daillère, R.; Vétizou, M.; Waldschmitt, N.; Yamazaki, T.; Isnard, C.; Poirier-Colame, V.; Duong, C.P.; Flament, C.; Lepage, P.; Roberti, M.P.; et al. Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity 2016, 45, 931–943.
- Alexander, J.L.; Wilson, I.D.; Teare, J.; Marchesi, J.R.; Nicholson, J.K.; Kinross, J.M. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 356–365.
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084.
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103.
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.-L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108.
- Routy, B.; Le Chatelier, E.; DeRosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 2018, 359, 91–97.
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089.
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 357, 1156–1160.
- Lehouritis, P.; Cummins, J.; Stanton, M.; Murphy, C.T.; McCarthy, F.O.; Reid, G.; Urbaniak, C.; Byrne, W.L.; Tangney, M. Local bacteria affect the efficacy of chemotherapeutic drugs. Sci. Rep. 2015, 5, 14554.
- Stein, A.; Voigt, W.; Jordan, K. Review: Chemotherapy-induced diarrhea: Pathophysiology, frequency and guideline-based management. Ther. Adv. Med. Oncol. 2010, 2, 51–63.
- Kodawara, T.; Higashi, T.; Negoro, Y.; Kamitani, Y.; Igarashi, T.; Watanabe, K.; Tsukamoto, H.; Yano, R.; Masada, M.; Iwasaki, H.; et al. The Inhibitory Effect of Ciprofloxacin on the β-Glucuronidase-mediated Deconjugation of the Irinotecan Metabolite SN-38-G. Basic Clin. Pharmacol. Toxicol. 2015, 118, 333–337.
- Kong, R.; Liu, T.; Zhu, X.; Ahmad, S.; Williams, A.L.; Phan, A.T.; Zhao, H.; Scott, J.E.; Yeh, L.-A.; Wong, S.T. Old Drug New Use—Amoxapine and Its Metabolites as Potent Bacterial β-Glucuronidase Inhibitors for Alleviating Cancer Drug Toxicity. Clin. Cancer Res. 2014, 20, 3521–3530.
- Cheng, K.W.; Tseng, C.H.; Tzeng, C.C.; Leu, Y.L.; Cheng, T.C.; Wang, J.Y.; Chang, J.M.; Lu, Y.C.; Cheng, C.M.; Chen, I.J.; et al. Pharma-cological inhibition of bacterial β-glucuronidase prevents irinotecan-induced diarrhea without impairing its antitumor effica-cy in vivo. Pharmacol. Res. 2019, 139, 41–49.
- Nakamura, H.; Omori, S.; Kitada, K.; Mochida, A. Prevention of drug interactions and its countermeasure. J. Toxicol. Sci. 1994, 19, 89–93.
- Nakayama, H.; Kinouchi, T.; Kataoka, K.; Akimoto, S.; Matsuda, Y.; Ohnishi, Y. Intestinal anaerobic bacteria hydrolyse sorivudine, producing the high blood concentration of 5-(E)-(2-bromovinyl)uracil that increases the level and toxicity of 5-fluorouracil. Pharmacogenetics 1997, 7, 35–43.
- Wilkinson, E.M.; Ilhan, Z.E.; Herbst-Kralovetz, M.M. Microbiota–drug interactions: Impact on metabolism and efficacy of therapeutics. Maturitas 2018, 112, 53–63.
- Diasio, R.B. Sorivudine and 5-fluorouracil; a clinically significant drug-drug interaction due to inhibition of dihydropyrimidine dehydrogenase. Br. J. Clin. Pharmacol. 1998, 46, 1–4.
- Higuchi, K.; Umegaki, E.; Watanabe, T.; Yoda, Y.; Morita, E.; Murano, M.; Tokioka, S.; Arakawa, T. Present status and strategy of NSAIDs-induced small bowel injury. J. Gastroenterol. 2009, 44, 879–888.
- Boelsterli, U.A.; Redinbo, M.R.; Saitta, K.S. Multiple NSAID-Induced Hits Injure the Small Intestine: Underlying Mechanisms and Novel Strategies. Toxicol. Sci. 2012, 131, 654–667.
- Yauw, S.T.; Arron, M.; Lomme, R.M.; Broek, P.V.D.; Greupink, R.; Bhatt, A.P.; Redinbo, M.R.; Van Goor, H. Microbial Glucuronidase Inhibition Reduces Severity of Diclofenac-Induced Anastomotic Leak in Rats. Surg. Infect. 2018, 19, 417–423.
- Dashnyam, P.; Mudududdla, R.; Hsieh, T.-J.; Lin, T.-C.; Lin, H.-Y.; Chen, P.-Y.; Hsu, C.-Y.; Lin, C.-H. β-Glucuronidases of opportunistic bacteria are the major contributors to xenobiotic-induced toxicity in the gut. Sci. Rep. 2018, 8, 1–12.
- Ervin, S.M.; Hanley, R.P.; Lim, L.; Walton, W.G.; Pearce, K.H.; Bhatt, A.P.; James, L.I.; Redinbo, M.R. Targeting Regorafenib-Induced Toxicity through Inhibition of Gut Microbial β-Glucuronidases. ACS Chem. Biol. 2019, 14, 2737–2744.
- Yang, B.; Liu, H.; Yang, J.; Gupta, V.K.; Jiang, Y. New insights on bioactivities and biosynthesis of flavonoid glycosides. Trends Food Sci. Technol. 2018, 79, 116–124.
- Falony, G.; Joossens, M.; Vieira-Silva, S.; Wang, J.; Darzi, Y.; Faust, K.; Kurilshikov, A.; Bonder, M.J.; Valles-Colomer, M.; Vandeputte, D.; et al. Population-level analysis of gut microbiome variation. Science 2016, 352, 560–564.
- Wright, B.; Spencer, J.P.; Lovegrove, J.A.; Gibbins, J.M. Insights into dietary flavonoids as molecular templates for the design of anti-platelet drugs. Cardiovasc. Res. 2012, 97, 13–22.
- Freedberg, D.E.; Lebwohl, B.; Abrams, J.A. The Impact of Proton Pump Inhibitors on the Human Gastrointestinal Microbiome. Clin. Lab. Med. 2014, 34, 771–785.
- Imhann, F.; Bonder, M.J.; Vila, A.V.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, S.; Cenit, M.C.; Harmsen, H.J.M.; et al. Proton pump inhibitors affect the gut microbiome. Gut 2016, 65, 740–748.
- Dial, S.; Alrasadi, K.; Manoukian, C.; Huang, A.; Menzies, D. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: Cohort and case-control studies. Can. Med. Assoc. J. 2004, 171, 33–38.
- Bruno, G.; Zaccari, P.; Rocco, G.; Scalese, G.; Panetta, C.; Porowska, B.; Pontone, S.; Severi, C. Proton pump inhibitors and dysbiosis: Current knowledge and aspects to be clarified. World J. Gastroenterol. 2019, 25, 2706–2719.
- Blackler, R.W.; Motta, J.-P.; Manko, A.; Workentine, M.; Bercik, P.; Surette, M.G.; Wallace, J.L. Hydrogen sulphide protects against NSAID-enteropathy through modulation of bile and the microbiota. Br. J. Pharmacol. 2014, 172, 992–1004.
- Davis, J.A.; Collier, F.; Mohebbi, M.; Stuart, A.L.; Loughman, A.; Pasco, J.A.; Jacka, F.N. Obesity, Akkermansia muciniphila, and Proton Pump Inhibitors: Is there a Link? Obes. Res. Clin. Pr. 2020, 14, 524–530.
- Wallace, J.L.; Syer, S.; Denou, E.; de Palma, G.; Vong, L.; McKnight, W.; Jury, J.; Bolla, M.; Bercik, P.; Collins, S.M.; et al. Proton Pump Inhibitors Exacerbate NSAID-Induced Small Intestinal Injury by Inducing Dysbiosis. Gastroenterology 2011, 141, 1314–1322.e5.
- Glossmann, H.H.; Lutz, O.M. Pharmacology of metformin—An update. Eur. J. Pharmacol. 2019, 865, 172782.
- Kim, C.H. Microbiota or short-chain fatty acids: Which regulates diabetes? Cell. Mol. Immunol. 2018, 15, 88–91.
- Forslund, K.; Hildebrand, F.; Nielsen, T.R.; Falony, G.; Le Chatelier, E.; Sunagawa, S.; Prifti, E.; Vieira-Silva, S.; Gudmundsdottir, V.; Pedersen, H.K.; et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 2015, 528, 262–266.
- Shin, N.R.; Lee, J.C.; Lee, H.Y.; Kim, M.S.; Whon, T.W.; Lee, M.S.; Bae, J.W. An increase in the Akkermansia spp. population in-duced by metformin treatment improves glucose homeostasis in dietinduced obese mice. Gut 2014, 63, 727–735.
- Wu, H.; Esteve, E.; Tremaroli, V.; Khan, M.T.; Caesar, R.; Mannerås-Holm, L.; Ståhlman, M.; Olsson, L.M.; Serino, M.; Planas-Fèlix, M.; et al. Metformin alters the gut microbiome of individuals with treatmentnaive type 2 diabetes, contributing to the thera-peutic effects of the drug. Nat. Med. 2017, 23, 850–858.
- De La Cuesta-Zuluaga, J.; Mueller, N.T.; Corrales-Agudelo, V.; Velásquez-Mejía, E.P.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Metformin Is Associated with Higher Relative Abundance of Mucin-DegradingAkkermansia muciniphilaand Several Short-Chain Fatty Acid–Producing Microbiota in the Gut. Diabetes Care 2017, 40, 54–62.
- Tilg, H.; Moschen, A.R. Microbiota and diabetes: An evolving relationship. Gut 2014, 63, 1513–1521.
- Harsch, I.A.; Konturek, P.C. The Role of Gut Microbiota in Obesity and Type 2 and Type 1 Diabetes Mellitus: New Insights into “Old” Diseases. Med. Sci. 2018, 6, 32.
