2. Engineered Metal Composites Implemented for Healing
The nanoceutical adjuvants are biological entities that use nanotechnology to enhance their properties and garner their usage in diverse nano-based regenerative medicine applications. Nanoceutical materials possess lucrative physicochemical characteristics, which makes them of particular interest in various biomedical applications. Though scientific evidence regarding safety or efficacy is still being explored, the number of commercially viable nanoceuticals has magnified to a large extent. The growth trend of nanoceutical products is expected to continue and facilitate personalized medicine, targeted therapies with reduced side effects, and artificial intelligence-aided patient monitoring. However, mega challenges persist, particularly regarding biodistribution, metabolism in organs, and excretion from the body. Nanoceutical materials comprise designed metal-based nanoparticles and biomaterials that offer an unmatched approach to accelerate wound repair and the tissue-remodeling process. Their dimensions and shape govern their specificity, biological efficacy, cellular response, penetrability, and targeted delivery to the site of injury. These are comparatively nontoxic and exhibit high antibacterial properties [
109]. In addition, other nanoceutical counterparts, such as the nanospheres, nano-capsules, nano-emulsions, nanocarriers, nano-scaffolds/composites, and nano-colloids, could serve as materials for wound tissue regeneration. The main focus here is metal-based nanoparticles as nanoceutical adjuvants for healing, followed by a brief overview of the other types.
Nanoparticles, both metallic and non-metallic, principally aid in wound repair and management by either possessing inbuilt inherent features that assist wound contraction or as delivery vehicles/carriers for assisted therapy. The most extensively studied ones are silver, gold, and zinc nanoparticles, owing to their unique dimensional, functional, chemical, and biochemical properties [
109]. Silver nanoparticles (AgNPs), the potential candidate of choice for wound repair, have a high surface:volume ratio, show excellent activities at low concentrations, and are superior to the traditional silver compounds that were formerly used. Neat AgNPs can regulate the release of anti-inflammatory cytokines that facilitate rapid non-hypertrophic scar-devoid wound contraction [
109,
110]. AgNPs can initiate proliferation and differentiation of keratinocytes to augment epidermal closure and re-epithelialization and antibacterial efficacy. Myofibroblast differentiation from normal fibroblasts to promote speedy tissue renewal is also facilitated by AgNPs [
109]. However, reports on their toxicity at increased concentrations by Szmyd et al. has shown that keratinocyte feasibility, absorption, migration, and differentiation are affected via specific cell death initiating the stimulus of caspase 3 and 7, ultimately leading to DNA mutilation [
111]. Therefore, it is recommended to use lower safe doses, together with antimicrobic preparations (in the form of heat, radiation, and natural and synthetic chemicals, viz., phytochemicals, antiseptics, antibiotics, polymers, etc.), to attain improved effectiveness. AgNPs in conglomeration with the polyketide antibiotic can significantly reduce the bacterial load in epidermal and deep dermal layers in a mice model and quicken healing [
110,
111]. Hence, the combination of nanoparticles with conventional antibacterial mediators or dressings can more competently be used to repair infected wounds. Micro-cellulose reinforced with AgNPs behaves as an antimicrobial coating for open wounds and has shown high antibacterial performance against Gram-negative pathogens [
112]. Experimental evidence by Chakrabarti et al. also depicted that coated polyester-nylon dressings with AgNPs can prevent biofilm formation and bacterial colonization, while upholding a low toxicity profile [
112]. AgNPs form sulfur bonds with the bacterial plasmalemma proteins or bind to enzymatic thiol moieties, resulting in respiratory chain reactions and cell death [
111]. Furthermore, these nanoparticles can hinder DNA synthesis and curb bacterial multiplication in the wounds. Experimental observations by Lu et al. showed that the incorporation of silica into AgNPs could give rise to nontoxic mesoporous disulfide structures, which can very efficiently adhere to open wounds and have outstanding bacteriostatic activity [
109]. Ag-infused veneers warrant quicker wound healing and evade microbial colonization on the wound site, as observed in an in vivo canine model [
109]. Commercially available silver-impregnated dressings, Acticoat ™ nano-sized AgNPs (<15 nm size) are currently being explored for their reparative, anti-infective, and pain-lessening facets for early burn wounds and may be complaint in evading burn wound infections upon their amalgamation with silver sulphadiazine and chlorhexidine digluconate formulation [
113].
Gold nanoparticles (AuNPs) show potency in tissue rejuvenation, targeted drug delivery, and wound repair due to their extraordinary biocompatibility. AuNPs nanomaterials, because of their stabilizing properties, can be used as reinforcements with many other nanomaterials. Since neat gold particles do not exhibit a substantial activity, they need to be incorporated or combined with some matrix or therapeutic agent, a carrier, biomolecules, etc., for efficient antimicrobial activity. The cross-linking of AuNPs with collagen, chitosan, gelatin, and alginate, and their incorporation with various polysaccharides, growth factors, peptides, and cell adhesion proteins enables their attachment onto the gold nanoparticle surface without any alteration in the structural conformation of the biomolecule. These conglomerated moiety-modified AuNPs display excellent biocompatibility and biodegradability and are suited for healing. Similar to collagen, gelatin and chitosan can also easily be incorporated with AuNPs, showing safe and positive effects in enhancing wound healing [
109,
110]. Additionally, by modifying the surface plasmon resonance of AuNP, these exhibit thermo-responsive behavior, which is supported by in vitro and in vivo experimental data [
109]. The mechanism of action of AuNPs follows either targeting the cell wall or binding to DNA to stall the double-helical structure from unwinding during replication or transcription, therefore contributing to bactericidal and bacteriostatic activities. They can thus show multidrug resistance to
Staphylococcus aureus and
Pseudomonas aeruginosa. AuNPs are also potent antioxidants [
114]. Low concentrations of AuNPs are associated with keratinocyte growth and differentiation [
109]. Observations made by Marza et al., on basic fibroblast growth factor AuNP-impregnated petroleum jelly mixtures showed enhanced angiogenesis and fibroblast proliferation, which aided speedy wound recovery [
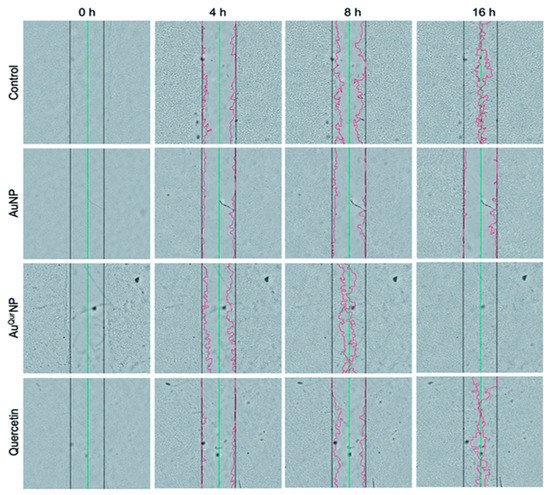
115]. The effect of colloidal AuNP coupled with quercetin (Au
QurNPs) on the fibroblast cell migration-assisted wound healing mechanism was depicted by Madhyastha et al. Au
QurNPs displayed enhanced cell proliferation and migration of keratinocytes, which was directed through the TGF-β1-dependent SMAD signaling pathway. This initial study on nanoceutical-engineered gold particles brings forth molecular and cellular evidence-based data to elevate the promising healing applications of Au
QurNPs in upcoming nanomedicine for skin etiology [
116] ().
Figure 3. In vitro wound assay of human keratinocyte cells treated with AuNP (5 μg l
−1), AuQurNP (5 μg l
−1), or pure quercetin (15 ng ml
−1) for different time periods (0, 4, 8, 16 h). Non-treated cells were used as control. Black, green, and red lines depict the start, end-point of cell migration, and migratory cell edge, respectively (magnification:10X). (reprinted with permission from reference [
116]. Reproduced with permission of The Royal Society of Chemistry).
Zinc-oxide nanoparticles (ZnONPs) exhibit potent antibacterial activity, and combined with hydrogel-based wound dressings [
22], can activate keratinocyte migration and improve re-epithelialization [
117] (). A recent study on the assessment of ZnONP-based chitosan hydrogel formulations presented high absorbency of wound exudates and aided hemostatic blood clotting and antibacterial effectivity simultaneously [
24]. ZnONPs and a collagen-based bioresorbable matrix with orange essential oil have been seen to substantially heal burn wounds, while also decreasing sepsis chances. This wound dressing was seen to augment angiogenesis, form new tissue, and exhibit biocompatibility and no cytotoxicity when evaluated in vitro and in vivo [
118]. Yet, their inherent toxicity makes them less used in wound healing therapies [
24]. ZnONP toxicity is dose-dependent, with higher doses it acts as a mitochondrial-dysfunctioning agent to release reactive oxygen species and block gene expression of superoxide dismutase and glutathione peroxidase in human keratinocytes, ultimately giving rise to oxidative stress and cell death. Creating core-shell nanocomposites by combining two metals, such as biogenic AuNPs with a thin coat of ZnO to form AuZnO core-shell nanocomposites, was assessed to evaluate the antibacterial and anti-biofilm efficacy against
Staphylococcus aureus and methicillin-resistant
Staphylococcus haemolyticus [
119]. ZnONPs also have good tissue adhesive properties, as exhibited in mice skin models [
109].
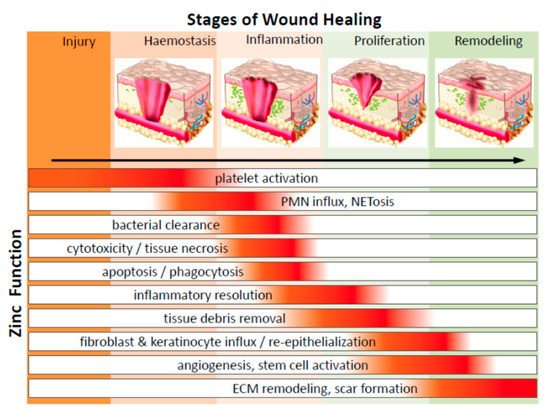
Figure 4. Diagrammatic representation of the significant function of zinc on cells in various phases of wound healing (PMN—polymorphonuclear leukocytes; NETosis—a novel form of programmed neutrophil death that resulted in the formation of neutrophil extracellular traps (NETs)) (adapted from Reference [
118] respectively, open access).
Nanoparticle-based composites: Renewable sources for nanoparticle synthesis and the development of its composites are gaining increased importance as they are cost-effective, consume less energy, and do not require additional sources for disposing of toxic by-products, unlike in nanoparticle synthesis via a chemical process. Several phytochemicals, viz., alkaloids, phenols, amino acids, proteins, etc., have been used to stabilize several nanoparticles like Ag ions in AgNPs.
Ocimum sanctum merged AgNPs embedded into a Carbopol gel base and attained ≈96% of wound contraction by the 14th post-wounding day, as revealed by Sood and coworkers [
120]. Additionally, they possessed activity against
Staphylococcus aureus and
Pseudomonas aeruginosa. Gelatin has in situ reductive properties to stabilize AgNPs, and the development of gelatin-chitosan-Ag porous composites with sizes 100–250µm that are biocompatible, biodegradable, and non-immunogenic, which upon cross-linking with tannic acid does exhibit therapeutic and antibacterial characteristics with low cytotoxicity [
121]. Shao et al. [
122] used
Barleria gibsoni leaf extracts to synthesize ZnONPs gel formulation that showed substantial effect against Gram-positive and Gram-negative infected burn wounds. Polymers such as chitosan can also serve as nanomaterial dressings or drug carriers due to their biocompatible polymeric networks to hold optimum moisture for a balanced wound environment. Chitosan, being cationic, attract most metals, proteins, and dyes to form complexes [
123], and its degradation products can activate ECM synthesis. Chitosan-assisted wound healing therapies include hydrogels, membranes, films, sponges, and scaffolds [
123]. Chitosan nanoparticles have immunomodulatory and nontoxic effects on human dermal cells, as revealed by Chen et al. in his assembled acellular porcine dermal matrix using a naturally-derived chitosan oligosaccharide [
124]. Additionally, the presence of the functional group aldehyde in AgNPs, formed during in situ reaction, confers the chitosan-AgNPs scaffolds with broad-spectrum antimicrobial properties against associated
Escherichia coli and
Staphylococcus aureus [
125]. A combination of a chitosan-poly (vinyl alcohol) (PVA) complex improved the antioxidant and antimicrobial efficacy compared to the polymer alone. It also conferred strong Gram-negative activity against
Klebsiella species and further enhanced in vivo wound repair by forming granulation tissue and re-epithelialization while demonstrating no cytotoxicity [
125]. An infrared-irradiation-triggered thermo-sensitive hydrogel-based drug delivery system loaded with ciprofloxacin was developed by Gao et al., triggered by near-infrared light stimulation. The mixture of polydopamine nanoparticles/glycol chitosan, being photothermally active, generated hyperthermia leading to bacterial cell leaching. Besides, polydopamine nanoparticles in combination with the drug ciprofloxacin exhibit a controlled released when stimulated with near-infrared light and showed minimal leakage under physiological conditions [
125]. Calreticulin (calcium-binding protein)-based AuNPs and chitosan/AuNP nanocomposites have been used for diabetic lesions. Calreticulins regulate the proper folding of proteins, and the nanocomposite endorses fibroblast-keratinocyte-endothelial cell growth, migration, division, and collagen formation without hindering cell proliferation [
126]. The biopolymer cellulose triggers repair via multiple local growth factors such as the epidermal growth factor and basic fibroblast growth factor [
127]. Nanocellulose dressings, due to their anti-infective properties and amplified tensile properties, have been explored as scaffolds [
24]. Bacterial cellulose mimics the skin structure with a high surface area per unit, increased biocompatibility, hydrophilicity, and no cytotoxicity. 3D porous networks of nanocellulose have high water retention capacity, ensuring a moist environment appropriate for healing [
24]. The wound healing potential of cellulose-ZnONPs composites was displayed by Khalid et al. [
127]. Bacterial nanocellulose derived from Gram-negative
Gluconacetobacter xylinus, and combined with silver nanoparticles, showed enhanced healing and reduced colonization of wound-associated
Staphylococcus aureus in vitro [
24].
Nanoparticle-embedded nano-scaffold systems: Nanoparticles in nano-scaffold systems for wound healing applications have escalated in the last few years. Nano-scaffolds, or better elaborated as nanofibrous scaffolds, are nano-systems premeditated to resemble the components of the cellular microenvironment or to reorientate cell behavior. The predicted tendency is to use biodegradable biomaterials, which help regenerate and repair damaged tissue [
128]. With advanced tissue engineering tools such as nano-architectonics approaches, in which materials are designed taking into account methods including organic synthesis, self-assembly/self-organization, molecular manipulation, and structural regulation upon stimuli, scaffolds are being tailor-made to have good biodegradability, mechanical properties, and ease in processing some polymers to better fit into different tissue engineering arenas. At present, only a few techniques can successfully produce nano-scaffolds, and their consequent nanomaterials, within the nanoscale frame [
129]. Electrospinning is among the few methods that results in uniform and stable morphological nanofibrous scaffolds [
130]. The amalgamation of bioactives by direct dissolution into functional polymer solutions helps progress healing at different phases. Further, nanopolymers like the dendrimers also show anti-inflammatory and antibacterial characteristics. Studies on a porcine model of superficial partial-thickness wounds displayed enhance healing potency of an electrospun polymer nanofiber dressing with the least risk of infection. Chitosan-poly-vinyl alcohol nanofibrous scaffolds upon application to rat diabetic wound models had improved healing rates compared to controls [
131]. An in vivo study in Wistar rats of a silver nanoparticle-spun nanofiber membrane exhibited numerous favorable effects of reduced cytotoxicity, broad-spectrum antibacterial action, abridged inflammation, and higher healing rates [
132]. Recombinant human epidermal growth factor, another nanocarrier, has been revealed to stimulate healing of full-thickness diabetic wounds. Nevertheless, their restricted use is due to the highly proteolytic environment they possess and the downregulation of associated growth factor receptors and signaling molecules in the case of chronic wounds [
133]. However, the results vary between experiments. Zhang et al. [
134] defined a hydrogel with Ca
2+ cross-linker as capable of releasing preloaded bFGF. Observations that both calcium and bFGF led to the growth and division of fibroblasts in the early re-epithelialization phases, persuading wound shrinkage on both in vitro and in vivo models. Nanofibrous mesh networks developed by electrospinning have been used for gene encapsulation in wound dressings. Gene-activated matrix therapy can simultaneously alter the expression of a target gene involved in regeneration and bridge the gap between tissue engineering and gene therapy. Wang et al. [
135] optimized a gene delivery system based on the antimicrobial peptide LL-37 embedded on ultra-small AuNPs, which increased the complete antibacterial action in the topical treatment of diabetic lesions. Furthermore, a LL37-AuNPs composite boosted cellular and nucleus diffusion, thus accomplishing high gene delivery efficacy. This system possessed biocompatibility, endorsed angiogenesis through the expression of VEGF expression, and improved re-epithelialization and granulation tissue formation [
136]. Nanoceria has scavenging activity due to the coexistence of two oxidation states (3+ and 4+) in the valence cerium atom. Hence, these nanoparticles may diminish oxidative stress and reinstate the balance between oxidants and antioxidant enzymes in diabetic lesions. A 100µg dose of cerium oxide nanoparticles-miR-146a combination enhanced diabetic wound healing without altering the wound tensile [
136]. Stem cell therapy, another feather in the cap of tissue engineering and regenerative medicine, represents another possible beneficiary of the nano scaffold technology due to their substantial stem cell migration and differentiation. These multifaceted nanomaterials with numerous enhancing properties represent advantages compared to standard treatment procedures adopted in clinical practice.