Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) is a protein that promotes transcription of numerous genes, particularly those responsible for the regulation of mitochondrial biogenesis. Evidence for a key role of PGC1α in bone metabolism is very recent. In vivo studies showed that PGC1α deletion negatively affects cortical thickness, trabecular organization and resistance to flexion, resulting in increased risk of fracture.
- mitochondria
- bone metabolism
- metabolic regulations
1. The Bone Phenotype of PGC1α Knock-Out Mice
Global deletion of PGC1α has a pronounced impact on bone phenotype, particularly in adulthood. PGC1α deficient mice aged 12 months showed a lower bone mass and strength than wild-type littermates [12]. PGC1α loss compromised long bones, especially the tibia, causing a reduction in cortical bone mass and strength [12]. While trabecular thickness (Tb. Th) decreased in PGC1α knock-out mice, trabecular number (Tb. N) increased compared to wild-type mice, thus enhancing anisotropy degree [12]. The anisotropy, notably in bone tissue, is a parameter also used in humans to detect the degree of material organization, thus defining the relationship between architectural structure and mechanical properties of bone [15]. Increased degree of anisotropy was observed in postmenopausal women with vertebral fracture compared with age-matched control cases, suggesting that fracture risk assessment can be improved after acquiring information related to the organization of trabecular bone architecture [16]. Moreover, PGC1α deficiency resulted in modification of the trabecular pattern and reduction of cortical thickness (Ct. Th) (Figure 1A). Furthermore, the deficiency also reduced resistance to flexion (~48.4%), implying PGC1α importance in preventing the risk of fracture [12]. Furthermore, the absence of PGC1α in vivo caused a reduction of the bone-matrix protein Osteocalcin (Ocn) (Figure 1B) [12], in accordance with a previous in vitro study, which showed that PGC1α contributed to the activity of osteoblasts, inducing together with Nurr1 the expression of Ocn [8]. Notably, Ocn promoter contains three Estrogen-related receptor alpha (ERRα) response elements, and ERRα was thought to cooperate with PGC1α to regulate gene expression involved in mitochondrial pathways and oxidative phosphorylation [17]. In addition, ERRα interacts with PGC1α to ameliorate the Ocn promoter functionality [9].
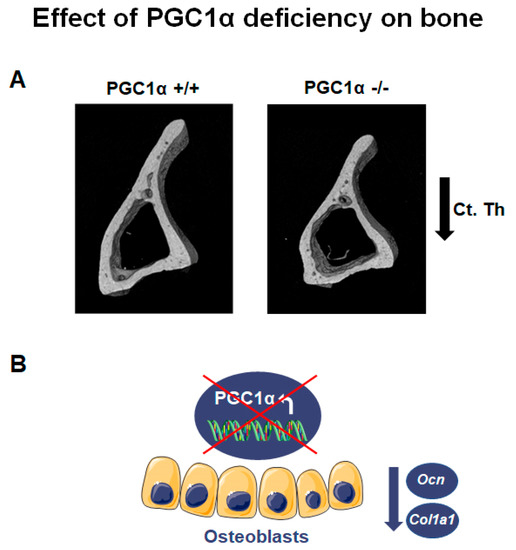
Figure 1. PGC1α deletion affects bone. (A) Representative images of micro-CT-generated sections of the tibia midshaft of PGC1α+/+ and PGC1α−/− mice show a reduction in cortical thickness (Ct. Th) in the absence of PGC1α. Adapted from [12]. (B) Schematic representation of the effect of PGC1α deletion in osteoblasts consisting of the reduction of Ocn and Col1a1 levels.
Moreover, bone marrow precursors of PGC1α deficient mice expressed a lower mRNA level of collagen type I α 1 (Col1a1), the most abundant bone matrix protein [18], than wild-type mice (Figure 1B) [12]. Consistently, bone marrow cells from PGC1α knock-out mice cultured ex vivo displayed a delayed differentiation of osteoblasts [12]. Interestingly, osteoclasts from PGC1α deficient mice, differentiated from pure monocyte cultures, also showed a delay in the differentiation process [12]. In contrast, when osteoclasts from PGC1α null mice were differentiated from a culture of whole bone marrow, an increased formation of multinucleated osteoclasts was observed [12]. This result suggested that the elevated Receptor activator of nuclear factor-kappa-Β ligand (RANKL) levels observed in the bone marrow of knock-out mice could be the indirect mechanism through which osteoblasts increase osteoclast formation and activity in vivo.
Moreover, in agreement with Lin and colleagues’ study of 2004 [19], PGC1α knock-out mice had 30% lower weight than control mice, lower ratio of inguinal white adipose tissue (iWAT)/body weight and strong decrease (~75%) in adipocyte area [12]. Colaianni and colleagues also evaluated, in iWAT, the uncoupling protein 1 (Ucp1) expression, considering its importance as a master gene involved in the trans-differentiation program from white adipocytes to adipocytes with a brown adipose tissue (BAT)-like phenotype [20]. PGC1α deficiency negatively affected Ucp1 expression also in iWAT and not only in the interscapular brown fat, as previously shown [12,21].
Although the overall results of this study highlight for the first time that PGC1α plays a critical role in the regulation of bone mass, one limitation may be that the characterization of the bone phenotype may have been masked by other secondary systemic effects due to whole-body PGC1α deletion. Similarly, it is plausible that the 30% reduction in body weight in PGC1α knock-out mice affected the mechanical loading on their skeleton. Therefore, the generation of conditional PGC1α knock-out models, with a specific deletion in osteoblasts or osteoclasts, will be required to provide a further understanding of the contribution of this transcription factor to bone metabolism.
2. PGC1α/β Role in Modulating Osteoblast and Osteocyte Gene Expression
Ding and colleagues, in 2017, published a study on the effect of PGC1α overexpression on Sirtuin 3 (SIRT3) knockdown in a murine osteoblast cell line (MC3T3-E1) [10]. The Sirtuins (SIRTs), which are characterized by a sirtuin core domain, are the family of NAD+-dependent deacetylase proteins that regulate numerous cellular processes including proliferation, apoptosis, autophagy, and DNA repair [22]. Among the members of this family of proteins, SIRT3-5, expressed in mitochondria, influence the metabolic activity of these organelles. In particular, SIRT3 acts by deacetylating many proteins and regulating mitochondrial biogenesis and reactive oxygen species homeostasis. Of note, SIRT3 is involved in the control of ATP production in mitochondria by acting on the respiratory chain, suggesting a key role of SIRT3 as a crucial mediator for cellular energy production [22].
SIRT3 exhibits deacetylase activity and affects the regulation of many proteins with a key role in osteoblastic differentiation, maintaining bone homeostasis [22]. SIRT3 knockdown negatively affected alkaline phosphatase (ALP) activity and expression of the major gene involved in osteoblastic differentiation, Runt-related transcription factor 2 (Runx2), Col1α1 and Ocn [10]. Moreover, in differentiated MC3T3-E1, SIRT3 knockdown inhibited mitochondrial function, evaluated by Complex I, II, III, IV, and V activity measurements, oxygen consumption and mitochondrial membrane potential level [10]. In addition, the expression of two key factors of mitochondrial biogenesis, Nrf1 and Tfam, was negatively affected by the absence of SIRT3 [10]. Of note, mitochondrial size increased, and mitochondrial density decreased by SIRT3 deletion [10]. This study also demonstrated that SIRT3 knockdown reduced the expression, at both mRNA and protein levels, of superoxide dismutase 2 (SOD2), an efficiently mitochondrial molecule with antioxidant activity that converts superoxide to the less reactive hydrogen peroxide (H2O2) [10,23]. Overexpression of SOD2 markedly reverted reduction of oxygen consumption, ALP staining and Runx2, Col1α1, and Ocn mRNA level [10]. These findings indicated a key role of SOD2 in SIRT3 knockdown-induced inhibition of osteogenic differentiation and mitochondrial activity [10].
PGC1α overexpression restored the reduction of mitochondrial density, mitochondrial membrane potential, Nrf1 and Tfam mRNA expression and ALP activity [10]. Moreover, PGC1α overexpression inverted the increase of mitochondrial size, highlighting a key role of PGC1α in SIRT3 activity on osteoblastic differentiation [10]. These findings were relevant to the most recent evidence confirming that the SIRT3-PGC1α-SOD2 interaction is the central pathway used by SIRT3 to regulate bone homeostasis [24].
Unlike SIRT3, SIRT4, and SIRT5, which are localized in the mitochondria, SIRT1, SIRT6, SIRT7 are localized predominantly in the nucleus. Specifically, SIRT1 deacetylates histones H3, H4, and H1, and modifies nonhistone proteins, such as the transcription factors p53, nuclear factor-κB (NF-κB), and the members of the class O of forkhead box transcription factors (FoxOs) [25]. The effects of SIRT1 on the skeleton have been extensively studied, and results obtained in mouse models have shown that SIRT1 increases trabecular bone mass by stimulating Wnt signaling in osteoblasts and osteocytes. During differentiation of these bone cells, SIRT1 deacetylates FoxOs by preventing FoxO association with β-catenin and potentiates Wnt signaling [25].
In a recent study, the role of PGC1α/β and its activators 5’ adenosine monophosphate-activated protein kinase (AMPK) and SIRT1 in osteocyte differentiation and reprogramming was investigated [11]. Preosteocytic cells (IDG-SW3), differentiated for 14 days in the presence of glucose, and femur-derived bone organotypic cultures, maintained in glucose media, were treated with 5-Aminoimidazole-4-carboxamide ribonucleotide (AICAR) and SRT2104, two chemical factors activating AMPK and SIRT1 pathway, respectively [11]. AMPK activation via AICAR treatment upregulated Runx2 and Osterix in IDG-SW3 cells and the osteocyte genes Dentin matrix acidic phosphoprotein 1 (Dmp1), Fibroblast growth factor 23 (Fgf23), and Sclerostin (Sost) in both IDG-SW3 cells and bone organotypic cultures [11]. In parallel, treatment with SRT2104 activating SIRT1 stimulated the expression of late osteocyte markers. All together, these results suggested that activation of AMPK/SIRT1 plays a key role in osteocyte differentiation [11].
To evaluate PGC1α/β role in modulating osteoblast and osteocyte gene expression, retroviral pMSCV-PGC1α was used for PGC1α overexpression in primary osteoblasts and IDG-SW3 cells [11]. PGC1α/β deletion was performed using retroviral pMSCV-puro-Cre-ERT2, pMSCV-puro, and pMSCV-GFP virus in primary osteoblasts and primary osteocytes derived from control mice [11]. Real-time quantitative polymerase chain reaction (qRT-PCR) analysis showed that PGC1α overexpression upregulated many key factors involved in osteoblast and osteocyte differentiation both in IDG-SW3 cells and primary osteoblasts, while PGC1α/β deletion strongly caused their reduction [11]. Moreover, micro-computed tomography analysis (μCT) of femurs from 8 week-old mice with specific deletion of Ppargc1α/β in osteoblasts (Ppargc1α/βf/f;Col1a1-Cre) showed a reduction of both cortical and trabecular parameters compared to control mice [11]. PGC1α/β deletion in osteoblasts decreased cortical bone volume (BV), bone area (B.Ar) and Ct. Th, while bone perimeter (B.Pm) was not affected [11]. In addition, BV/total volume (TV), Tb. N and Tb. Th were lower in the absence of PGC1α/β, while trabecular space increased [11].
Although a limitation of this study was that Pgc1α/β was deleted in both osteoblasts and osteocytes, and therefore the relative contribution of each transcription factors in the two bone cell types could not be deciphered, the overall results suggested a central role of PGC1s in bone metabolism and osteoblast and osteocyte differentiation.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22094670
This entry is offline, you can click here to edit this entry!
