2.1. Magnetic Nanoparticles (MNPs)
In recent years, magnetic nanoparticles (MNPs) have received considerable attention among researchers due to their wide use in the preparation of recyclable catalysts. These nanoparticles can be directly used as catalysts or as magnetic carriers for the catalytic species immobilized on their surface. Utilizing magnetic nanoparticles as catalysts or supports helps to combine the advantages of both nano-sized particles and magnetic materials. The large surface-to-volume ratio of these types of materials allows excellent loading of the homogeneous catalyst and then obtaining a magnetic catalyst with high catalytic activity.
Magnetic nanoparticles (MNPs) usually exhibit a phenomenon known as superparamagnetism. Superparamagnetism is a form of magnetism that takes place when the size of ferromagnetic or ferrimagnetic nanoparticles is as low as 10–20 nm [
41]. As a consequence of an externally applied magnetic field, superparamagnetic and paramagnetic materials become magnetized up to their saturation magnetization. As soon as the field goes to zero, any residual magnetic interaction disappears. In comparison with paramagnetic materials, superparamagnetic nanoparticles have a higher magnetic susceptibility, rendering them highly sensitive to externally applied magnetic fields [
42].
If nanoparticles are sufficiently small, each of them can be considered as characterized by a single magnetic domain able to act as a “single super spin”. Unlike ferromagnetic materials, in the absence of the magnetic field, these nanoparticles randomly change the orientation of their spins even at room temperature. Instead, the ordered magnetic moments of ferromagnetic materials become disordered in response to thermal fluctuations only above the Curie Temperature (CT), or Curie point, losing their permanent magnetism.
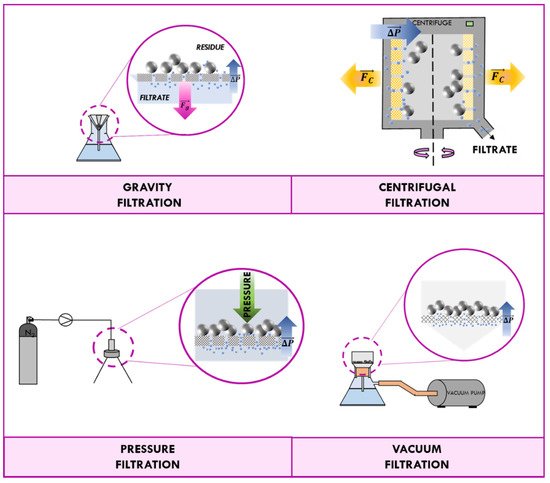
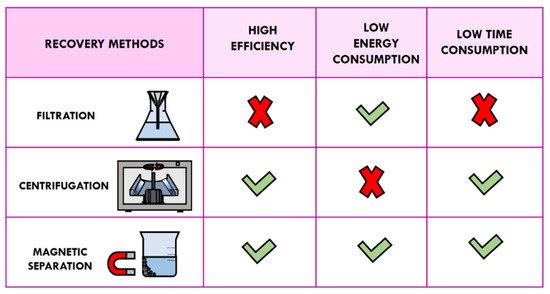
The magnetic component of MNPs provides a fast separation from the liquid phase by application of an external magnetic field and an easy redispersion in the absence of an applied magnetic field, avoiding in this way the conventional filtration, centrifugation, or other tedious workup processes. Most magnetic-supported catalysts can be reused to several runs almost keeping their initial activity [
43].
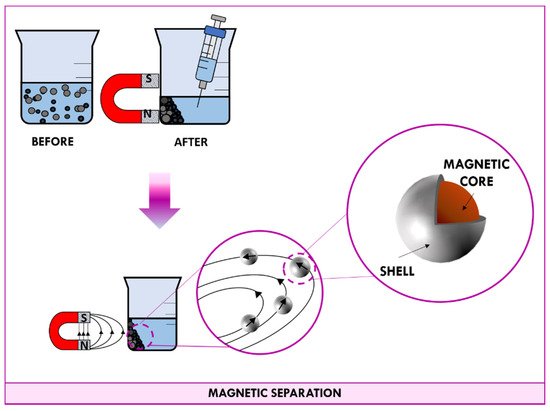
When a permanent magnet is placed in contact with a beaker containing a suspension of magnetic nanomaterial, magnetic dipoles of nanoparticles point in the same direction of the field lines. As shown in , the applied magnetic field induces nanoparticles to migrate towards the magnet. After a few minutes, all particles end up concentrated at the wall in correspondence with the magnet; in this way, the supernatant can be easily aspirated with a syringe. When the magnet is removed, the random orientation of nanoparticle is so fast that the measured magnetic moment gets down to zero quickly and nanoparticles behave as no-magnetic materials.
Figure 5. Schematic representation of the magnetic separation.
Properties such as large surface area, relatively low preparation cost and toxicity, good stability, coercivity (the field required to bring the magnetism to zero), low Curie temperature [
44], and rapid recovery make them ideal supports in the field of catalysis. Thanks to these particular properties, these materials are also applied in a variety of fields such as biotechnology/biomedicine [
45], magnetic resonance imaging (MRI) [
46], controlled drug delivery [
46], biosensors [
44], hyperthermia [
47], gene therapy [
48], gas sensor [
49], data storage [
50], adsorbent materials [
51], batteries [
52], pigments [
53], water purification [
54], tissue repair [
55], detoxification of biological fluids [
56], and labeling and separation of cells [
57].
The most common MNPs are pure metal nanoparticles (Fe, Co, Ni), metal alloys (FePt, CoPt), iron oxides (FeO, Fe
2O
3, Fe
3O
4), or ferrites MFe
2O
4 (M = Co, Mn, Cu, Zn) [
24]. These materials can be easily modified and functionalized without altering their magnetic properties. Appropriate surface changes are required to improve the dispersion, the catalytic activity, and the physicochemical and mechanical properties of MNPs, and make them biocompatible [
58].
For example, cobalt (0) nanoparticles supported on ceria were used as catalyst in the hydrolysis of ammonia borane for hydrogen production. The presence of cerium(III) defects improved the catalytic activity of metal nanoparticles through a more favorable substrate–metal interaction [
59]. After completion of reaction, the catalyst was isolated by a permanent magnet and then redispersed for a subsequent run of hydrolysis retaining its initial catalytic activity even after the fifth use [
60].
Among the magnetic nanoparticles, iron oxides are widespread used in the field of MNPs catalysis owing to their optical, electrical, magnetic, and catalytic properties [
61]. Up to now, 16 pure phases of iron oxides, in the form of oxides, hydroxides, and oxyhydroxides have been found [
62]. Particularly, magnetite (Fe
3O
4), an important type of ferrite, is highly regarded and applied due to their low cost, availability, nontoxicity, and easy functionalization with other metallic species or organocatalysts [
38]. Fe
3O
4 is characterized by strong superparamagnetic and electron properties due to the presence of iron cations in both capacities (Fe
2+, Fe
3+) [
62]. Their superparamagnetic behavior makes magnetite NPs possible to use such as the magnetic core to an eventual core–shell structure. Adding Fe
3O
4 into catalysts endows the catalysts with excellent magnetic properties and improves even further the catalytic performances [
38].
2.3. Magnetically Recoverable Orthoferrites of Rare-Earth Nanoparticles
The rare earths (REEs) are a class of seventeen transition metal elements, including 15 lanthanides, scandium (Sc), and yttrium (Y), widely applied in various fields such as metallurgy, electronic devices, high-performance alloys, glass, ceramics, and the phosphorous industries. They are also used to make permanent magnets (PMs) [
66,
67]. The variable valence states and electronic structures of rare earth ions allow their use for several catalytic applications. For example, same rare earth elements are applied in catalysis as dopants or components to modulate the specific physical and chemical properties of different materials [
68], cerium-based materials act as catalysts in various reactions such as CO
2 hydrogenation [
10], Fischer–Tropsch synthesis [
69], and CO
2 methanation [
9,
11,
13,
70] due to redox properties and excellent oxygen mobility.
Orthoferrites of rare-earth elements (REE) with the formula RFeO
3 (R = Sc, Y, La-Lu) are a novel class of acid-base catalysts of great interest for researchers due to their high activity and the possibility of magnetic recovery. They are applied mainly in redox and photoinduced processes [
40]. For example, K.D Martinson and his research group successfully synthetized a phase-pure porous magnetic nanopowder based on holmium orthoferrites by glycine-nitrate combustion approach for the first time. The porous holmium orthoferrite has been tested for n-hexane conversion at 500 °C and 1 atm. The observed catalytic activity can be attributed to high concentration of acid-base sites on its surface. After reaction, the spent sample was separated by an external magnetic field of a permanent magnet from the reaction followed by heat treatment at 600 °C for 1 h, with an efficiency of 97% [
40].
2.5. Ionic Liquid Coated Magnetic Nanoparticles
Recently, Ionic liquids (ILs)-coated MNPs have attracted growing interest as catalysts for a wide range of new industrial applications. Ionic liquids (ILs) are commonly defined as salts composed of organic cations and organic or inorganic anions with melting point below the boiling point of water. According to the nature of the cation and/or the counter anion moiety, the ionic liquid may behave as an acidic, basic, or organ catalyst [
73]. Many of these ILs have been investigated in a variety of organic transformations in which ILs can act as solvents and/or catalysts [
74]. They have a number of advantages such as wide liquid range and potential window, extremely low vapor pressure, high ionic conductivity, and extraordinary ability to functionalization [
75]. However, their industrial applications are hampered by their high viscosity [
71], high cost, large consumption, and homogeneousness (difficulty to separate) [
76]. To overcome these drawbacks, supported ionic liquid catalysts (SIL) could be a valid alternative to homogeneous analogues since they maintain the excellent catalytic properties of ionic liquids, but also manifest the advantages of easy separation and recovery typical of the heterogeneous catalyst. On the basis of this awareness, different support materials, such as amorphous or ordered mesoporous silica, zeolites, polystyrene, magnetic nanoparticles, and many others have been used as carrier for loading ionic liquids [
77]. Particularly, ionic liquids immobilized on the magnetic nanoparticles surface, have emerged as good catalysts because of their facile separation by an external magnet.
Cano et al. synthesised a new, highly active, and efficiently recoverable catalytic system modifying nano-magnetite materials with a paramagnetic halometallate-based ionic liquid (MILs), Fe
3O
4@SiO
2@(mim)[FeCl
4] (mim: methylimidazolium). This system was applied in the glycolysis of PET into BHET under conventional heating. The catalyst exhibited an excellent recycling behaviour, achieving nearly 100% yield and selectivity over twelve consecutive reaction cycles at 180 °C and was easily recovered with an external magnetic field avoiding tedious work-up or purification processes. Further analyses by ICP revealed that the amount of catalyst lost after each cycle was minimal and the amount of Fe in the purified product was negligible [
76].
Ren et al. synthesized an alkaline ionic liquid immobilized on protective copolymers coated magnetic nanoparticles ([Im][OH]/P(VBC-DVB) @MNPs). The catalyst consisted of three layers: a magnetic core, an intermediate protective shell, and an outer catalytic layer. The magnetic core, mainly composed of CoFe
2O
4 magnetic nanoparticles (CFNPs), makes easier separation of the catalyst from reaction medium. The protective layer, formed by the copolymer of divinylbenzene (DVB) and vinyl benzyl chloride (VBC), incorporated into the catalyst, on the other hand, prevents the corrosion of magnetic nanoparticle when the catalyst is used in polar solvents, thus preserving recyclability and improving its stability. The catalytic layer, which consisted of 1-propyl-3-alkylimidazole hydroxide ionic liquid, is where the reaction takes part. This catalyst showed an excellent catalytic activity for Knoevenagel condensation between benzaldehyde and ethyl cyanoacetate due to the large amount of OH
− ions loading on the surface and a lower activation energy than of homogeneous catalyst NaOH. The catalyst was conveniently recovered upon applying an external magnetic field, and it was recycled at least six times without significant changes [
23]. However, since the loading of ILs on the surface cannot be too high, catalytic activity turns out to be limited owing to the relatively low number of catalytic units compared to the large surface area of magnetic nanoparticles [
71]. To improve the catalytic activity, it is necessary to increase the amount of catalyst used for the catalytic process, and this could require a greater use of solvents as well as expensive recovery cost and possible toxicological concern [
23]. This problem could be solved by the surface modification of the magnetic nanoparticles with different polymer grafting methods [
78]. The introduction of polymer onto the MNPs increases the loading content of grafted homogenous material since more catalytic units can be attached onto magnetic support [
78]. On the other hand, the polymer layer could prevent the formation of clusters in high temperature processes [
79]. Arghan et al. developed a novel poly (ionic liquid) (PIL) coated magnetic nanoparticles catalyst, noted as n-Fe
3O
4/PVAm-SO
3H, and used it as a magnetic heterogeneous acid catalyst for the one-pot synthesis of tri and tetrasubstituted imidazole derivatives under optimized condition in excellent yields. The catalyst was synthesized by directly grafting polyvinyl amine layer onto surface of Fe
3O
4 without using any bridged organosilane compounds. In the following, the sulfonation treatment was carried out by covalent grafting of chlorosulfonic acid on amine groups to convert n-Fe
3O
4/PVAm into an efficient acid catalyst. In addition, the resulted catalyst was readily separated in a green way by using a magnetic force and reused eight times without any significant deterioration in catalytic activity. The study results show that this new acid magnetic catalyst exhibits more advantages than soluble acids [
78].
Nowadays, dicationic ionic liquids (DILs), a new subclass of ILs, have attracted significant attention. Typically, these molecules contain two cationic head groups connected together with a rigid or flexible spacer, associated with two counter anions, respectively [
75]. Modifying the length of the chain and the type of spacer or the cation, their physicochemical properties can be tuned [
71]. In comparison to various reported monocationic ionic liquid in the literature, dicationic ionic liquids exhibit several interesting advantages such as higher thermal stability, surface activity, more extensive liquid range, and higher selectivity [
75]. These ILs can be used in many applications from the “classical” use as solvents, catalysts, or catalytic supports in organic reactions, to more specific uses as high temperature lubricants/heat transfer fluids, including a variety of roles in analytical sciences [
71].
Fahimeh Rezaei and co-workers designed Brønsted acidic dicationic ionic liquid immobilized on silica-coated iron oxide support with core–shell structure and applied it as a recyclable nanocatalyst under solvent-free conditions in the synthesis of pyrazole derivatives [
80]. The catalyst has maintained his catalytic activity at least five times. In addition, Reyhaneh Karimi-Chayjani demonstrated that magnetic γFe
2O
3@SiO
2 NPs-supported Bis[(3-aminopropyl) triethoxysilane] dichloride bis-dicationic ionic liquid (DIL) catalyst can catalyse the Knoevenagel condensation achieving high reaction rates and yields. The synthesized nanomagnetic catalyst possesses a high superparamagnetic characteristic and can be simply recovered and reused applying an external magnetic field at least six times without loss of catalytic activity [
44].
Metal-organic frameworks (MOFs), also known as porous coordination polymers (PCPs), are a class of porous crystalline materials, highly investigated in the last twenty years. Several features of MOFs such as controllable composition, large surface areas, regular and accessible pores, and crystalline open structures allows them to encapsulate within their frameworks magnetic nanoparticles, making them promising heterogeneous catalysts or catalytic carriers of various species including ionic liquids [
81].
For example, very recently, Xie et al. developed an efficient and magnetically recyclable acidic catalyst based on the amino-functionalized metal-organic framework structures, for the biodisel production via simultaneous transesterification-esterifications of low-quality oil feedstock. To prepare the acidic catalyst with Brönsted–Lewis acid sites, the POM-ILs with sulfonated organic cations and Keggin POM counter anions were immobilized on the CoFe
2O
4/MIL-88B(Fe)-NH
2 support combining in this way the advantages of ILs and heterogeneous character of MOFs. The catalyst showed a good stability, probably, due to the charge interaction of acidic groups of POM-based sulfonated ILs with amino groups of the magnetic support. On the other hand, the encapsulation of magnetic nanoparticles (CoFe
2O
4) into cavities of the metal organic framework nanocomposites gives magnetic properties to catalysts, improving its separation performances. In order to investigate the reusability of the solid catalyst, its recovery was conveniently carried out by using a permanent magnet. The catalyst was reused for five transesterification runs under the optimized reaction conditions without significant decay of the catalytic activity [
82].