Sudden cardiac death (SCD) is the leading cause of cardiovascular mortality in patients with coronary artery disease without severe systolic dysfunction and in heart failure with preserved ejection fraction. From a global health perspective, while risk may be lower, the absolute number of SCDs in patients with left ventricle ejection fraction >35% is higher than in those with severely reduced left ventricle ejection fraction (defined as ≤35%). Despite these observations and the high amount of available data, to date there are no clear recommendations to reduce the sudden cardiac death burden in the population with mid-range or preserved left ventricle ejection fraction. Ongoing improvements in risk stratification based on electrophysiological and imaging techniques point towards a more precise identification of patients who would benefit from ICD implantation, which is still an unmet need in this subset of patients.
- sudden cardiac death
- preserved ejection fraction
- ICD
- sudden cardiac arrest
1. Introduction
Sudden cardiac death (SCD) is an event of presumed cardiac origin occurring suddenly and unexpectedly in an otherwise stable patient [1]. From a healthcare perspective, with a rate as high as 183,000 deaths per year, SCD represents a major social issue [2]. Despite several improvements in the treatment of cardiovascular diseases, SCD still accounts for 2.04 million or 40–50% of the potential years of life lost [2][3]. Among men, death rate from SCD (76 per 100,000) exceeds all other individual causes of death including lung cancer, accident, chronic lower respiratory disease, cerebrovascular disease, diabetes mellitus, prostate cancer and colorectal cancer [3].
The implantable cardioverter defibrillator (ICD) represented a turning point in the prevention of SCD, with several landmark trials demonstrating its efficacy in selected populations [4][5]. Building on these studies, ICD is currently recommended to reduce the risk of death in patients with severely reduced (≤35%) left ventricle ejection fraction (LVEF) (primary prevention) and in cardiac arrest survivors (secondary prevention) [6][7]. Notably, while patients with LVEF ≤ 35% are at the highest absolute risk of death, more than 70% of SCD in patients with coronary artery disease (CAD) occur in patients with LVEF >35%, leaving most subjects at risk largely uncaptured by an LVEF-centered risk stratification [8]. Furthermore, SCD is the most common cause of cardiovascular death in patients with CAD without severe systolic dysfunction and in patients with heart failure with preserved ejection fraction (HFpEF [9]). While absolute risk may be low in the LVEF > 35% population, the large number of patients at risk and the devastating consequences of SCD pose an intriguing clinical challenge.
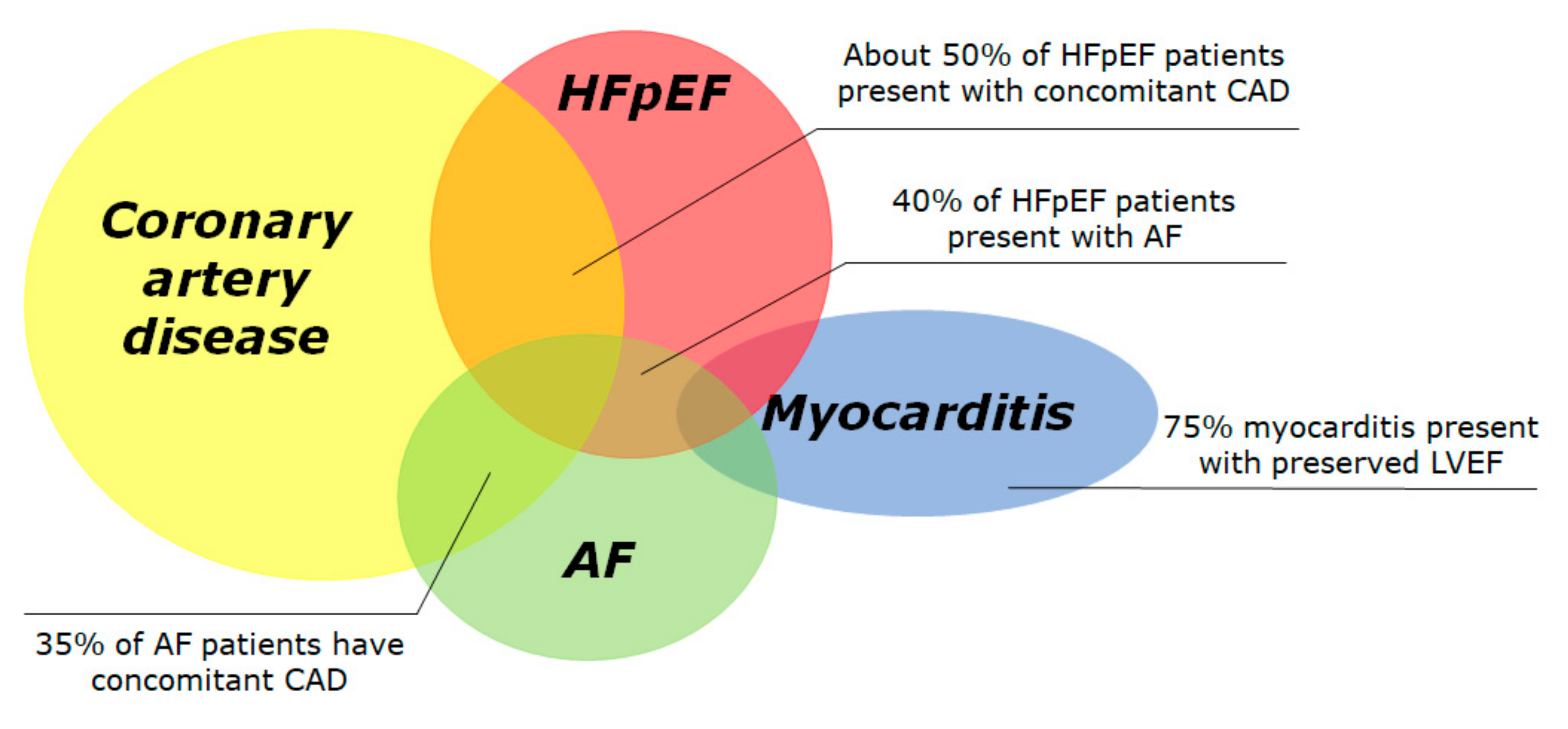
In the following sections, we will review the current approach to risk stratification of SCD in the population with LVEF > 35%, in the four most common subsets: CAD, HFpEF, atrial fibrillation (AF) and myocarditis (Figure 1). In the real world, significant overlap exists between these conditions, since 50% of HFpEF patients present with concomitant CAD [10][11], 40% of HFpEF patients present with AF [12] and 35% of AF patients have concomitant CAD [13]. We reported about currently published studies and clinical trials, selected after a systematic research on PubMed including the keywords “sudden cardiac death” and “preserved” or “mid-range”.
Other conditions at high risk of SCD with preserved ejection fraction including idiopathic dilated cardiomyopathy, hypertrophic cardiomyopathy (HCM), arrhythmogenic right ventricular cardiomyopathy (ARVC), channelopathies and valvular diseases.
2. Emerging Risk Factors: Genetics, Biomarkers and Obstructive Sleep Apnea
3. Conclusions
Risk stratification in patients with pLVEF, although relevant for the prevention of SCD, is still hampered by several difficulties. A light in the shade is provided by the PRESERVE EF study [36], which proposed a fascinating two-step algorithm for patients with ICM and pLVEF. However, before applying the model in current clinical practice, we have to weigh the possible advantages against the increased numbers of invasive procedure. Based on 2005–2014 ARIC study [37], 720,000 acute MI per year are expected in the US, with 77% of them in presence of LVEF > 35%, accounting for 554,400 MI patients per year with pLVEF. Clinical application of the PRESERVE EF algorithm would lead to EPS in 194,040 patients per year, with 52,390 of them inducible for malignant arrhythmia. Given that 22% of implanted patients in PRESERVE EF had a major arrhythmic event, 11,525 patients per year in the US would receive an appropriate treatment from the ICD; if applied in clinical practice, this would lead to a further 35% increase to the 150,000 ICDs annually implanted in the United States [38]. Additionally, among considered risk factors, only NSVT and late potentials were more frequent in inducible patients; no events occurred in patients with an LVEF >50%. Further analysis should evaluate if limitation of EPS only to patients with LVEF ≤50% and the use of weighted risk scores instead of the mere presence of prespecified risk factors could provide effective stratification without increasing the number of EPS and ICD implants.
Effective risk stratification in patients with HFpEF is hampered by the substrate heterogeneity and future studies including big data from well-characterized population might improve our understanding.
This entry is adapted from the peer-reviewed paper 10.3390/jcm10091823
References
- Solomon, S.D.; Zelenkofske, S.; McMurray, J.J.; Finn, P.V.; Velazquez, E.; Ertl, G.; Harsanyi, A.; Rouleau, J.L.; Maggioni, A.; Kober, L.; et al. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N. Engl. J. Med. 2005, 352, 2581–2588.
- Albert, C.M.; Stevenson, W.G. The Future of Arrhythmias and Electrophysiology. Circulation 2016, 133, 2687–2696.
- Stecker, E.C.; Reinier, K.; Marijon, E.; Narayanan, K.; Teodorescu, C.; Uy-Evanado, A.; Gunson, K.; Jui, J.; Chugh, S.S. Public health burden of sudden cardiac death in the United States. Circ. Arrhythm. Electrophysiol. 2014, 7, 212–217.
- Moss, A.J.; Hall, W.J.; Cannom, D.S.; Daubert, J.P.; Higgins, S.L.; Klein, H.; Levine, J.H.; Saksena, S.; Waldo, A.L.; Wilber, D.; et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N. Engl. J. Med. 1996, 335, 1933–1940.
- Bardy, G.H.; Lee, K.L.; Mark, D.B.; Poole, J.E.; Packer, D.L.; Boineau, R.; Domanski, M.; Troutman, C.; Anderson, J.; Johnson, G.; et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N. Engl. J. Med. 2005, 352, 225–237.
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm. Society. Circulation 2018, 138, e210–e271.
- Priori, S.G.; Blomstrom-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2015, 36, 2793–2867.
- Chatterjee, N.A.; Moorthy, M.V.; Pester, J.; Schaecter, A.; Panicker, G.K.; Narula, D.; Lee, D.C.; Goldberger, J.J.; Kadish, A.; Cook, N.R.; et al. Sudden Death in Patients with Coronary Heart Disease Without Severe Systolic Dysfunction. JAMA Cardiol. 2018, 3, 591–600.
- Vaduganathan, M.; Patel, R.B.; Michel, A.; Shah, S.J.; Senni, M.; Gheorghiade, M.; Butler, J. Mode of Death in Heart Failure with Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2017, 69, 556–569.
- Zile, M.R.; Gaasch, W.H.; Anand, I.S.; Haass, M.; Little, W.C.; Miller, A.B.; Lopez-Sendon, J.; Teerlink, J.R.; White, M.; McMurray, J.J.; et al. Mode of death in patients with heart failure and a preserved ejection fraction: Results from the Irbesartan in Heart Failure with Preserved Ejection Fraction Study (I-Preserve) trial. Circulation 2010, 121, 1393–1405.
- Lam, C.S.; Donal, E.; Kraigher-Krainer, E.; Vasan, R.S. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2011, 13, 18–28.
- Pellicori, P.; Urbinati, A.; Kaur, K.; Zhang, J.; Shah, P.; Kazmi, S.; Capucci, A.; Cleland, J.G.F.; Clark, A.L. Prevalence and Incidence of Atrial Fibrillation in Ambulatory Patients with Heart Failure. Am. J. Cardiol. 2019, 124, 1554–1560.
- Kralev, S.; Schneider, K.; Lang, S.; Suselbeck, T.; Borggrefe, M. Incidence and severity of coronary artery disease in patients with atrial fibrillation undergoing first-time coronary angiography. PLoS ONE 2011, 6, e24964.
- Ackerman, M.J.; Priori, S.G.; Willems, S.; Berul, C.; Brugada, R.; Calkins, H.; Camm, A.J.; Ellinor, P.T.; Gollob, M.; Hamilton, R.; et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies: This document was developed as a partnership between the Heart Rhythm. Society (HRS) and the European Heart Rhythm. Association (EHRA). Europace 2011, 13, 1077–1109.
- Mellor, G.; Laksman, Z.W.M.; Tadros, R.; Roberts, J.D.; Gerull, B.; Simpson, C.S.; Klein, G.J.; Champagne, J.; Talajic, M.; Gardner, M.; et al. Genetic Testing in the Evaluation of Unexplained Cardiac Arrest: From the CASPER (Cardiac Arrest Survivors With Preserved Ejection Fraction Registry). Circ. Cardiovasc. Genet 2017, 10.
- Havmoller, R.; Chugh, S.S. Plasma biomarkers for prediction of sudden cardiac death: Another piece of the risk stratification puzzle? Circ. Arrhythm. Electrophysiol. 2012, 5, 237–243.
- Albert, C.M.; Ma, J.; Rifai, N.; Stampfer, M.J.; Ridker, P.M. Prospective study of C-reactive protein, homocysteine, and plasma lipid levels as predictors of sudden cardiac death. Circulation 2002, 105, 2595–2599.
- Empana, J.P.; Jouven, X.; Canoui-Poitrine, F.; Luc, G.; Tafflet, M.; Haas, B.; Arveiler, D.; Ferrieres, J.; Ruidavets, J.B.; Montaye, M.; et al. C-reactive protein, interleukin 6, fibrinogen and risk of sudden death in European middle-aged men: The PRIME study. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2047–2052.
- Kucharska-Newton, A.M.; Couper, D.J.; Pankow, J.S.; Prineas, R.J.; Rea, T.D.; Sotoodehnia, N.; Chakravarti, A.; Folsom, A.R.; Siscovick, D.S.; Rosamond, W.D. Hemostasis, inflammation, and fatal and nonfatal coronary heart disease: Long-term follow-up of the atherosclerosis risk in communities (ARIC) cohort. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 2182–2190.
- Berger, R.; Huelsman, M.; Strecker, K.; Bojic, A.; Moser, P.; Stanek, B.; Pacher, R. B-type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation 2002, 105, 2392–2397.
- Peacock, J.M.; Ohira, T.; Post, W.; Sotoodehnia, N.; Rosamond, W.; Folsom, A.R. Serum magnesium and risk of sudden cardiac death in the Atherosclerosis Risk in Communities (ARIC) Study. Am. Heart J. 2010, 160, 464–470.
- Deo, R.; Sotoodehnia, N.; Katz, R.; Sarnak, M.J.; Fried, L.F.; Chonchol, M.; Kestenbaum, B.; Psaty, B.M.; Siscovick, D.S.; Shlipak, M.G. Cystatin C and sudden cardiac death risk in the elderly. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 159–164.
- Cappuccio, F.P.; Cooper, D.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Sleep duration predicts cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. Eur. Heart J. 2011, 32, 1484–1492.
- Kwok, C.S.; Kontopantelis, E.; Kuligowski, G.; Gray, M.; Muhyaldeen, A.; Gale, C.P.; Peat, G.M.; Cleator, J.; Chew-Graham, C.; Loke, Y.K.; et al. Self-Reported Sleep Duration and Quality and Cardiovascular Disease and Mortality: A Dose-Response Meta-Analysis. J. Am. Heart Assoc. 2018, 7, e008552.
- Gottlieb, D.J.; Yenokyan, G.; Newman, A.B.; O’Connor, G.T.; Punjabi, N.M.; Quan, S.F.; Redline, S.; Resnick, H.E.; Tong, E.K.; Diener-West, M.; et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: The sleep heart health study. Circulation 2010, 122, 352–360.
- Garvey, J.F.; Pengo, M.F.; Drakatos, P.; Kent, B.D. Epidemiological aspects of obstructive sleep apnea. J. Thorac. Dis. 2015, 7, 920–929.
- Deo, R.; Albert, C.M. Epidemiology and genetics of sudden cardiac death. Circulation 2012, 125, 620–637.
- Morand, J.; Arnaud, C.; Pepin, J.L.; Godin-Ribuot, D. Chronic intermittent hypoxia promotes myocardial ischemia-related ventricular arrhythmias and sudden cardiac death. Sci. Rep. 2018, 8, 2997.
- Chadda, K.R.; Fazmin, I.T.; Ahmad, S.; Valli, H.; Edling, C.E.; Huang, C.L.; Jeevaratnam, K. Arrhythmogenic mechanisms of obstructive sleep apnea in heart failure patients. Sleep 2018, 41.
- Nakamura, T.; Chin, K.; Hosokawa, R.; Takahashi, K.; Sumi, K.; Ohi, M.; Mishima, M. Corrected QT dispersion and cardiac sympathetic function in patients with obstructive sleep apnea-hypopnea syndrome. Chest 2004, 125, 2107–2114.
- Raghuram, A.; Clay, R.; Kumbam, A.; Tereshchenko, L.G.; Khan, A. A systematic review of the association between obstructive sleep apnea and ventricular arrhythmias. J. Clin. Sleep Med. 2014, 10, 1155–1160.
- Koehler, U.; Fus, E.; Grimm, W.; Pankow, W.; Schafer, H.; Stammnitz, A.; Peter, J.H. Heart block in patients with obstructive sleep apnoea: Pathogenetic factors and effects of treatment. Eur. Respir. J. 1998, 11, 434–439.
- Mehra, R.; Benjamin, E.J.; Shahar, E.; Gottlieb, D.J.; Nawabit, R.; Kirchner, H.L.; Sahadevan, J.; Redline, S.; Sleep Heart Health, S. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am. J. Respir. Crit. Care Med. 2006, 173, 910–916.
- Schlatzer, C.; Bratton, D.J.; Craig, S.E.; Kohler, M.; Stradling, J.R. ECG risk markers for atrial fibrillation and sudden cardiac death in minimally symptomatic obstructive sleep apnoea: The MOSAIC randomised trial. BMJ Open 2016, 6, e010150.
- Wang, X.; Zhang, Y.; Dong, Z.; Fan, J.; Nie, S.; Wei, Y. Effect of continuous positive airway pressure on long-term cardiovascular outcomes in patients with coronary artery disease and obstructive sleep apnea: A systematic review and meta-analysis. Respir. Res. 2018, 19, 61.
- Bulluck, H.; Dharmakumar, R.; Arai, A.E.; Berry, C.; Hausenloy, D.J. Cardiovascular Magnetic Resonance in Acute ST-Segment-Elevation Myocardial Infarction: Recent Advances, Controversies, and Future Directions. Circulation 2018, 137, 1949–1964.
- Aro, A.L.; Reinier, K.; Rusinaru, C.; Uy-Evanado, A.; Darouian, N.; Phan, D.; Mack, W.J.; Jui, J.; Soliman, E.Z.; Tereshchenko, L.G.; et al. Electrical risk score beyond the left ventricular ejection fraction: Prediction of sudden cardiac death in the Oregon Sudden Unexpected Death Study and the Atherosclerosis Risk in Communities Study. Eur. Heart J. 2017, 38, 3017–3025.
- Kremers, M.S.; Hammill, S.C.; Berul, C.I.; Koutras, C.; Curtis, J.S.; Wang, Y.; Beachy, J.; Blum Meisnere, L.; Conyers del, M.; Reynolds, M.R.; et al. The National ICD Registry Report: Version 2.1 including leads and pediatrics for years 2010 and 2011. Heart Rhythm. 2013, 10, e59–e65.
