“Immuno-PET” merges the high target selectivity and specificity of antibodies and engineered fragments toward a given tumor cell surface marker with the high spatial resolution, sensitivity, and quantitative capabilities of positron emission tomography (PET) imaging techniques. In this review, we detail and provide examples of the clinical limitations of current imaging techniques for diagnosing PDAC.
- PDAC
- pancreatic cancer
- diagnostic imaging
- immuno-PET
1. Introduction
Despite multiple diagnostic and therapeutic advances, pancreatic ductal adenocarcinoma (PDAC) presents a high mortality rate, representing the fourth cause of cancer death in developing countries [1][2]. This lethality can be associated with a late diagnosis, caused by the absence of symptoms at an early stage of the disease. Most cases of PDAC are located in the head of the pancreas (70%), followed in frequency by the uncinate process (18.66%), body (10–20%), and tail (5–10%) [3][4]. At present, complete surgical resection is the only potentially curative treatment for these tumors. However, only the initial stages benefit from surgery, representing only 10–15% of patients [5][6][7]. In only 10% of cases, the lesion is limited to the pancreatic gland and surrounded by normal pancreatic tissue [5][8]. At the time of diagnosis, 40–50% of cases present distant metastases, and approximately 40% of patients present signs of locally advanced disease; therefore, surgery in these cases is not indicated.
Several imaging techniques for PDAC diagnosis are available, including computed tomography (CT), magnetic resonance imaging (MRI), or endoscopic ultrasound (EUS) [9][10]. While they are widely used in the clinic and are very useful for the diagnosis of PDAC, they present several limitations.
Unlike other neoplastic processes (breast, colon, prostate…) there are no effective diagnostic screening methods for PDAC. Furthermore, due to the absolute low risk of developing this disease, population screening is not indicated. Only, in those groups [11] considered to be at-risk population, monitoring by pancreatic MRI or Cholangio-MRI, and EUS is indicated to detect small precursor lesions, such as cystic neoplasms. In these cases, CT would provide a suboptimal degree of lesion detection, compared to EUS and MRI, besides being a source of radiation [11]. Additionally, the probability of detecting lesions using these techniques is low, no more than 20% [12][13].
The development of “omics” has identified potentially relevant alterations in PDAC that still need to be integrated into the clinical management of PDAC patients. This is due, in part, to the deficiency of non-invasive imaging biomarkers [14]. “Immunotargeted imaging” represents a novel, innovative, and attractive option that combines the target specificity and selectivity of antibodies, and their variants, toward a biomarker with given imaging technique capabilities.
2. Novel Non-Invasive Immunotargeted Imaging Methods for PDAC
The revolution in cancer genomics has uncovered clinically relevant alterations that have yet to be integrated into patients’ clinical management, in part due to the lack of non-invasive imaging biomarkers [14]. An innovative and attractive option is termed “immunotargeted imaging”. This approach combines the target selectivity and specificity of antibodies and engineered fragments toward a given tumor cell surface marker with the capabilities of a given imaging technique.
2.1. Immunotargeted Imaging Features
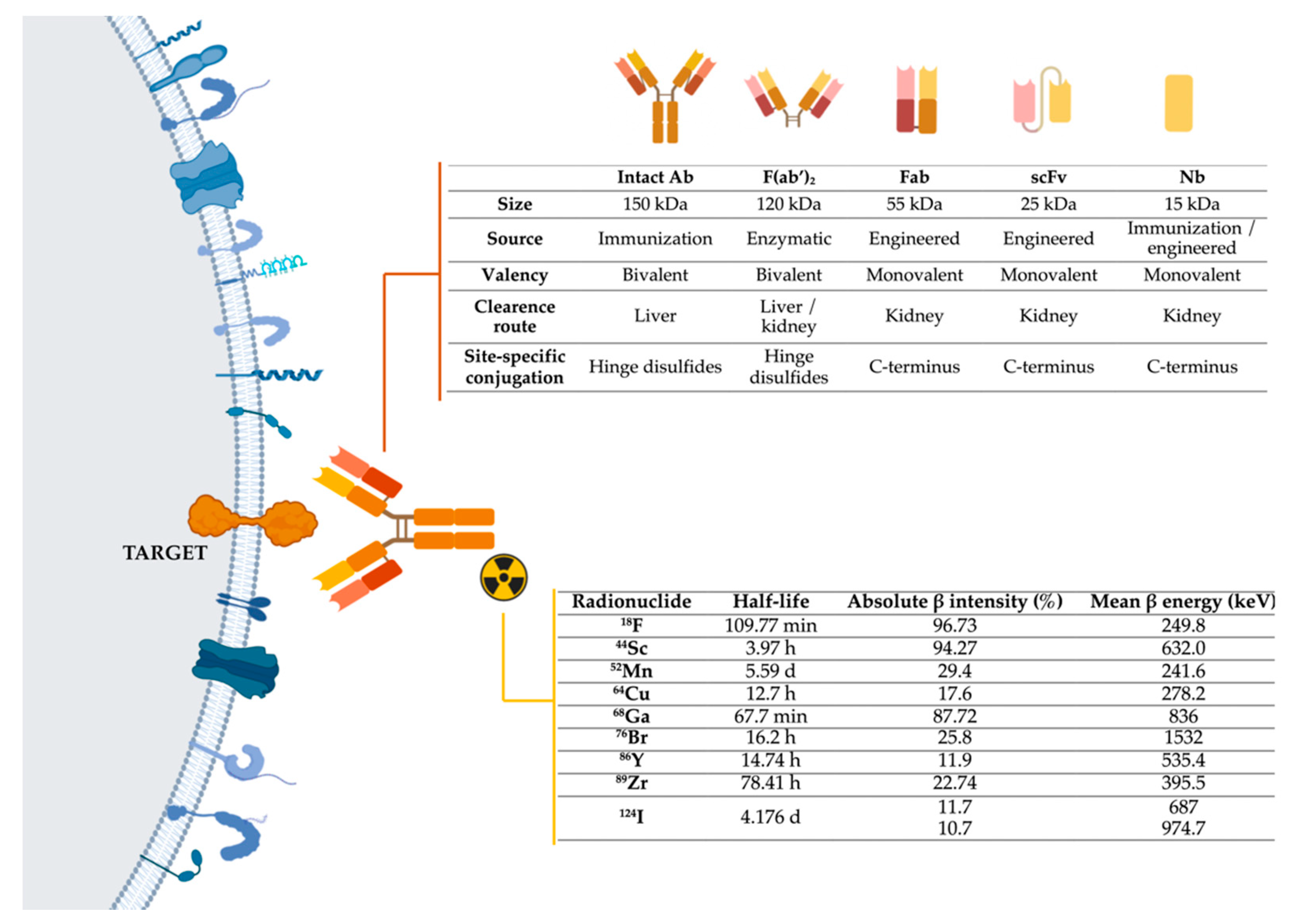
To develop immunotargeted imaging, three features must be taken into account (Figure 1): I)Selection of a specific molecular target for imaging; II) Selection of the optimally engineered antibodies for imaging applications; III) Selection of a suitable radionuclide modality-specific imaging agent. For immunoPET. it is important to match the physical half-life of the positron-emitting radionuclide with the biological half-life of the antibody or fragment being used.
Figure 1. Representation of the three main components of immuno-PET techniques: target, antibodies, and radionuclides. Abbreviations: Ab-Antibody; Fab-Fragment antigen-binding; F(ab’)2-Fab dimer; scFv- single-chain variable fragment; Nb-Nanobody, 18F-fluorine; 44Sc-scandium; 52Mn-manganese; 64Cu-copper; 68Ga-gallium; 76Br-bromine; 86Y-yttrium; 89Zr-zirconium; 124I-iodine [15][16]. Image generated with BioRender.
As shown in Table 1, membrane proteins that are overexpressed on tumor or tumor-associated cells have been potentially suitable for tumor-targeted imaging; other components of the tumor microenvironment, such as extracellular matrix proteins, have also been promising candidates for the development of diagnostic approaches in PDAC.
Table 1. Immuno-PET applications in PDAC.
| PET Imaging Probes | Conjugation Strategy | Targets | Hallmark | Models | References |
|---|---|---|---|---|---|
| [64Cu]Cu-DOTA-anti-PD-1 [64Cu]Cu-NOTA-anti-PD-1 [64Cu]Cu-NOTA-anti-PD-L1 |
Lysine-based random | PD-1/PD-L1 | Imaging of immune checkpoints | Orthotopic KRAS murine PDAC | [17] |
| [89Zr]Zr-Df-10D7 (anti-CDCP1 mAb) |
Lysine-based random | CUB Domain-Containing Protein 1 (CDCP1) | CDCP1 regulates migration, invasion, and extracellular matrix degradation | Patient-derived subcutaneous and orthotopic xenografts (PDX) mice | [18] |
| [64Cu]Cu-PCTA-cetuximab | Lysine-based random | Epidermal Growth Factor Receptor (EGFR) | EGFR is overexpressed in a wide variety of cancers | Resectable orthotopic xenograft mouse model with human PC XPA-1 cells | [19] |
| [89Zr]Zr-Df-MVT-2163 (human HuMab-5B1 Ab) |
Lysine-based random | CA19-9 (Sialyl Lewis A) | CA19-9 is the most commonly used serum tumor marker for PDAC | Patients with primary PDAC and metastases (Phase 1) |
[20][21] |
| [64Cu]Cu-NOTA-NJB2 (nanobody) |
Sortase-Mediated Radiolabeling | Alternatively spliced EIIIB (EDB) domain of fibronectin tumor extracellular matrix and neovasculature | Fibronectin is a glycoprotein that forms a major constituent of tumor extracellular matrix and neovasculature | (K-rasLSL.G12D/+; p53R172H/+; PdxCre) KPC mouse models of PDAC | [22] |
| [89Zr]Zr-Df-LEM2/15 (anti-MM1-MMP mAb) |
Lysine-based random | MT1-MMP | Metalloprotease MT1-MMP is overexpressed in many tumors and associates with tumor growth, invasion, metastasis, and poor prognosis | Subcutaneous xenograft mouse model with Capan-2 cells, and subcutaneous and orthotopic PDX mice. | [23] |
| [89Zr]Zr-Df-MEHD7945A (duligotuzumab) |
Lysine-based random | EGFR and Receptor tyrosine-proteinase kinase erbB-3 (HER3) | EGFR and HER3 are highly expressed in PDAC, marking this aggressive disease with poor survival rates | Subcutaneous xenograft mouse model with AsPC-1 cells | [23] |
| [124I]-A2cDb (anti-PSCA 2B3 A2 cys-diabody) [124I]-A11 Mb (anti-PSCA minibody) |
Direct iodination | Prostate stem cell antigen (PSCA) | PSCA is also overexpressed in pancreatic carcinoma | Subcutaneous PDX mice | [24] |
| [64Cu]Cu-NOTA-3B4 (single chain Fv) |
Lysine-based random | Receptor for advanced glycation end products (RAGE) | RAGE is overexpressed in human pancreatic tumors; it is a critical promoter in the transition of premalignant epithelial precursors (PanIN) to PDAC | Balb c/nude mice bearing Panc02 tumors. No PET study, only ex vivo biodistribution. | [25] |
| [89Zr]Zr-Df-ALT-836 (anti-human TF mAb) |
Lysine-based random | Tissue factor (TF) | Overexpression of TF in pancreatic cancer has been correlated with high tumor grade, the primary disease’s extent, and local and distant metastatic invasion. | Subcutaneous xenograft mouse model with BxPC-3 or PANC-1 cells | [26] |
| [64Cu]Cu-NOTA-heterodimer-ZW800 (bispecific immunoconjugate of CD105 and TF Fab′ antibody fragments) |
Lysine-based random | Endoglobin (CD105) and TF | CD105 is a cell surface glycoprotein expressed on endothelial cells, and its overexpression in cancer has been linked to angiogenesis, metastasis, and cancer progression | Subcutaneous xenograft mouse model with BxPC-3 or PANC-1 cells | [27] |
| [89Zr]Zr-Df-5B1 (anti-CA19.9 mAb) |
Lysine-based random | CA19-9 | CA19-9 is the most commonly used serum tumor marker for PDAC | Orthotopic xenograft mouse model with CAPAN-2 cells | [28] |
| [89Zr]Zr-Df-1A2G11 (anti-IGF-1R mAb) |
Lysine-based random | Insulin-like growth factor-1 receptor (IGF-1R) | IGF-1R is a transmembrane receptor of the tyrosine kinase class involved in cell growth, apoptosis, and tumor invasion in cancer | Subcutaneous xenograft mouse model with MIA PaCa-2 or BxPC-3 cells | [28][29] |
| [64Cu]Cu-DOTA-MAb159 (anti-GRP78 mAb) |
Lysine-based random | Glucose-regulated protein (GRP78) | Cell-surface GRP78 expression, an immuno-globulin heavy-chain binding protein, has been detected in pancreatic cancer. | Subcutaneous xenograft mouse model with BxPC-3 cells | [30] |
| [64Cu]Cu-DOTA-11-25 (anti-Mesothelin mAb) |
Lysine-based random | Mesothelin (MSLN) | MSLN is a cell differentiation-associated glycoprotein, overexpressed in various cancers, including PDAC | Subcutaneous xenograft mouse model with CFPAC-1 or BxPC-3 cells | [31] |
| [89Zr]Zr-Df-TSP-A01 (anti-transferrin receptor mAb) |
Lysine-based random | Transferrin receptor (TfR) | TfR is upregulated on the cell surface of many cancer types, including pancreatic cancer | Subcutaneous xenograft mouse model with MIA PaCa-2 cells | [32] |
| [89Zr]Zr-Df-059-053 (human anti-CD147 mAb) |
Lysine-based random | CD147 | CD147 (so-called EMMPRIN) is a transmembrane protein of the immunoglobulin superfamily and is expressed in many types of tumors, including PDAC | Subcutaneous xenograft mouse model with MIA PaCa-2 cells | [33] |
| [64Cu]Cu-NOTA-panitumumab-F(ab′)2 | Lysine-based random | EGFR | EGFR is overexpressed in a wide variety of cancers | Subcutaneous xenograft mouse model with PANC-1 cells, and subcutaneous and orthotopic PDX OCIP23 mice | [34] |
| [89Zr]Zr-Df-5B1 (anti-CA19.9 mAb) |
Lysine-based random | CA19-9 | CA19-9 is the most commonly used serum tumor marker for PDAC | Subcutaneous xenograft mouse model with BxPC3 cells | [35] |
| [124I]-A2cDb (anti-CA19.9 diabody) |
Direct iodination | CA19-9 | CA19-9 is the most commonly used serum tumor marker for PDAC | Subcutaneous xenograft mouse model with BxPC3 or CAPAN-2 cells | [36] |
| [64Cu]Cu-NOTA-ALT-836 (anti-human TF mAb) |
Lysine-based random | Tissue factor (TF) | Overexpression of TF in pancreatic cancer has been correlated with high tumor grade, the primary disease’s extent, and local and distant metastatic invasion. | Subcutaneous xenograft mouse model with BxPC-3, PANC-1, or ASPC-1 cells | [37] |
| [64Cu]Cu-DOTA-2A3 (2A3 is an anti-CEACAM6 nanobody) [64Cu]Cu-DOTA-2A3-mFc (2A3 fused with a murine Fc fragment) [64Cu]Cu-DOTA-9A6 (anti-CEACAM6 murine mAb) |
Lysine-based random | Carcinoembryonic antigen-related cell adhesion molecule 6 (CEACAM-6) | CEACAM-6 is a cell surface glycoprotein known to be highly expressed in most cancers | Subcutaneous xenograft mouse model with BxPC3 cells | [38] |
| [124I]-H310A (anti-CEA scFv-Fc) |
Direct iodination | Carcinoembryonic antigen (CEA) | CEA is a GPI-linked glycoprotein overexpressed in gastrointestinal epithelial tumors, including PDAC | Subcutaneous xenograft mouse model with BxPC-3, CAPAN-1, or HPAF-II cells | [37] |
Antibody-based PET imaging probes for PDAC ordered by the most recent publication date. Bioconjugation strategy has been categorized into three methods: lysine-based random, site-specific via sortase-mediated reaction, and direct iodination. Antibody-based PET imaging probes reaching clinical trials are highlighted in bold.
With the rise of immunotherapy in recent years, PET imaging of immune checkpoint inhibitors (ICIs) may serve as a robust biomarker to predict and monitor responses to ICIs, complementing the existing immunohistochemical techniques [39][40]; it has been described that PET imaging using antibodies against the programmed cell death receptor 1 (PD-1)/programmed cell death ligand 1 (PD-L1) pathway can be a useful method for evaluating PD-L1 expression in orthotopic pancreatic cancer models [41]. To date, most of the PET imaging probes have been designed to target PDAC tumors in preclinical models (Figure 2), and only one study has been conducted with an [89Zr]Zr-labeled human monoclonal antibody in patients with pancreatic cancer or other CA19-9 positive malignancies [42].
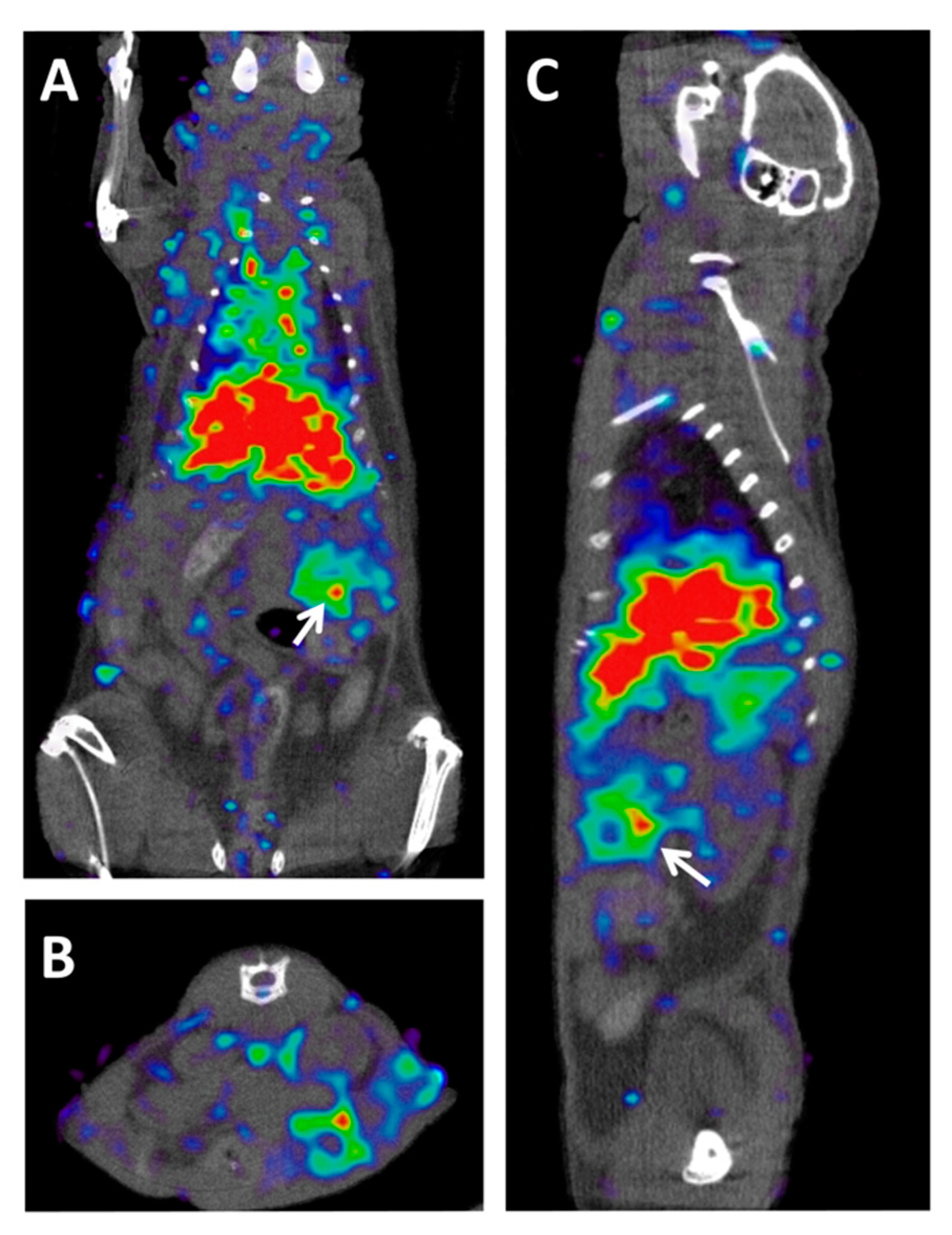
Figure 2. ImmunoPET–CT of MT1-MMP metalloproteinase in a preclinical model of PDAC. (A) Coronal, (B) axial, and (C) sagittal views of fused Immuno-PET and CT images of an orthotopic pancreatic patient-derived xenograft mouse. White arrows indicate tumor location. The imaging probe used was [89Zr]Zr-DFO-LEM2/15, a mAb developed against the MT1-MMP metalloproteinase [23]. Owing to the central role that this metalloproteinase plays in collagen-induced gemcitabine resistance, this probe could be used for the early prediction of resistance to gemcitabine in metastatic PDAC patients.
This entry is adapted from the peer-reviewed paper 10.3390/jcm10061151
References
- Gómez, M.C.; Sabater, L.; Ferrández, A. Protocolo detallado, estudio e informe anatomopatológico de las piezas de duodenopancreatectomía cefálica por carcinoma de páncreas. Rev. Esp. Patol. 2010, 43, 207–214.
- Altekruse, S.F.; Kosary, C.L.; Krapcho, M.; Neyman, N.; Aminou, R.; Waldron, W.; Ruhl, J.; Howlader, N.; Tatalovich, Z.; Cho, H.; et al. SEER Cancer Statistics Review, 1975–2007. Natl. Cancer Instit. 2010. Available online: (accessed on 23 December 2020).
- de la Santa, L.G. Radiology of pancreatic neoplasms: An update. World J. Gastrointest. Oncol. 2014, 6, 330.
- McIntyre, C.A.; Winter, J.M. Diagnostic evaluation and staging of pancreatic ductal adenocarcinoma. Semin. Oncol. 2015, 42, 19–27.
- Cassinotto, C.; Cortade, J.; Belleannée, G.; Lapuyade, B.; Terrebonne, E.; Vendrely, V.; Laurent, C.; Sa-Cunha, A. An evaluation of the accuracy of CT when determining resectability of pancreatic head adenocarcinoma after neoadjuvant treatment. Eur. J. Radiol. 2013, 82, 589–593.
- van Veldhuisen, E.; van den Oord, C.; Brada, L.J.; Walma, M.S.; Vogel, J.A.; Wilmink, J.W.; Del Chiaro, M.; van Lienden, K.P.; Meijerink, M.R.; van Tienhoven, G.; et al. Locally advanced pancreatic cancer: Work-up, staging, and local intervention strategies. Cancers 2019, 11, 976.
- Varadhachary, G.R. Preoperative therapies for resectable and borderline resectable pancreatic cancer. J. Gastrointest. Oncol. 2011, 2, 136–13642.
- Mazzeo, S.; Cappelli, C.; Battaglia, V.; Caramella, D.; Caproni, G.; Contillo, B.P.; Del Chiaro, M.; Boggi, U.; Funel, N.; Pollina, L.; et al. Multidetector CT in the evaluation of retroperitoneal fat tissue infiltration in ductal adenocarcinoma of the pancreatic head: Correlation with histopathological findings. Abd. Imaging 2010, 35, 465–470.
- Toft, J.; Hadden, W.J.; Laurence, J.M.; Lam, V.; Yuen, L.; Janssen, A.; Pleass, H. Imaging modalities in the diagnosis of pancreatic adenocarcinoma: A systematic review and meta-analysis of sensitivity, specificity and diagnostic accuracy. Eur. J. Radiol. 2017, 92, 17–23.
- Elbanna, K.Y.; Jang, H.J.; Kim, T.K. Imaging diagnosis and staging of pancreatic ductal adenocarcinoma: A comprehensive review. Insights Imaging 2020, 11.
- Canto, M.I.; Harinck, F.; Hruban, R.H.; Offerhaus, G.J.; Poley, J.W.; Kamel, I.; Nio, Y.; Schulick, R.S.; Bassi, C.; Kluijt, I.; et al. International cancer of the pancreas screening (CAPS) consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 2013, 62, 339–347.
- Zhang, L.; Sanagapalli, S.; Stoita, A. Challenges in diagnosis of pancreatic cancer. World J. Gastrointest. 2018, 24, 2047–2060.
- Montejo Gañán, I.; Ángel Ríos, L.F.; Sarría Octavio de Toledo, L.; Martínez Mombila, M.E.; Ros Mendoza, L.H. Staging pancreatic carcinoma by computed tomography. Radiologia 2018, 60, 85–89.
- Pandit-Taskar, N.; Postow, M.A.; Hellmann, M.D.; Harding, J.J.; Barker, C.A.; O’Donoghue, J.A.; Ziolkowska, M.; Ruan, S.; Lyashchenko, S.K.; Tsai, F.; et al. First-in-Humans Imaging with 89Zr-Df-IAB22M2C Anti-CD8 Minibody in Patients with Solid Malignancies: Preliminary Pharmacokinetics, Biodistribution, and Lesion Targeting. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2020, 61, 512–519.
- Kerdjoudj, R.; Pniok, M.; Alliot, C.; Kubíček, V.; Havlíčková, J.; Rösch, F.; Hermann, P.; Huclier-Markai, S. Scandium(III) complexes of monophosphorus acid DOTA analogues: A thermodynamic and radiolabelling study with 44Sc from cyclotron and from a 44Ti/44Sc generator. Dalton Trans. 2016, 45, 1398–1409.
- Romero, E.; Martínez, A.; Oteo, M.; Ibañez, M.; Santos, M.; Morcillo, M.Á. Development and long-term evaluation of a new 68Ge/68Ga generator based on nano-SnO2 for PET imaging. Sci. Rep. 2020, 10, 1–10.
- Zhao, J.; Wen, X.; Li, T.; Shi, S.; Xiong, C.; Wang, Y.A.; Li, C. Concurrent Injection of Unlabeled Antibodies Allows Positron Emission Tomography Imaging of Programmed Cell Death Ligand 1 Expression in an Orthotopic Pancreatic Tumor Model. ACS Omega 2020, 5, 8474–8482.
- Kryza, T.; Khan, T.; Puttick, S.; Li, C.; Sokolowski, K.A.; Tse, B.W.C.; Cuda, T.; Lyons, N.; Gough, M.; Yin, J.; et al. Effective targeting of intact and proteolysed CDCP1 for imaging and treatment of pancreatic ductal adenocarcinoma. Theranostics 2020, 10, 4116–4133.
- Yoshii, Y.; Tashima, H.; Iwao, Y.; Yoshida, E.; Wakizaka, H.; Akamatsu, G.; Yamaya, T.; Matsumoto, H.; Yoshimoto, M.; Igarashi, C.; et al. Immuno-OpenPET: A novel approach for early diagnosis and image-guided surgery for small resectable pancreatic cancer. Sci. Rep. 2020, 10, 1–11.
- O’Reilly, E.M.; Borazanci, E.H.; Yu, K.H.; Varghese, A.M.; Estrella, H.; Kamins, D.; Melink, T.; Dorr, K.; Maffuid, P.; Gutheil, J.; et al. HuMab-5B1 (MVT-5873), a mAb targeting sLea, in combination with first-line gemcitabine plus nab-paclitaxel (gem/nab-P) for patients with pancreatic cancer (PDAC) and other CA19-9 positive malignancies. J. Clin. Oncol. 2018, 36, 16235.
- Lohrmann, C.; O’Reilly, E.M.; O’Donogue, J.A.; Pandit-Taskar, N.; Carrasquillo, J.A.; Lyashchenki, S.K.; Ruan, S.; Teng, R.; Scholz, W.; Maffuid, P.W.; et al. Retooling a Blood-Based Biomarker: Phase I Assessment of the High-Affinity CA19-9 Antibody HuMab-5B1 for Immuno-PET Imaging of Pancreatic Cancer. Clin. Cancer Res. 2019, 25, 7014–7023.
- Jailkhani, N.; Ingram, J.R.; Rashidian, M.; Rickelt, S.; Tian, C.; Mak, H.; Jiang, Z.; Ploegh, H.L.; Hynes, R.O. Noninvasive imaging of tumor progression, metastasis, and fibrosis using a nanobody targeting the extracellular matrix. Proc. Natl. Acad. Sci. USA 2019, 116, 14181–14190, Erratum in Proc. Natl. Acad. Sci. USA 2019, 116, 18745, doi:10.1073/pnas.1913962116.
- Morcillo, M.Á.; De Lucas, Á.G.; Oteo, M.; Romero, E.; Magro, N.; Ibáñez, M.; Martínez, A.; Garaulet, G.; Arroyo, A.G.; López-Casas, P.P.; et al. MT1-MMP as a PET Imaging Biomarker for Pancreas Cancer Management. Contrast Media Mol. Imaging 2018, 2018.
- Zettlitz, K.A.; Tsai, W.T.K.; Knowles, S.M.; Kobayashi, N.; Donahue, T.R.; Reiter, R.E.; Wu, A.M. Dual-modality immuno-PET and near-infrared fluorescence imaging of pancreatic cancer using an anti-prostate stem cell antigen cys-diabody. J. Nucl. Med. 2018, 59, 1398–1405.
- Kim, H.Y.; Wang, X.; Kang, R.; Tang, D.; Boone, B.A.; Zeh, H.J.; Lotze, M.T.; Barry Edwards, W. RAGE-specific single chain Fv for PET imaging of pancreatic cancer. PLoS ONE 2018, 13, e0192821.
- Hernandez, R.; England, C.G.; Yang, Y.; Valdovinos, H.F.; Liu, B.; Wong, H.C.; Barnhart, T.E.; Cai, W. ImmunoPET imaging of tissue factor expression in pancreatic cancer with 89Zr-Df-ALT-836. J. Control. Release 2017, 264, 160–168.
- Luo, H.; England, C.G.; Goel, S.; Graves, S.A.; Ai, F.; Liu, B.; Theuer, C.P.; Wong, H.C.; Nickles, R.J.; Cai, W. ImmunoPET and Near-Infrared Fluorescence Imaging of Pancreatic Cancer with a Dual-Labeled Bispecific Antibody Fragment. Mol. Pharm. 2017, 14, 1646–1655.
- Houghton, J.L.; Abdel-Atti, D.; Scholz, W.W.; Lewis, J.S. Preloading with Unlabeled CA19.9 Targeted Human Monoclonal Antibody Leads to Improved PET Imaging with 89Zr-5B1. Mol. Pharm. 2017, 14, 908–915.
- England, C.G.; Kamkaew, A.; Im, H.J.; Valdovinos, H.F.; Sun, H.; Hernandez, R.; Cho, S.Y.; Dunphy, E.J.; Lee, D.S.; Barnhart, T.E.; et al. ImmunoPET Imaging of Insulin-Like Growth Factor 1 Receptor in a Subcutaneous Mouse Model of Pancreatic Cancer. Mol. Pharm. 2016, 13, 1958–1966.
- Wang, H.; Li, D.; Liu, S.; Liu, R.; Yuan, H.; Krasnoperov, V.; Shan, H.; Conti, P.S.; Gill, P.S.; Li, Z. Small-animal PET imaging of pancreatic cancer xenografts using a 64Cu-labeled monoclonal antibody, MAb159. J. Nucl. Med. 2015, 56, 908–913.
- Kobayashi, K.; Sasaki, T.; Takenaka, F.; Yakushiji, H.; Fujii, Y.; Kishi, Y.; Kita, S.; Shen, L.; Kumon, H.; Matsuura, E. A novel PET imaging using 64cu-labeled monoclonal antibody against mesothelin commonly expressed on cancer cells. J. Imunol. Res. 2015, 2015.
- Sugyo, A.; Tsuji, A.B.; Sudo, H.; Nagatsu, K.; Koizumi, M.; Ukai, Y.; Kurosawa, G.; Zhang, M.R.; Kurosawa, Y.; Saga, T. Preclinical evaluation of 89Zr-labeled human antitransferrin receptor monoclonal antibody as a PET probe using a pancreatic cancer mouse model. Nucl. Med. Commun. 2015, 36, 286–294.
- Sugyo, A.; Tsuji, A.B.; Sudo, H.; Nagatsu, K.; Koizumi, M.; Ukai, Y.; Kurosawa, G.; Zhang, M.R.; Kurosawa, Y.; Saga, T. Evaluation of 89Zr-Labeled Human Anti-CD147 Monoclonal Antibody as a Positron Emission Tomography Probe in a Mouse Model of Pancreatic Cancer. PLoS ONE 2013, 8.
- Boyle, A.J.; Cao, P.J.; Hedley, D.W.; Sidhu, S.S.; Winnik, M.A.; Reilly, R.M. MicroPET/CT imaging of patient-derived pancreatic cancer xenografts implanted subcutaneously or orthotopically in NOD-scid mice using 64Cu-NOTA-panitumumab F(ab’)2 fragments. Nucl. Med. Biol. 2015, 42, 71–77.
- Viola-Villegas, N.T.; Rice, S.L.; Carlin, S.; Wu, X.; Evans, M.J.; Sevak, K.K.; Drobjnak, M.; Ragupathi, G.; Sawada, R.; Scholz, W.W.; et al. Applying PET to broaden the diagnostic utility of the clinically validated CA19.9 serum biomarker for oncology. J. Nucl. Med. 2013, 54, 1876–1882.
- Girgis, M.D.; Kenanova, V.; Olafsen, T.; McCabe, K.E.; Bergara, F.; Wu, A.M.; Tomlinson, J.S. Anti-CA19-9 Diabody As A PET Imaging Probe For Pancreas Cancer. J. Surg. Res. 2011, 165, 258–259.
- Girgis, M.D.; Olafsen, T.; Kenanova, V.; McCabe, K.E.; Wu, A.M.; Tomlinson, J.S. Targeting CEA in pancreas cancer xenografts with a mutated scFv-Fc antibody fragment. EJNMMI Res. 2011, 1, 1–10.
- Niu, G.; Murad, Y.M.; Gao, H.; Hu, S.; Guo, N.; Jacobson, O.; Nguyen, T.D.; Zhang, J.; Chen, X. Molecular targeting of CEACAM6 using antibody probes of different sizes. J. Control. Release 2012, 161, 18–24.
- Rodríguez, E.; Arqués, J.L.; Rodríguez, R.; Nuñez, M.; Medina, M.; Talarico, T.L.; Casas, I.A.; Chung, T.C.; Dobrogosz, W.J.; Axelsson, L.; et al. We are IntechOpen, the world ’ s leading publisher of Open Access books Built by scientists, for scientists TOP 1%. Intech 1989, 32, 137–144.
- Zhao, J.; Yuan, Q.; Wang, H.; Liu, W.; Liao, X.; Su, Y.; Wang, X.; Yuan, J.; Li, T.; Li, J.; et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin. Infect. Dis. 2020, 71, 2027–2034.
- Wen, B.; Zhao, L.; Wang, Y.; Qiu, C.; Xu, Z.; Huang, K.; Zhu, H.; Li, Z.; Li, H. Nanobodies targeting the interaction interface of programmed death receptor 1 (PD-1)/PD-1 ligand 1 (PD-1/PD-L1). Prep. Biochem. Biotech. 2020, 50, 252–259.
- Phase 1 Imaging Study of 89Zr-DFO-HuMab-5B1 With HuMab-5B1—Full Text View—ClinicalTrials. Available online: (accessed on 14 January 2021).