Advanced, unresectable hepatocellular carcinoma has a dismal outcome. Multiple immune checkpoint inhibitors (ICIs) targeting the programmed-cell death 1 pathway (PD-1/L1) have been approved for the treatment of advanced HCC. However, outcomes remain undesirable and unpredictable on a patient-to-patient basis. The combination of anti-PD-1/L1 with alternative agents, chiefly cytotoxic T-lymphocyte antigen-4 (CTLA-4) ICIs or agents targeting other oncogenic pathways such as the vascular endothelial growth factor (VEGF) pathway and the c-MET pathway, has, in addition to the benefit of directly targeting alterative oncogenic pathways, in vitro evidence of synergism through altering the genomic and function signatures of T cells and expression of immune checkpoints. Several trials have been completed or are underway evaluating such combinations. Finally, studies utilizing transcriptomics and organoids are underway to establish biomarkers to predict ICI response.
- hepatocellular carcinoma
- biomarker
- checkpoint inhibitors
- combination therapy
- targeted therapy
- precision medicine
- ICI response
- transcriptomics
1. Introduction
Accounting for up to 90% of all primary liver malignancy, hepatocellular carcinoma (HCC) is a highly aggressive cancer type and the fourth most common cause of cancer-related deaths worldwide [1]. HCC develops within an immune-tolerant niche, as the liver has multiple distinct mechanisms to suppress unwanted immune activation from bypassing antigen and bacteria channeled through the portal vein system, including the upregulation of immune checkpoints such as the programmed death-1 (PD-1) pathway [2][3]. In addition, HCC is nearly always accompanied by chronic hepatic inflammation and cirrhosis. Consequently, HCCs have been theorized, and indeed proven, to be responsive to immune-checkpoint inhibitors (ICIs). The first single agent ICI to be approved in HCC was the anti-PD-1 nivolumab, which demonstrated an objective response rate (ORR) of 15% in the phase I/II CheckMate-040 trial [4]. In addition, pembrolizumab, another anti-PD-1, was approved as a single agent with the results of the phase I/II Keynote-224 trial, which demonstrated an ORR of 17% [5]. However, in spite of the successful phase I/II trials of nivolumab and pembrolizumab, both agents did not reach their primary endpoints in their phase III trials (Checkmate-459 and Keynote-240), demonstrating a median OS of 16.4 months and 13.9 months, respectively [6][7].
The definite but limited clinical benefit of single agent anti-PD-1 gave fresh impetus to the search for strategies to optimize ICI treatment. These include attempting to enhance response by combining ICIs with other ICIs, tyrosine kinase inhibitors (TKIs), anti-vascular endothelial growth factor antibodies (anti-VEGFs), and other agents. In addition, biomarkers are currently being searched for to predict responders.
2. Immune Checkpoints and the Programmed Death-1 Pathway
In an adaptive immune response, two signals are required for T cell activation. Through MHC peptides, antigen-presenting cells present unique antigens and provide specificity to T cell response. A co-regulatory signal then either stimulates or inhibits T cell activation. Immune checkpoints are intrinsic to the immune system to prevent autoreactivity. Amongst the most studied immune checkpoints are the programmed death-1 pathway (PD-1) and the cytotoxic T-lymphocyte associated protein-4 (CTLA-4) pathway. Knocking out PD-1 in mice leads to the development of diverse autoreactivity phenomena including type 1 diabetes mellitus and autoimmune encephalomyelitis [8][9]. Meanwhile, knockout of CTLA-4 in mice leads to massive and fatal lymphoproliferation [10]. However, these pathways are often upregulated in the tumor microenvironment (TME), leading to T cell suppression and tumor immune escape. Last but not least, a variety of other immune co-regulatory signals have been studied in the context of tumor immune response (Figure 1).
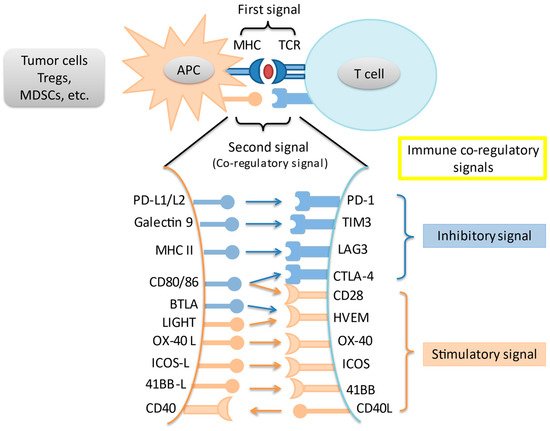
Figure 1. Interaction of main immune co-stimulatory/co-inhibitory molecules and their corresponding receptors. Cancer cells and other cells in the tumor microenvironment express a variety of inhibitory and stimulatory ligands that bind to their cognate receptors on immune cells, thereby leading to immune-modulation. These ligand-receptor pairs are known as immune checkpoints. Tregs = regulatory T cells; MDSC = myeloid-derived suppressor cell; APC = antigen presenting cell; TCR = T-cell receptor.
The programmed death-1 pathway consists of the programmed cell death protein 1 (PD-1) and programmed death ligand 1 (PD-L1). PD-1 is an immune co-inhibitory receptor expressed during inflammation or infection on CD8+ T cells and other immune cells [11]. By contrast, PD-L1, the primary ligand of PD-1, is constitutively expressed on the surface of antigen-presenting cells (APCs) as well as other tissues, including on tumor cells [12]. Upon binding of PD-L1 to its receptor PD-1, the PD-1/PD-L1 signaling pathway is activated, resulting in a suppression of antigen-specific T cell activation through a variety of mechanisms [13]. Several pathways have been implicated in the upregulation of PD-L1 expression in cancer. An upregulation of PD-L1 messenger RNA transcription has been attributed to a loss-of-function mutation of the phosphatase and tensin homolog (PTEN) [14]. The activation of the transcription factor STAT3, as well as the NF-kB pathway can also contribute to an enhanced PD-L1 mRNA transcription [15][16]. In addition, activated T cells facilitate PD-L1 expression by releasing distinct pro-inflammatory proteins during an immune response, which have a positive impact on the transcription of PD-L1 mRNA [17]. The most prominent pro-inflammatory molecule that has been ascribed to this process is interferon-gamma (IFN-γ), which is secreted by tumor-associated antigen specific T cells in the TME. Upon interaction of IFN-γ with its receptor (IFNGR) on tumor cells, the downstream Jak/Stat signaling cascade is triggered, resulting in an upregulation of PD-L1 transcription (Figure 2). Anti-PD-1/L1 antibodies block either PD-1 or PD-L1 to increase CD8+ T cell proliferation and partially reverse T cell anergy, resulting in increased anti-tumor immune response [13].
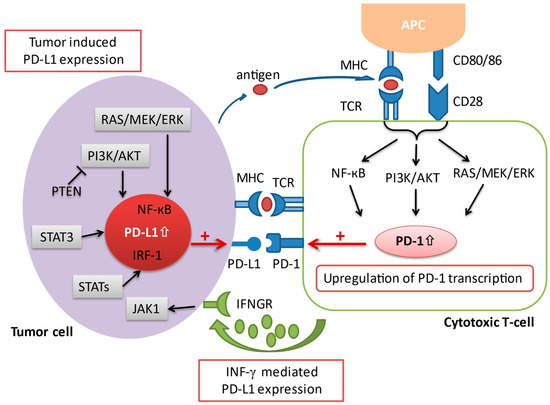
Figure 2. A schematic overview illustrating the various aspects involved in the regulation of PD-1/PD-L1 transcription. Co-stimulation via CD28 in conjunction with the TCR initiates the activation of several signaling pathways, including the Ras/MEK/ERK, NF-κB, and PI3K/AKT cascade, which in turn leads to an upregulation of PD-1 transcription. On the other hand, the expression of PD-L1 on tumor cells is regulated via 1) oncogenic activation of different signaling pathways and by 2) the presence of inflammatory signaling molecules (i.e., interferon-γ) released by effector immune cells such as the cytotoxic T cell in the tumor microenvironment. AKT = protein kinase B; APC = antigen presenting cell; ERK = extracellular signal-regulated kinase; IFNGR = interferon-gamma receptor; IFN-γ = interferon-gamma; IRF-1 = interferon regulatory factor-1; JAK = janus tyrosine kinase; MEK = mitogen-activated protein kinase; MHC = major histocompatibility complex; NF-κB = nuclear factor kappa-light-chain-enhancer of activated B cells; PD-1 = programmed cell death protein 1; PD-L1 = programmed cell death-ligand 1; PI3K = phosphatidylinositol-3 kinase; PTEN = phosphatase and tensin homolog; STAT = signal transducer and activator of transcription; TCR = T cell receptor.
3. Immune Checkpoint Inhibitor Combinations
3.1. Anti-PD-1/L1 and Anti-CTLA-4
Apart from the PD-1 pathway, CTLA-4 is another immune checkpoint that has been successfully targeted. Unlike PD-1 which suppresses T cells at a later stage in the immune response and in peripheral tissues, CTLA-4 mediates T cell proliferation early in an immune response and within lymph nodes [12]. It is a co-inhibitory molecule which competes with the co-stimulatory CD28 for binding to CD80/86. By having a higher affinity for CD80/86 than CD28, CTLA-4 effectively impairs the activation of effector T cells. Furthermore, CTLA-4 is expressed constitutively on regulatory T cells (Tregs) and thus plays an essential role in the immunosuppressive activity of Tregs in the TME. Consequently, ICIs targeting the CTLA-4 pathway aims to counter-act the ability of tumor cells to escape the host immune surveillance by promoting and reactivating tumor-specific T cells (Figure 3). Combined blockade of PD-1/L1 and CTLA-4 offers several distinct advantages over single PD-1 pathway blockade. Firstly, blockade of PD-1/L1 is associated with upregulation of CTLA-4, and thus a direct additional blockade may prevent further immune escape [18]. Secondly, myeloid-derived suppressor cells (MDSCs) can severely restrict T cell activity within the TME, but combined blockade can synergistically increase the proportion of CD8+ effector T cells relative to MDSCs [19]. Thirdly, combined blockade increases production of inflammatory cytokines such as TNF-alpha and IFN-gamma and decreases T cell anergy [19]. Finally, combined blockade can lead to the expansion of memory T cells (Tmem), which can confer longer-term anti-tumor immunity compared to effector T-cells [20][21]. In fact, in melanoma patients with exceptional responses to ICIs, Tmem have been known to persist both in skin and blood for up to a remarkable 9 years. The persistent Tmem also preferentially expressed a high IFN-gamma/TNF signature, which strongly prognosticates melanoma patients [22]. These findings suggest that Tmem are critical in long-term anti-tumor immunity, which anti-CTLA-4 is uniquely positioned to enhance. Consequently, anti CTLA-4 and anti-PD-1/L1 have been established in multiple tumor types such as melanoma, lung cancer, urothelial carcinoma, renal cell carcinoma, esophagogastric cancer, and MSI-high colorectal cancer [23][24][25][26][27][28][29].
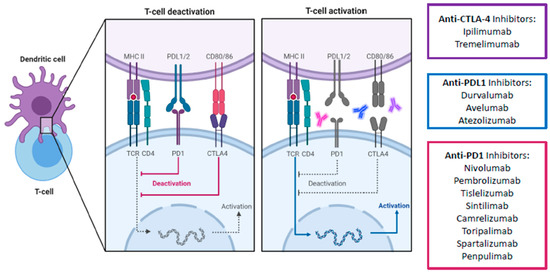
Figure 3. Schematic overview of main immune checkpoint inhibitors including their mechanism of action for advanced HCC treatment. The signaling cascade of the PD-1/PD-L1 and CTLA4 pathway and the corresponding therapeutical immune-checkpoint inhibitors. Due to the higher affinity of CTLA-4 to CD80/86 than the co-stimulatory CD28, CTLA-4 successfully binds to CD80/86 and antagonizes the stimulatory signal by the interaction of CD28 with CD80/86. As a result, CTLA-4 causes an inhibition of T cell activation. Similarly, the interaction of PD-L1 with its receptor PD-1 also disrupts the activation of T cells, therefore influencing immune activity in a negative way. CTLA-4, cytotoxic T-lymphocyte-associated protein 4; PD1, programmed death-1 protein; PDL1, programmed death- ligand 1; MHC II, major histocompatibility complex II; TCR, toll-cell receptor; CD4, cluster of differentiation 4. Created with biorender.com.
In HCC, two CTLA-4 ICIs, ipilimumab and tremelimumab, have been evaluated in combination with anti-PD-1/L1 agents. Ipilimumab–nivolumab is the only combination-ICI regime to be approved by the US FDA for use in HCC so far, having been approved in sorafenib-treated advanced HCC patients based on the results from cohort 4 of the phase I/II CheckMate-040 study [30]. In this study, ipilimumab–nivolumab was given to a total of 148 patients in one of three treatment arms: arm A with nivolumab 1 mg/kg + ipilimumab 3 mg/kg every 3 weeks for 4 doses; arm B with nivolumab 3 mg/kg + ipilimumab 1 mg/kg every 3 weeks for 4 doses, with each arm being followed by single agent nivolumab 240 mg every 2 weeks; or arm C with nivolumab 3 mg/kg Q2W + ipilimumab 1 mg/kg every 6 weeks continuously. In terms of clinical benefit, the ORRs were 32% in arm A, 31% in arm B, and 31% in arm C, the CR rates were 8%, 6%, and 0%, and the median OS was 22.8, 12.5, and 12.7 months, respectively. Recently published long term results showed a similar median OS, with the 3-year survival rates of arms A, B, and C being 42, 26, and 30 months, respectively [31]. In general, ipilimumab–nivolumab was well tolerated despite having higher rates of adverse events (AEs) compared to nivolumab monotherapy. All grade and grade ≥3 AEs were 94% and 55% in arm A, 71% and 29% in arm B, and 79% and 31% in arm C. Of note, although the study was not powered to compare outcomes across different arms, patients in arm A, who had the highest ipilimumab dose, had the longest median overall survival and highest long term survival rates, but also the highest incidence of AEs. This interesting phenomenon has been further explored in a dose exposure–response analysis based on the trial population. Using population pharmacokinetics, ipilimumab and nivolumab exposure were generated and outcomes analyzed [32]. High ipilimumab exposure, especially for patients in arm A, but not nivolumab exposure, was associated with better overall survival. Overall, the results of the CheckMate-040 trial, especially for arm A, compares favorable against other treatment options in the post-sorafenib setting. Of particular note is that although cross-trial comparisons are difficult, the median OS of arm A represents the longest amongst all regimes approved in this setting. Although the overall toxicity was higher, the overall profile of AEs was similar to other ICIs. About 50% of the patients in arm A needed systemic steroids treatment. However, immune-mediated AEs (IRAE) mostly resolved across all treatment arms, and intriguingly, for patients who were rechallenged after experiencing an IRAE, few experienced recurrence of the event.
Aside from ipilimumab, tremelimumab has also been evaluated with durvalumab (an anti-PD-L1) in advanced HCC. In the phase I/II Study 22, tremelimumab was investigated either in direct combination with durvalumab, or as a one-off priming therapy to enhance the anti-tumor activity of durvalumab without significant added toxicity [33]. In this study, patients who were previously treated with sorafenib were randomized to one of the following arms: arm A = tremelimumab 300 mg (1 dose) plus durvalumab 1500 mg every 4 weeks; arm B = tremelimumab 75 mg (4 doses) plus durvalumab 1500 mg every 4 weeks; arm C = durvalumab monotherapy 1500 mg every 4 weeks; arm D = tremelimumab monotherapy 750 mg every 4 weeks (7 doses, then every 12 weeks thereafter). In general, patients in arm A had better outcomes, such as a higher ORR (arm A, 24%; arm B, 9.5%; arm C, 10.6%; arm D, 7.2%) and a longer median OS (arm A, 18.7 months; arm B, 11.3 months; arm C, 13.6 months; arm D, 15.1 months). In terms of safety profile, all grade treatment-related AEs (TRAEs) and grade ≥3 TRAEs occurred in 82.4% and 35.1% of patients in arm A, 69.5% and 23.2% in arm B, 60.4% and 17.8% in arm C, and 84.1% and 43.5% in arm D. Specifically, the incidence of TRAEs requiring systemic steroids for arm A was 24.3%, and the safety profile appeared favorable. Interestingly, the proliferative CD8+Ki67+ T-cell count was associated with radiological response regardless of treatment received but was highest for arm A, supporting the proposed mechanism of enhanced immune activation with higher dose tremelimumab.
Based on the encouraging results of the CheckMate-040 cohort 4 and Study 22, ipilimumab–nivolumab and tremelimumab–durvalumab are both currently being investigated in phase III trials as first-line treatment in advanced HCC. The CheckMate-9DW study aims to compare ipilimumab–nivolumab to lenvatinib or sorafenib, while the HIMALAYA study aims to compare tremelimumab–durvalumab either as combination or with tremelimumab as a priming agent (as per Study 22) with durvalumab monotherapy or sorafenib [34][35]. The results of these trials would further define the role of anti-CTLA-4 and anti-PD-1/L1 combinations in different settings in advanced HCC.
Apart from being used as combination or priming therapy, anti-CTLA-4 has also been studied as salvage therapy for patients who are refractory to anti-PD-1/L1. We recently published the first study on the use of ipilimumab–nivolumab/pembrolizumab in patients with PD-1 pathway ICI refractory HCC [36]. All 25 patients received ipilimumab 1 mg/kg with nivolumab 3 mg/kg or pembrolizumab 2 mg/kg every 3 weeks. Importantly, the ORR was 16%, CR rate was 12%, and median OS was 10.9 months. There was no difference in ORR between those with prior response to ICIs compared to those without. Prospective trials will be conducted to validate the addition of anti-CTLA-4 for those with HCCs refractory to PD-1 pathway ICI, which is an expanding and ever more important patient population.
3.2. ICIs and TKIs/Anti-VEGFs
Aside from ICIs, the other major group of systemic HCC therapeutics are tyrosine kinase inhibitors (TKI)/anti-VEGF monoclonal antibodies. Approved agents include the TKIs sorafenib and lenvatinib as first line treatment, as well as regorafenib and cabozantinib as post-sorafenib second-line treatment. In addition, the anti-VEGF-2 monoclonal antibody ramucirumab is approved for use in post sorafenib patients with alpha-fetoprotein ≥400 ng/mL (Figure 4). All such agents target the VEGF pathway, long implicated in HCC pathogenesis and progression [37]. In addition, individual TKIs target other pathways such as FGF receptors (1–4), KIT, platelet-derived growth factor receptor alpha, c-MET, RET, and B-RAF pathways. Although all these agents have been licensed as monotherapy, there is ample in vitro evidence to support their use with ICIs for synergistic effect [38]. VEGF may downregulate adhesion molecules needed for T cell trafficking such as ICAM-1 in the TME. An increased VEGF level is also associated with increased tumor vessel formation, which is paradoxically associated with increased hypoxia and acidosis. This hypoxic TME leads to recruitment of immunosuppressive cells such as Tregs and MDSCs. Blockade of such pathways thus improves T cell infiltration and activity in the TME. Individual TKIs may additionally have specific immunomodulatory effects. Sorafenib and lenvatinib have been shown to be able to either deplete or alter the phenotype of tumor-associated macrophages (TAMs) from a M2 polarized immunosuppressive state to a M1, pro-anti-tumor immunity state [38][39][40]. Regorafenib has been shown to increase T cell cytokine production, reduce co-inhibitory receptor expression, and increase the expression of CD25 and CD28 on T cells [41]. Finally, cabozantinib specifically blocks the c-MET pathway, which, in addition to being a direct pathogenesis pathway for HCC, mediates direct phosphorylation and activation of GSK3B, causing decreased expression of PDL1 [42][43]. Based on these results, multiple ICI and TKI/anti-VEGF combinations have been considered in the treatment of HCCs (Figure 5 and Figure 6).
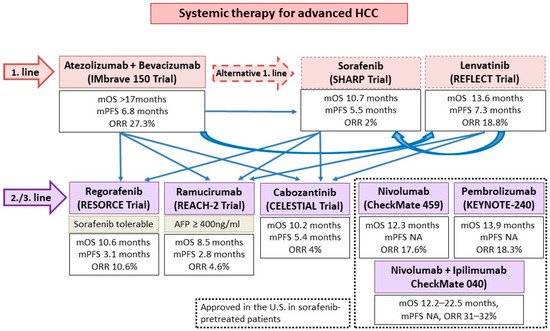
Figure 4. The current proposed clinical practice algorithm in terms of systemic therapies and sequencing options in advanced HCC. Adapted from [44]. NA, not available; mOS, median overall survival; mPFS, median progression free survival; ORR, overall response rate.
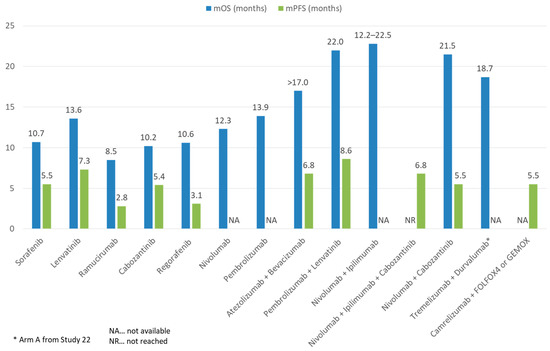
Figure 5. Comparison of mOS and mPFS of ICI and TKI monotherapy or combination therapy with other treatment modalities in advanced HCC.
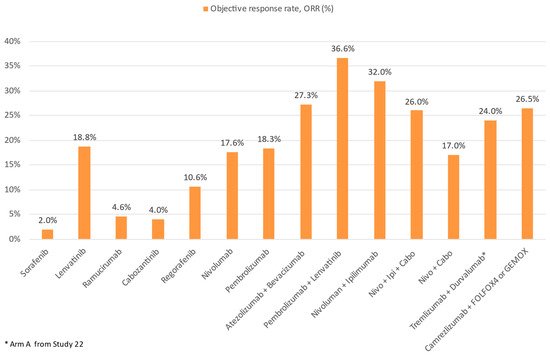
Figure 6. Comparison of ORR of ICI and TKI monotherapy or combination therapy with other treatment modalities in advanced HCC.
In the landmark IMBrave150 trial (the first ever successful phase III trial of ICIs in advanced HCC), atezolizumab (an anti-PD-L1) combined with bevacizumab (an anti-VEGF monoclonal antibody) was compared against sorafenib in the first-line setting. A total of 501 patients were randomized in a 2:1 ratio to atezolizumab–bevacizumab or sorafenib. In the primary analysis, the atezolizumab–bevacizumab arm had superior overall survival (hazard ratio for death 0.58, 95% CI 0.42–0.79, p < 0.001), progression free survival (hazard ratio for disease progression or death 0.59, 95% CI 0.47–0.76, p < 0.001), and ORR by RECIST 1.1 (27.3% vs. 11.9%, p < 0.001) compared to the sorafenib arm. AEs of any grade, grade 3–4, and grade 5 were reported in 98.2%, 56.5%, and 4.6% in the atezolizumab–bevacizumab arm, and 98.7%, 55.1%, and 5.8% in the sorafenib arm. Most frequent AEs were those related to anti-VEGF such as hypertension [45]. A follow-up long-term analysis showed a median OS 19.2 months of the atezolizumab–bevacizumab arm vs. 13.4 months of the sorafenib (p = 0.0009). Survival at 18 months was 52% with atezolizumab–bevacizumab and 40% with sorafenib. No new safety signals were identified [46]. The success of IMbrave150 has several significant impacts on the treatment landscape of HCC: firstly, atezolizumab–bevacizumab has now been established as arguable the first-line treatment of choice; secondly, ICI and VEGF targeting agent combinations have been proven to be safe and efficacious; and finally, future clinical research of second-line treatment should be focused on anti-PD-1/L1 resistant patients.
Besides atezolizumab–bevacizumab, ICI has also been evaluated with TKIs in advanced HCC. In the phase I/II CheckMate-040 cohort 6 study, the efficacy and safety of nivolumab–cabozantinib with or without ipilimumab (doublet and triplet) in sorafenib-naïve or experienced patients were evaluated [47]. The ORR was 17% and 26%, and disease control rate (DCR) was 81% and 83% for the doublet and triplet arms, respectively. Median PFS was 5.5 mo for the doublet arm and 6.8 mo for the triplet arm, whereas median OS was not reached in either treatment group. Nevertheless, more frequent Grade 3–4 TRAEs were reported, with the incidence reaching 42% and 71% of patients in the doublet and triplet arms, respectively. In addition, the combination of lenvatinib and pembrolizumab was evaluated in the phase Ib KEYNOTE-524 trial [48]. Most patients had no prior systemic therapy. By RECIST 1.1, an ORR of 36% and median PFS of 8.6 months was achieved. The median OS was impressive (22.0 months). In total, 99% and 67% of patients experienced any and grade ≥3 AEs, respectively. Camrelizumab, another anti-PD-1, was evaluated with the anti-VEGF-2 TKI apatinib in the RESCUE trial in both first and second line (to TKI) settings. In total, 75 first line and 120 s line patients were enrolled. The ORR was 34.3% in the first line and 22.5% in the second line. Median PFS was 5.7 and 5.5 months, and 12-month survival rates were 74.7% and 68.2%, respectively. Grade ≥3 TRAEs occurred in 77.4% of patients [49]. Finally, the VEGF Liver 100 trial evaluated the anti-PD-L1 avelumab in combination with axitinib (VEGF receptor 1/2/3 inhibitor) in treatment-naïve advanced HCC patients [50]. Out of 22 patients, the ORR was 13.6% by RECIST 1.1 (31.8% by mRECIST) and median PFS was 5.5 months by RECIST 1.1. The OS data were yet to be mature.
In the setting of advanced HCC, three ongoing phase III trials evaluating ICI-TKI combinations in the first line are underway. The COSMIC-312 trial aims to evaluate the atezolizumab–cabozantinib combination compared to sorafenib and cabozantinib monotherapies [51]. Additionally, pembrolizumab–lenvatinib is being evaluated in the LEAP-002 study compared against lenvatinib monotherapy [52]. Finally, camrelizumab is being evaluated with apatinib compared to sorafenib [53]. The results of these trials would enlighten the HCC community as to the synergism between TKIs targeting multiple pathways and ICIs.
In general, only limited real-world data on the use of ICI-TKI combinations exist. We recently reported the use of cabozantinib with ICIs in 11 patients, with 45.5% having stable disease and a median OS of 12.3 months [54]. ICI-TKIs have also been investigated as a potential treatment strategy to downstage advanced HCC to resectable HCC. In a recently published real-world study, 63 patients with advanced HCC received either lenvatinib– pembrolizumab or camrelizumab–apatinib. In total, 15.9% of patients were able to receive curative resection following systemic treatment [55].
3.3. Anti-PD-1 and Anti-LAG3
Lymphocyte activation gene-3 (LAG3) is the third and latest immune checkpoint which has garnered sufficient interest to be exploited therapeutically. LAG3 has diverse but incompletely understood functions both intracellularly and extrinsically [56][57]. It is expressed on CD4+ and CD8+ T cells as well as NK cells during activation phase [58]. Overall, LAG3 is a negative regulator of T cell activation and function, as well as a key contributor to T cell exhaustion. Knockout of LAG3 in mouse models leads to deficiencies in cell cycle arrest/death of T cells, splenomegaly, and autoreactive phenomena such as accelerated diabetes [59][60]. In vitro studies show that blockade of LAG3 leads to enhanced proliferation of CD4+ and CD8+ T cells and increase inflammatory cytokine release such as IFNγ [61][62][63][64]. Converse to its expression on effector T cells and NK cells, LAG3 is constitutively expressed on thymic-derived Tregs [65]. It has been shown to promotes Treg differentiation and suppressive activity, and LAG3 blockade both inhibits Treg induction and proliferation [65][66]. Finally, LAG3 has a number of inhibitory functions on dendritic cells and NK cells, although further studies are needed to map out such pathways [56].
LAG3 is commonly co-expressed with other immune checkpoints such as PD-1, resulting in an anergic, dysfunctional T cell state [56]. Interestingly, single target blockade of LAG3 had a limited effect on both resolution of chronic infection and tumor clearance in mouse models with exhausted T cells. However, combination blockade with anti-LAG3 and anti-PD1 dramatically increased viral and tumor clearance [67][68]. Based on these results, the first-in-class anti-LAG3 relatlimab is currently being evaluated in a number of solid-organ tumors in various trials including in HCC [69][70]. The first results, from the RELATIVITY-047 trial of nivolumab–relatlimab in treatment naïve melanoma, met its primary endpoint of progression-free survival [71]. The details of the RELATIVITY-047 trial and the results of other ongoing trials will further clarify the role of anti-LAG3 in HCC and other solid organ tumors.
3.4. ICI and Chemotherapy
Chemotherapy is well known to have immunomodulatory effect on cancers. This is achieved through multiple mechanisms: firstly, certain chemotherapy agents such as oxaliplatin mediate the release of tumor antigens and enhance signaling for phagocytosis such as CRT and HMGB1. This activates the NRLP3 inflammasome, leading to the production of IL1 beta, dendritic cell maturation, and eventually, CD8+ T cell recruitment and activation [72][73]. Furthermore, chemotherapy can upregulate tumor antigens directly, as well as upregulate co-stimulatory molecules and downregulate immune checkpoints [72][74]. Finally, chemotherapy has been known to restrict Treg and MDSC activities, thus reducing immunosuppression and enhancing anti-tumor immunity [75][76]. Therefore, chemotherapy and ICIs represent another promising option to achieve synergistic effect. To date, one trial exists investigating ICI–chemotherapy combinations, which is a phase II study containing 34 HCC patients who received camrelizumab with FOLFOX (5-Fluorouracil, leucovorin, and oxaliplatin) or GEMOX (gemcitabine–oxaliplatin) as first line therapy [77]. The ORR was 26.5% and DCR was 79.4%. Median PFS was 5.5 months with median OS not reached. Grade ≥3 TRAEs occurred in 85.3% of patients. A phase III study is underway to investigate camrelizumab with FOLFOX in treatment for naïve advanced HCC patients [78].
3.5. ICI and Locoregional Therapies or Radiotherapy
Locoregional therapies such as transarterial chemoembolization (TACE) and ablation have been shown to stimulate tumor antigen specific CD8+ T cells, Tmem, and NK cell responses [79][80][81]. Based on these findings, a number of locoregional therapies have been evaluated in combination with ICIs. In a phase I/II single arm study, tremelimumab was evaluated in combination with TACE or RFA for treatment of 32 patients with advanced HCC [82]. In total, 19 patients had evaluable response with 5 (26.3%) having partial response. Interestingly, a significant increase in CD3+, CD4+, and CD8+ T cells was observed in peripheral blood and intratumorally after treatment, with responders having a higher number of CD3+/CD8+. Furthermore, in the published results of the PETAL study, 6 patients were given pembrolizumab following TACE. Of 4 patients with evaluable responses, 3 had stable disease. No synergistic toxicity was reported [83]. Several ongoing phase III trials are underway evaluating ICIs with TACE, including CheckMate-74W, EMERALD-1, and LEAP-012 trials. In these 3 studies, ipilimumab–nivolumab or nivolumab, durvalumab–bevacizumab or durvalumab, and pembrolizumab–lenvatinib are being evaluated against TACE, respectively. The results of these trials will further shine light on whether ICIs can be used effectively beyond the advanced HCC setting [84][85][86].
Finally, radiotherapy (RT) may play a role in overcoming ICI resistance due to augmented tumor antigen release, T cell activation/ infiltration, and MHC class I expression [87]. Furthermore, ICIs may enhance the well-known abscopal effect of RT: the immune-mediated phenomenon of tumor regression outside of the irradiated field. To date, two published trials combining ICIs with RT exist. In a phase II single center study, Y-90 radioembolization was combined with nivolumab for treatment of 40 patients. Of 36 evaluable patients, the ORR was 31% and DCR was 58.3% The median PFS and OS were 4.6 months and 15.1 months, respectively. In total, 11% of patients had ≥grade 3 treatment-related AEs (TRAEs) [88]. In another phase II trial including 42 patients, who were treated with selective internal radiation therapy (SIRT) with subsequent nivolumab, the ORR was 38%, DCR was 81%, time-to-progression (TTP) was 9.3 months, and median OS was 20.6 months. Grade ≥3 TRAEs were found in 48% of patients [89]. Several other phase II clinical trials investigating the combined approach of ICIs and radiotherapy are underway, including the combined use of durvalumab–tremelimumab with RT and stereotactic body RT (SBRT) with ipilimumab [90][91].
This entry is adapted from the peer-reviewed paper 10.3390/cancers13081949
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018, 68, 394–424.
- Knolle, P.A.; Thimme, R. Hepatic immune regulation and its involvement in viral hepatitis infection. Gastroenterology 2014, 146, 1193–1207.
- Hong, Y.P.; Li, Z.D.; Prasoon, P.; Zhang, Q. Immunotherapy for hepatocellular carcinoma: From basic research to clinical use. World J. Hepatol. 2015, 7, 980–992.
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.H., 3rd; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502.
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952.
- Yau, T.; Park, J.W.; Finn, R.S.; Cheng, A.-L.; Mathurin, P.; Edeline, J.; Kudo, M.; Han, K.-H.; Harding, J.J.; Merle, P.; et al. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann. Oncol. 2019, 30, v874–v875.
- Finn, R.S.; Ryoo, B.Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab As Second-Line Therapy in Patients with Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202.
- Keir, M.E.; Liang, S.C.; Guleria, I.; Latchman, Y.E.; Qipo, A.; Albacker, L.A.; Koulmanda, M.; Freeman, G.J.; Sayegh, M.H.; Sharpe, A.H. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J. Exp. Med. 2006, 203, 883–895.
- Carter, L.L.; Leach, M.W.; Azoitei, M.L.; Cui, J.; Pelker, J.W.; Jussif, J.; Benoit, S.; Ireland, G.; Luxenberg, D.; Askew, G.R.; et al. PD-1/PD-L1, but not PD-1/PD-L2, interactions regulate the severity of experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2007, 182, 124–134.
- Chambers, C.A.; Sullivan, T.J.; Allison, J.P. Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ T cells. Immunity 1997, 7, 885–895.
- Bardhan, K.; Anagnostou, T.; Boussiotis, V.A. The PD1: PD-L1/2 pathway from discovery to clinical implementation. Front. Immunol. 2016, 7, 550.
- Kudo, M. Immune Checkpoint Inhibition in Hepatocellular Carcinoma: Basics and Ongoing Clinical Trials. Oncology 2017, 92, 50–62.
- Tsang, J.; Wong, J.S.L.; Kwok, G.G.W.; Li, B.C.W.; Leung, R.; Chiu, J.; Cheung, T.t.; Yau, T. Nivolumab + Ipilimumab for patients with hepatocellular carcinoma previously treated with Sorafenib. Expert Rev. Gastroenterol. Hepatol. 2021, 1–10.
- Parsa, A.T.; Waldron, J.S.; Panner, A.; Crane, C.A.; Parney, I.F.; Barry, J.J.; Cachola, K.E.; Murray, J.C.; Tihan, T.; Jensen, M.C.; et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 2007, 13, 84–88.
- Chen, J.; Jiang, C.C.; Jin, L.; Zhang, X.D. Regulation of PD-L1: A novel role of pro-survival signalling in cancer. Ann. Oncol. 2016, 27, 409–416.
- Ostrand-Rosenberg, S.; Horn, L.A.; Haile, S.T. The Programmed Death-1 Immune-Suppressive Pathway: Barrier to Antitumor Immunity. J. Immunol. 2014, 193, 3835–3841.
- Kythreotou, A.; Siddique, A.; Mauri, F.A.; Bower, M.; Pinato, D.J. PD-L1. J. Clin. Pathol. 2018, 71, 189–194.
- Koyama, S.; Akbay, E.A.; Li, Y.Y.; Herter-Sprie, G.S.; Buczkowski, K.A.; Richards, W.G.; Gandhi, L.; Redig, A.J.; Rodig, S.J.; Asahina, H.; et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 2016, 7.
- Curran, M.A.; Montalvo, W.; Yagita, H.; Allison, J.P. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl. Acad. Sci. USA 2010, 107, 4275–4280.
- Das, R.; Verma, R.; Sznol, M.; Boddupalli, C.S.; Gettinger, S.N.; Kluger, H.; Callahan, M.; Wolchok, J.D.; Halaban, R.; Dhodapkar, M.V.; et al. Combination Therapy with Anti–CTLA-4 and Anti–PD-1 Leads to Distinct Immunologic Changes In Vivo. J. Immunol. 2015, 194, 950–959.
- Pauken, K.E.; Sammons, M.A.; Odorizzi, P.M.; Manne, S.; Godec, J.; Khan, O.; Drake, A.M.; Chen, Z.; Sen, D.R.; Kurachi, M.; et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 2016, 354, 1160–1165.
- Han, J.; Zhao, Y.; Shirai, K.; Molodtsov, A.; Kolling, F.W.; Fisher, J.L.; Zhang, P.; Yan, S.; Searles, T.G.; Bader, J.M.; et al. Resident and circulating memory T cells persist for years in melanoma patients with durable responses to immunotherapy. Nat. Cancer 2021, 2, 300–311.
- Larkin, J.; Hodi, F.S.; Wolchok, J.D. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 1270–1271.
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031.
- Antonia, S.J.; López-Martin, J.A.; Bendell, J.; Ott, P.A.; Taylor, M.; Eder, J.P.; Jäger, D.; Pietanza, M.C.; Le, D.T.; de Braud, F.; et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): A multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016, 17, 883–895.
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290.
- Janjigian, Y.Y.; Bendell, J.; Calvo, E.; Kim, J.W.; Ascierto, P.A.; Sharma, P.; Ott, P.A.; Peltola, K.; Jaeger, D.; Evans, J.; et al. CheckMate-032 study: Efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J. Clin. Oncol. 2018, 36, 2836–2844.
- Sharma, P.; Siefker-Radtke, A.; de Braud, F.; Basso, U.; Calvo, E.; Bono, P.; Morse, M.A.; Ascierto, P.A.; Lopez-Martin, J.; Brossart, P.; et al. Nivolumab alone and with ipilimumab in previously treated metastatic urothelial carcinoma: CheckMate 032 nivolumab 1 mg/kg plus ipilimumab 3 mg/kg expansion cohort results. J. Clin. Oncol. 2019, 37, 1608–1616.
- Lenz, H.-J.J.; Van Cutsem, E.; Limon, M.L.; Wong, K.Y.; Hendlisz, A.; Aglietta, M.; Garcia-Alfonso, P.; Neyns, B.; Luppi, G.; Cardin, D.; et al. Durable clinical benefit with nivolumab (NIVO) plus low-dose ipilimumab (IPI) as first-line therapy in microsatellite instability-high/mismatch repair deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC). Ann. Oncol. 2018, 29, viii714.
- Yau, T.; Kang, Y.K.; Kim, T.Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab plus Ipilimumab in Patients with Advanced Hepatocellular Carcinoma Previously Treated with Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020, 6.
- El-Khoueiry, A.B.; Yau, T.; Kang, Y.-K.; Kim, T.-Y.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.-M.; Matilla, A.; et al. Nivolumab (NIVO) plus ipilimumab (IPI) combination therapy in patients (Pts) with advanced hepatocellular carcinoma (aHCC): Long-term results from CheckMate 040. J. Clin. Oncol. 2021, 39, 269.
- Sangro, B.; Yau, T.; El-Khoueiry, A.B.; Kudo, M.; Shen, Y.; Tschaika, M.; Urvi, A.; Feng, Y.; Gao, L. Exposure-response analysis for nivolumab + ipilimumab combination therapy in patients with advanced hepatocellular carcinoma. In Proceedings of the 14th Annual Conference of the International Liver Cancer Association (ILCA), Madrid, Spain, 11–13 September 2020.
- Kelley, R.K.; Abou-Alfa, G.K.; Bendell, J.C.; Kim, T.-Y.; Borad, M.J.; Yong, W.-P.; Morse, M.; Kang, Y.-K.; Rebelatto, M.; Makowsky, M.; et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. J. Clin. Oncol. 2017, 35, 4073.
- A Study of Nivolumab in Combination with Ipilimumab in Participants with Advanced Hepatocellular Carcinoma (CheckMate 9DW). Case Med. Res. 2019.
- Kelley, R.K.; Furuse, J.; Darilay, A.; Armstrong, J.; Vlahovic, G.; Sangro, B.; Chan, S.L.; Galle, P.R.; Negro, A.; Abou-Alfa, G.K.; et al. A randomized, multicenter phase 3 study of durvalumab (D) and tremelimumab (T) as first-line treatment in patients with unresectable hepatocellular carcinoma (HCC): HIMALAYA study. J. Clin. Oncol. 2018, 36, TPS4144.
- Wong, J.S.L.; Kwok, G.W.; Tang, V.; Li, B.; Leung, R.C.-Y.; Chiu, J.W.Y.; Ma, K.W.; She, W.-H.; Tsang, W.Y.J.; Cheung, T.T.; et al. Ipilimumab and nivolumab/pembrolizumab in advanced hepatocellular carcinoma refractory to prior immune checkpoint inhibitors. J. Clin. Oncol. 2021, 39, 330.
- Morse, M.A.; Sun, W.; Kim, R.; He, A.R.; Abada, P.B.; Mynderse, M.; Finn, R.S. The role of angiogenesis in hepatocellular carcinoma. Clin. Cancer Res. 2019, 25, 912–920.
- Lin, Y.Y.; Tan, C.T.; Chen, C.W.; Ou, D.L.; Cheng, A.L.; Hsu, C. Immunomodulatory Effects of Current Targeted Therapies on Hepatocellular Carcinoma: Implication for the Future of Immunotherapy. Semin. Liver Dis. 2018, 38, 379–388.
- Makker, V.; Cohn, A.L.; Taylor, M.H.; Minoshima, Y.; Kato, Y.; Dairiki, R.; Kanekiyo, M.; Yachie, A.; Kodama, K.; Sachdev, P.; et al. Biomarker results and preclinical rationale for combination lenvatinib and pembrolizumab in advanced endometrial carcinoma. J. Clin. Oncol. 2018, 36, 5597.
- Kato, Y.; Tabata, K.; Kimura, T.; Yachie-Kinoshita, A.; Ozawa, Y.; Yamada, K.; Ito, J.; Tachino, S.; Hori, Y.; Matsuki, M.; et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8 + T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS ONE 2019, 14, e0212513.
- Tsai, A.K.; Khan, A.Y.; Worgo, C.E.; Wang, L.L.; Liang, Y.; Davila, E. A multikinase and DNA-PK inhibitor combination immunomodulates melanomas, suppresses tumor progression, and enhances immunotherapies. Cancer Immunol. Res. 2017, 5, 790–803.
- Li, H.; Li, C.W.; Li, X.; Ding, Q.; Guo, L.; Liu, S.; Liu, C.; Lai, C.C.; Hsu, J.M.; Dong, Q.; et al. MET Inhibitors Promote Liver Tumor Evasion of the Immune Response by Stabilizing PDL1. Gastroenterology 2019, 156, 1849–1861.e13.
- Rimassa, L.; Assenat, E.; Peck-Radosavljevic, M.; Pracht, M.; Zagonel, V.; Mathurin, P.; Rota Caremoli, E.; Porta, C.; Daniele, B.; Bolondi, L.; et al. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): A final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol. 2018, 19, 682–693.
- Pinter, M.; Scheiner, B.; Peck-Radosavljevic, M. Immunotherapy for advanced hepatocellular carcinoma: A focus on special subgroups. Gut 2021, 70, 204–214.
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905.
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Lim, H.Y.; Kudo, M.; Breder, V.V.; Merle, P.; et al. IMbrave150: Updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). J. Clin. Oncol. 2021, 39, 267.
- Yau, T.; Zagonel, V.; Santoro, A.; Acosta-Rivera, M.; Choo, S.P.; Matilla, A.; He, A.R.; Cubillo Gracián, A.; El-Khoueiry, A.B.; Sangro, B.; et al. Nivolumab (NIVO) + ipilimumab (IPI) + cabozantinib (CABO) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): Results from CheckMate 040. J. Clin. Oncol. 2020, 38, 478.
- Finn, R.S.; Ikeda, M.; Zhu, A.X.; Sung, M.W.; Baron, A.D.; Kudo, M.; Okusaka, T.; Kobayashi, M.; Kumada, H.; Kaneko, S.; et al. Phase ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J. Clin. Oncol. 2020, 38, 2960–2970.
- Xu, J.; Shen, J.; Gu, S.; Zhang, Y.; Wu, L.; Wu, J.; Shao, G.; Zhang, Y.; Xu, L.; Yin, T.; et al. Camrelizumab in Combination with Apatinib in Patients with Advanced Hepatocellular Carcinoma (RESCUE): A Nonrandomized, Open-label, Phase II Trial. Clin. Cancer Res. 2021, 27, 1003–1011.
- Kudo, M.; Motomura, K.; Wada, Y.; Inaba, Y.; Sakamoto, Y.; Kurosaki, M.; Umeyama, Y.; Kamei, Y.; Yoshimitsu, J.; Fujii, Y.; et al. First-line avelumab + axitinib in patients with advanced hepatocellular carcinoma: Results from a phase 1b trial (VEGF Liver 100). J. Clin. Oncol. 2019, 37, 4072.
- Kelley, R.K.; Oliver, J.W.; Hazra, S.; Benzaghou, F.; Yau, T.; Cheng, A.L.; Rimassa, L. Cabozantinib in combination with atezolizumab versus sorafenib in treatment-naive advanced hepatocellular carcinoma: COSMIC-312 Phase III study design. Futur. Oncol. 2020, 16, 1525–1536.
- Safety and Efficacy of Lenvatinib (E7080/MK-7902) in Combination with Pembrolizumab (MK-3475) versus Lenvatinib as First-Line Therapy in Participants with Advanced Hepatocellular Carcinoma (MK-7902-002/E7080-G000-311/LEAP-002). Available online: (accessed on 10 April 2021).
- A Study to Evaluate SHR-1210 in Combination with Apatinib as First-Line Therapy in Patients with Advanced HCC. Available online: (accessed on 10 April 2021).
- Dong, Y.; Leung, T.W.-T.; Kwok, G.W.; Tang, V.; Li, B.; Leung, R.C.-Y.; Chiu, J.W.Y.; Wong, J.S.L.; Ma, K.W.; She, W.H.; et al. The use of cabozantinib in advanced hepatocellular carcinoma (HCC): Hong Kong multi-center experience. J. Clin. Oncol. 2021, 39, 316.
- Zhu, X.D.; Huang, C.; Shen, Y.H.; Ji, Y.; Ge, N.L.; Qu, X.D.; Chen, L.; Shi, W.K.; Li, M.L.; Zhu, J.J.; et al. Downstaging and Resection of Initially Unresectable Hepatocellular Carcinoma with Tyrosine Kinase Inhibitor and Anti-PD-1 Antibody Combinations. Liver Cancer 2021.
- Andrews, L.P.; Marciscano, A.E.; Drake, C.G.; Vignali, D.A.A. LAG3 (CD223) as a cancer immunotherapy target. Immunol. Rev. 2017, 276, 80–96.
- Maruhashi, T.; Sugiura, D.; Okazaki, I.M.; Okazaki, T. LAG-3: From molecular functions to clinical applications. J. Immunother. Cancer 2020, 8.
- Triebel, F.; Jitsukawa, S.; Baixeras, E.; Roman-Roman, S.; Genevee, C.; Viegas-Pequignot, E.; Hercend, T. LAG-3, a novel lymphocyte activation gene closely related to CD4. J. Exp. Med. 1990, 171, 1393–1405.
- Bettini, M.; Szymczak-Workman, A.L.; Forbes, K.; Castellaw, A.H.; Selby, M.; Pan, X.; Drake, C.G.; Korman, A.J.; Vignali, D.A.A. Cutting Edge: Accelerated Autoimmune Diabetes in the Absence of LAG-3. J. Immunol. 2011, 187, 3493–3498.
- Workman, C.J.; Cauley, L.S.; Kim, I.-J.; Blackman, M.A.; Woodland, D.L.; Vignali, D.A.A. Lymphocyte Activation Gene-3 (CD223) Regulates the Size of the Expanding T Cell Population Following Antigen Activation In Vivo. J. Immunol. 2004, 172, 5450–5455.
- Grosso, J.F.; Kelleher, C.C.; Harris, T.J.; Maris, C.H.; Hipkiss, E.L.; De Marzo, A.; Anders, R.; Netto, G.; Getnet, D.; Bruno, T.C.; et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J. Clin. Investig. 2007, 117, 3383–3392.
- Grosso, J.F.; Goldberg, M.V.; Getnet, D.; Bruno, T.C.; Pyle, K.J.; Hipkiss, E.; Vignali, D.A.A.; Pardoll, D.M.; Drake, G. Functionally Distinct LAG-3 and PD-1 Subsets on Activated and Chronically Stimulated CD8 T cells. J. Immunol. 2011, 182, 6659–6669.
- Huard, B.; Tournier, M.; Hercend, T.; Triebel, F.; Faure, F. Lymphocyte-activation gene 3/major histocompatibility complex class II interaction modulates the antigenic response of CD4+ T lymphocytes. Eur. J. Immunol. 1994, 24, 3216–3221.
- Workman, C.J.; Vignali, D.A.A. The CD4-related molecule, LAG-3 (CD223), regulates the expansion of activated T cells. Eur. J. Immunol. 2003, 33, 970–979.
- Huang, C.-T.; Workman, C.J.; Flies, D.; Pan, X.; Marson, A.L.; Zhou, G. Role of in regulatory T cells. Immunity 2004, 21, 503–513.
- Durham, N.M.; Nirschl, C.J.; Jackson, C.M.; Elias, J.; Kochel, C.M.; Anders, R.A.; Drake, C.G. Lymphocyte activation gene 3 (LAG-3) modulates the ability of CD4 T-cells to be suppressed In Vivo. PLoS ONE 2014, 9, e0109080.
- Blackburn, S.D.; Shin, H.; Haining, W.N.; Zou, T.; Workman, C.J.; Polley, A.; Betts, M.R.; Freeman, G.J.; Vignali, D.A.A.; Wherry, E.J. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2009, 10, 29–37.
- Woo, S.R.; Turnis, M.E.; Goldberg, M.V.; Bankoti, J.; Selby, M.; Nirschl, C.J.; Bettini, M.L.; Gravano, D.M.; Vogel, P.; Liu, C.L.; et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012, 72, 917–927.
- A Study of Relatlimab in Combination with Nivolumab in Participants with Advanced Liver Cancer Who Have Never Been Treated with Immuno-oncology Therapy after Prior Treatment with Tyrosine Kinase Inhibitors. Available online: (accessed on 10 April 2021).
- Feasibility and Efficacy of Perioperative Nivolumab with or without Relatlimab for Patients with Potentially Resectable Hepatocellular Carcinoma (HCC). Available online: (accessed on 10 April 2021).
- Power, T. Bristol Myers Squibb Announces RELATIVITY-047, a Trial Evaluating Anti-LAG-3 Antibody Relatlimab and Opdivo (nivolumab) in Patients with Previously Untreated Metastatic or Unresectable Melanoma, Meets Primary Endpoint of Progression-Free Survival; Bristol Myers Squibb: New York, NY, USA, 2021.
- Emens, L.A.; Middleton, G. The interplay of immunotherapy and chemotherapy: Harnessing potential synergies. Cancer Immunol. Res. 2015, 3, 436–443.
- Ghiringheli, F.; Petoh, I.; Tesniere, A. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β-dependent adaptive immunity against tumors. Nat. Med. 2009, 15, 1170–1179.
- Ghebeh, H.; Lehe, C.; Barhoush, E.; Al-Romaih, K.; Tulbah, A.; Al-Alwan, M.; Hendrayani, S.F.; Manogaran, P.; Alaiya, A.; Al-Tweigeri, T.; et al. Doxorubicin downregulates cell surface B7-H1 expression and upregulates its nuclear expression in breast cancer cells: Role of B7-H1 as an anti-apoptotic molecule. Breast Cancer Res. 2010, 12.
- Vincent, J.; Mignot, G.; Chalmin, F.; Ladoire, S.; Bruchard, M.; Chevriaux, A.; Martin, F.; Apetoh, L.; Rébé, C.; Ghiringhelli, F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010, 70, 3052–3061.
- Ghiringhelli, F.; Larmonier, N.; Schmitt, E.; Parcellier, A.; Cathelin, D.; Garrido, C.; Chauffert, B.; Solary, E.; Bonnotte, B.; Martin, F. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur. J. Immunol. 2004, 34, 336–344.
- Qin, S.; Chen, Z.D.; Liu, Y.; Xiong, J.P. A phase II study of anti-PD1 antibody camrelizumab plus FOLFOX4 or GEMOX systemic chemotherapy as first-line therapy for advanced hepatocellular carcinoma or biliary tract cancer. J. Clin. Oncol. 2019, 37, 4074.
- A Trial of SHR-1210 (an Anti-PD-1 Inhibitor) in Combination with FOLFOX4 in Subjects with Advanced HCC Who Have Never Received Prior Systemic Treatment. Available online: (accessed on 10 April 2021).
- Mizukoshi, E.; Nakamoto, Y.; Arai, K.; Yamashita, T.; Sakai, A.; Sakai, Y.; Kagaya, T.; Yamashita, T.; Honda, M.; Kaneko, S. Comparative analysis of various tumor-associated antigen-specific t-cell responses in patients with hepatocellular carcinoma. Hepatology 2011, 53, 1206–1216.
- Zerbini, A.; Pilli, M.; Laccabue, D.; Pelosi, G.; Molinari, A.; Negri, E.; Cerioni, S.; Fagnoni, F.; Soliani, P.; Ferrari, C.; et al. Radiofrequency Thermal Ablation for Hepatocellular Carcinoma Stimulates Autologous NK-Cell Response. Gastroenterology 2010, 138.
- Hiroishi, K.; Eguchi, J.; Baba, T.; Shimazaki, T.; Ishii, S.; Hiraide, A.; Sakaki, M.; Doi, H.; Uozumi, S.; Omori, R.; et al. Strong CD8+ T-cell responses against tumor-associated antigens prolong the recurrence-free interval after tumor treatment in patients with hepatocellular carcinoma. J. Gastroenterol. 2010, 45, 451–458.
- Duffy, A.G.; Ulahannan, S.V.; Makorova-Rusher, O.; Rahma, O.; Wedemeyer, H.; Pratt, D.; Davis, J.L.; Hughes, M.S.; Heller, T.; ElGindi, M.; et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J. Hepatol. 2017, 66, 545–551.
- Pinato, D.J.; Cole, T.; Bengsch, B.; Tait, P.; Sayed, A.A.; Abomeli, F.; Gramenitskaya, D.; Allara, E.; Thomas, R.; Ward, C.; et al. A phase Ib study of pembrolizumab following trans-arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): PETAL. Ann. Oncol. 2019, 30, v288.
- Sangro, B.; Harding, J.J.; Johnson, M.; Palmer, D.H.; Edeline, J.; Abou-Alfa, G.K.; Cheng, A.-L.; Decaens, T.; El-Khoueiry, A.B.; Finn, R.S.; et al. A phase III, double-blind, randomized study of nivolumab (NIVO) and ipilimumab (IPI), nivo monotherapy or placebo plus transarterial chemoembolization (TACE) in patients with intermediate-stage hepatocellular carcinoma (HCC). J. Clin. Oncol. 2021, 39, TPS349.
- Sangro, B.; Kudo, M.; Qin, S.; Ren, Z.; Chan, S.; Joseph, E.; Arai, Y.; Mann, H.; Morgan, S.; Cohen, G.; et al. P-347 A phase 3, randomized, double-blind, placebo-controlled study of transarterial chemoembolization combined with durvalumab or durvalumab plus bevacizumab therapy in patients with locoregional hepatocellular carcinoma: EMERALD-1. Ann. Oncol. 2020, 31, S202–S203.
- Safety and Efficacy of Lenvatinib (E7080/MK-7902) with Pembrolizumab (MK-3475) in Combination with Transarterial Chemoembolization (TACE) in Participants with Incurable/Non-metastatic Hepatocellular Carcinoma (MK-7902-012/E7080-G000-318/LEAP-012). Available online: (accessed on 10 April 2021).
- Lee, Y.H.; Tai, D.; Yip, C.; Choo, S.P.; Chew, V. Combinational Immunotherapy for Hepatocellular Carcinoma: Radiotherapy, Immune Checkpoint Blockade and Beyond. Front. Immunol. 2020, 11.
- Tai, W.M.D.; Loke, K.S.H.; Gogna, A.; Tan, S.H.; Ng, D.C.E.; Hennedige, T.P.; Irani, F.; Lee, J.J.X.; Too, C.W.; Ng, M.C.H.; et al. A phase II open-label, single-center, nonrandomized trial of Y90-radioembolization in combination with nivolumab in Asian patients with advanced hepatocellular carcinoma: CA 209-678. J. Clin. Oncol. 2020, 38, 4590.
- Sangro, B. Nivolumab After Selective Internal Radiation Therapy (Sirt) Using Sir-Spheres Resin Microspheres in Patients with Hepatocellular Carcinoma: The Nasir-Hcc Trial. In Proceedings of the 14th Annual Conference of the International Liver Cancer Association (ILCA), Madrid, Spain, 11–13 September 2020.
- Ipilimumab and Stereotactic Body Radiation Therapy (SBRT) in Advanced Solid Tumors (NCT02239900). 2014. Available online: (accessed on 10 April 2021).
- Durvalumab (MEDI4736) and Tremelimumab and Radiation Therapy in Hepatocellular Carcinoma and Biliary Tract Cancer. Available online: (accessed on 10 April 2021).
