Dry eye disease (DED) is a common condition, which usually causes discomfort, but it can also be an origin of ocular pain and visual disturbances. Ocular surface inflammation is thought to be the main factor in the pathogenesis of DED. It has many overlapping causes, such as ocular surgery, environmental triggers, medication use and systemic diseases. Ophthalmic surgery may induce or worsen existing DED symptoms usually for a short-term period .
- dry eye disease
- ocular surface dysfunction
- cataract surgery
- phacoemulsification
- refractive surgery
- trabeculectomy
- vitrectomy
1. Introduction
Tear Film and Ocular Surface Society (TFOS) Dry Eye WorkShop (DEWS) II defines DED as follows “Dry eye is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles” [1]. DED can be classified into two categories: aqueous-deficient and evaporative. Aqueous-deficient DED may be associated with lacrimal gland dysfunction, while the evaporative type occurs with conditions affecting the eyelids or ocular surface. Eye structures and accessory organs of the eye, such as cornea, conjunctiva, eyelids, eyelashes, lacrimal and Meibomian glands, define the ocular surface. The tear film is a very important structure, as it is the interface of the ocular surface and the environment. Its function is to protect, lubricate the ocular exterior and form a stable refracting surface [2]. Chen et al. measured precorneal tear film thickness to be approximately 1.9 ± 0.9 μm using ultrahigh resolution optical coherent tomography [3]. Tear film is thought to comprise three layers: mucin, aqueous and lipid. Transmembrane mucins which are attached to epithelial cells extend to aqueous components containing various peptides, and proteins protect epithelial cells [4]. The tear film lipid layer is outermost, approximately 42 nm thick and ensures resistance to evaporation [5].
Disturbance of the balance of the tear film may be among the main factors in the development of the DED (Figure 1). Desiccation of the ocular surface, changes in tear composition and osmolarity lead to the activated production of inflammatory mediators and inflammatory cell recruitment. These processes trigger the dissolution of cell junctions, death of epithelial cells and destabilization of the tear film, which creates a cycle of inflammation [6]. Ocular surface inflammation alters the sensitization of nociceptors and causes a sensation of pain and dryness of the eye. Long-term inflammation and nerve injury can cause neuropathic pain on the ocular surface [7].
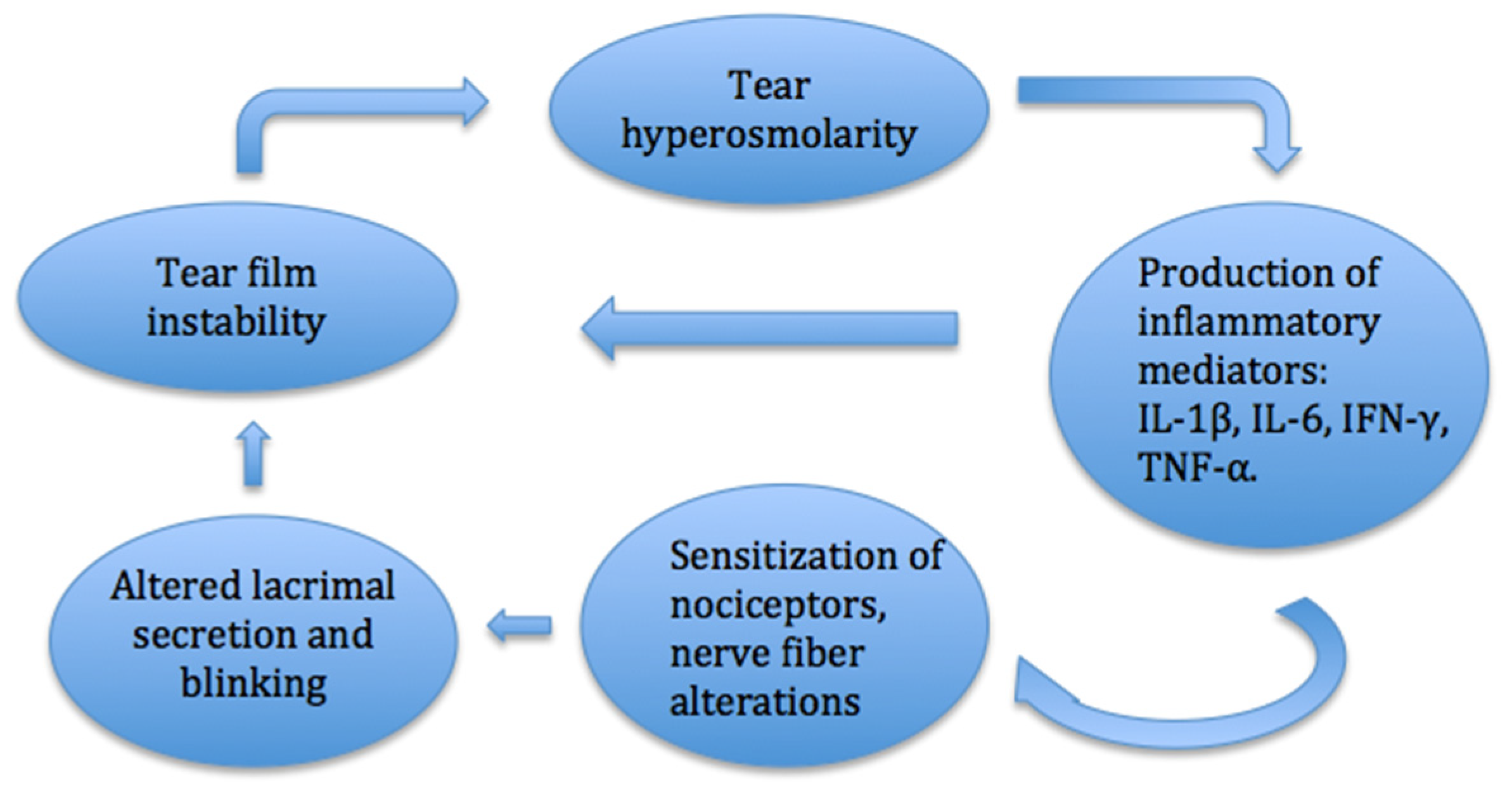
Homeostasis of the ocular surface is controlled by the physiological system and corneal innervation. The cornea is the most densely innervated organ in the human body. The ocular surface is supplied by sensory neurons of the trigeminal nerve (cranial nerve V). Sensory fibers travel to the cornea via the nasociliary branch of the ophthalmic nerve and via long ciliary nerves [7]. Long and short ciliary nerves form the pericorneal plexus at the corneal limbus. Corneal nerve fibers distribute into stromal and subepithelial plexuses below Bowman’s layer after entering the cornea in the corneoscleral limbus. Marfurt et al. analyzed the corneal nerve architecture of donor corneas. Nerve bundles entering the cornea through the corneoscleral limbus distribute uniformly around the cornea with a mean distance of 0.48 ± 0.40 mm [8]. Fibers end in the corneal epithelium as unmyelinated nerve endings which serve as nociceptors. Nociceptors are sensory to mechanical, chemical and thermal stimulation and transmit information to the brain. Facial nerves contain motor and autonomic fibers which control blinking and stimulate tear secretion in the lacrimal gland. Sensory nerve endings are able to respond to environmental changes and alter the intensity of tear production by trigeminal–parasympathetic reflex. Neural response mechanisms also regulate the secretion of compounds produced by goblet cells and Meibomian glands [9]. Due to reduced tear secretion, the tear film thins, and the corneal surface becomes vulnerable and exposed to unfavorable conditions. Corneal epithelium and terminal nerve branches may become injured. This initiates a cycle of nerve alternation which leads to the abnormal structure of corneal and conjunctival nerve fibers [7]. In a comparative study conducted by Labbe et al., the ocular surfaces of patients with non-Sjogren DED were analyzed with in vivo confocal microscopy and had a lower density and increased tortuosity and number of beadings of subbasal corneal nerves compared to control subjects. Corneal nerve density correlated with age, the Oxford scale and central corneal sensitivity. Alterations of subbasal corneal nerves were shown to be related to the severity of DED [10]. In another study, corneal sensitivity was significantly decreased in patients with DED and correlated positively with the density and number of subbasal nerves [11]. Subbasal nerve fiber length (SNFL) was also found to be significantly lower in patients with DED compared with controls. Patients with almost normal SNFL were shown to respond better to DED treatment (artificial tears and loteprednol etabonate with or without tobramycin 0.3% daily for 4 weeks) than subjects with low baseline SNFL [12]. As SNFL might help in evaluating the response to treatment, corneal subbasal nerve density, on the other hand, was found to correlate with endothelial cell loss. Endothelial cell density was lower in subjects with DED, and it correlated with the severity of the disease [13]. In a study conducted by Kheirkhan et al., patients with DED had lower densities of corneal endothelial cells and subbasal nerves compared to controls at their initial visit. The mean follow-up time was 33.2 months (±10.2). At the last visit, corneal subbasal nerve density did not change significantly, but there was a decrease in endothelial cell density, which correlated negatively with initial subbasal nerve density. Therefore, patients with low subbasal nerve density were at higher risk for endothelial cell loss [14].
In a study conducted by Kheirkhan et al., patients with DED had a higher density and size of dendritic cells and a higher number of dendrites compared to controls. These changes in the density and morphology of corneal epithelial immune dendritic cells reflected inflammation in DED [15]. The inflammatory component in the etiology of contact lens-induced DED was suggested by the increased mean Langerhans cell density in the central cornea and nasal bulbar conjunctiva found in symptomatic contact lens-wearing patients [16]. Inflammation is thought to be very important in the pathogenesis of DED. The release of mediators such as eicosanoids, bradykinin, histamine, purines and cytokines–interleukins contributes to the activation and sensitization of nociceptor terminals. Persistent exposure to inflammatory components may damage corneal nerves, while acute nerve injury causes local inflammation. These overlapping mechanisms increase the activity of sensory neurons and trigger a pain sensation, which can persist for prolonged periods of time. Twelve inflammatory cytokines (IL-1β, IL-6, IFN-γ and TNF-α) are most frequently detected in the tears of dry eye patients and are reliably used as biomarkers of DED [17]. The conjunctival expression of matrix metalloproteinase 9 and transglutaminase 2 was shown to be higher in patients with Meibomian gland dysfunction and showed inflammation of epithelial damage due to impaired tear secretion [18]. Patients with Meibomian gland dysfunction-related DED also had higher levels of inflammatory molecules, such as IL-6, IL-8, IFN-γ and ICAM-1, in their tears compared to control subjects [19]. In a study conducted by Macri et al., patients with DED were also shown to have increased levels of oxidative stress by assessing lipid peroxidation with the LP-CHOLOX test [20]. In another study, the expression of lipid peroxidation markers, such as hexanoyl-lysine, 4-hydroxy-2-nonenal and malondialdehyde, were measured in tears using enzyme-linked immunosorbent assays and, in the conjunctiva, using imunohistochemistry. In DED patients, concentrations of these markers were elevated and correlated with the severity of the disease [21].
2. Influence of Dry Eye Disease on Quality of Vision
Healthy and stable tear film is important for good optical quality, as tear film is the first surface for light to pass into the eye. Visual acuity is often normal, but other tests may show abnormalities, such as increased optical aberrations and decreased corneal sensitivity, in patients with DED [22]. The objective scatter index (OSI) is used to quantify ocular transparency. The scatter of light moving in the direction of the retina can be caused by unstable tear film. Su et al. analyzed the tear film objective scatter index (TF-OSI) in DED. The mean value and dispersion of TF-OSI were higher in patients with DED than in healthy subjects [23]. Another study conducted by Ma et al. examined visual quality in DED. The OSI was seen to correlate with clinical symptoms and signs, such as OSDI and TBUT. OSI was also found to correlate with corneal nerve length, which suggests that nerve changes may influence poor visual quality in DED patients [24]. Herbaut et al. also found that OSI correlated with OSDI, TBUT and Schirmer’s test results in DED patients [25]. Gao et al. examined optical quality in patients before and after dry eye treatment (hyaluronate and fluorometholone eye drops for two weeks). The OSI, contrast sensitivity, corneal high order aberrations, standard deviation of corneal power and surface asymmetry index improved after the treatment [26]. Similar results were seen in a study conducted by Lu et al. Optical quality was seen to improve by evaluating high-order aberrations of corneal surface before and after DED treatment (artificial eye drops for two weeks) [27]. On the other hand, Arikan et al. compared contrast sensitivity and corneal aberrations in patients with primary Sjogren syndrome (DED) and healthy participants. Contrast sensitivity function and corneal high-order aberrations did not differ between patients with Sjogren syndrome and the control group [28]. Ocular surface dysfunction might also affect preoperative cataract surgery planning due to topography and keratometry measures [29]. Increased osmolarity of the tear film as a result of ocular surface disease has been shown to influence keratometry results and, therefore, intraocular lens (IOL) power calculations. Hyperosmolarity is associated with tear film instability and rapid breakup after blinking. Epitropolous et al. compared the keratometry value, corneal astigmatism and IOL power between hyperosmolar (osmolarity more than 316 mOsm/Lin at least one eye) and normal (osmolarity less than 308 mOsm/L in both eyes) patients. A higher variability in the keratometry value and a greater difference in the measured corneal astigmatism and IOL power were seen in the hyperosmolar group [30]. Variability of keratometry values in dry eyes was also shown in a study conducted by Roggla et al. [31]. The instillation of artificial tears before keratometry may show more stable results, although in other studies, eye drops did not the change variability of keratometry values [32].
This entry is adapted from the peer-reviewed paper 10.3390/jcm10081642
References
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283.
- Willcox, M.D.; Argüeso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U.; et al. The Ocular Surface TFOS DEWS II Tear Film Report. Ocul. Surf. 2017, 15, 366–403.
- Chen, Q.; Wang, J.; Tao, A.; Shen, M.; Jiao, S.; Lu, F. Ultrahigh-Resolution Measurement by Optical Coherence Tomography of Dynamic Tear Film Changes on Contact Lenses. Investig. Opthalmol. Vis. Sci. 2010, 51, 1988–1993.
- Zhou, L.; Zhao, S.Z.; Koh, S.K.; Chen, L.; Vaz, C.; Tanavde, V.; Li, X.R.; Beuerman, R.W. In-depth analysis of the human tear proteome. J. Proteom. 2012, 75, 3877–3885.
- King-Smith, P.E.; Hinel, E.A.; Nichols, J.J. Application of a Novel Interferometric Method to Investigate the Relation between Lipid Layer Thickness and Tear Film Thinning. Investig. Opthalmol. Vis. Sci. 2010, 51, 2418–2423.
- Pflugfelder, S.C.; De Paiva, C.S.; States, U. The pathophysiology of dry eye disease: What we know and future directions for research. Ophthalmology 2017, 124 (Suppl. S11), S4–S13.
- Belmonte, C.; Nichols, J.J.; Cox, S.M.; Brock, J.A.; Begley, C.G.; Bereiter, D.A.; Dartt, D.A.; Galor, A.; Hamrah, P.; Ivanusic, J.J.; et al. The Ocular Surface TFOS DEWS II pain and sensation report. Ocul. Surf. 2017, 15, 404–437.
- Marfurt, C.F.; Cox, J.; Deek, S.; Dvorscak, L. Anatomy of the human corneal innervation. Exp. Eye Res. 2010, 90, 478–492.
- Labetoulle, M.; Baudouin, C.; Calonge, M.; Merayo-Lloves, J.; Boboridis, K.G.; Akova, Y.A.; Aragona, P.; Geerling, G.; Messmer, E.M.; Benítez-Del-Castillo, J. Role of corneal nerves in ocular surface homeostasis and disease. Acta Ophthalmol. 2019, 97, 137–145.
- Labbé, A.; Liang, Q.; Wang, Z.; Zhang, Y.; Xu, L.; Baudouin, C.; Sun, X. Corneal Nerve Structure and Function in Patients with Non-Sjögren Dry Eye: Clinical Correlations. Investig. Opthalmol. Vis. Sci. 2013, 54, 5144–5150.
- Labbé, A.; Alalwani, H.; Van Went, C.; Brasnu, E.; Georgescu, D.; Baudouin, C. The Relationship between Subbasal Nerve Morphology and Corneal Sensation in Ocular Surface Disease. Investig. Opthalmol. Vis. Sci. 2012, 53, 4926–4931.
- Kheirkhah, A.; Dohlman, T.H.; Amparo, F.; Arnoldner, M.A.; Jamali, A.; Hamrah, P.; Dana, R. Effects of Corneal Nerve Density on the Response to Treatment in Dry Eye Disease. Ophthalmology 2015, 122, 662–668.
- Kheirkhah, A.; Saboo, U.S.; Abud, T.B.; Dohlman, T.H.; Arnoldner, M.A.; Hamrah, P.; Dana, R. Reduced Corneal Endothelial Cell Density in Patients with Dry Eye Disease. Am. J. Ophthalmol. 2015, 159, 1022–1026.e2.
- Kheirkhah, A.; Satitpitakul, V.; Hamrah, P.; Dana, R. Patients With Dry Eye Disease and Low Subbasal Nerve Density Are at High Risk for Accelerated Corneal Endothelial Cell Loss. Cornea 2017, 36, 196–201.
- Kheirkhah, A.; Darabad, R.R.; Cruzat, A.; Hajrasouliha, A.R.; Witkin, D.; Wong, N.; Dana, R.; Hamrah, P. Corneal Epithelial Immune Dendritic Cell Alterations in Subtypes of Dry Eye Disease: A Pilot In Vivo Confocal Microscopic Study. Investig. Opthalmol. Vis. Sci. 2015, 56, 7179–7185.
- Alzahrani, Y.; Colorado, L.H.; Pritchard, N.; Efron, N. Longitudinal changes in Langerhans cell density of the cornea and conjunctiva in contact lens-induced dry eye. Clin. Exp. Optom. 2017, 100, 33–40.
- Wei, Y.; Gadaria-Rathod, N.; Epstein, S.; Asbell, P. Tear Cytokine Profile as a Noninvasive Biomarker of Inflammation for Ocular Surface Diseases: Standard Operating Procedures. Investig. Opthalmol. Vis. Sci. 2013, 54, 8327–8336.
- Aragona, P.; Aguennouz, M.; Rania, L.; Postorino, E.; Sommario, M.S.; Roszkowska, A.M.; De Pasquale, M.G.; Pisani, A.; Puzzolo, D. Matrix Metalloproteinase 9 and Transglutaminase 2 Expression at the Ocular Surface in Patients with Different Forms of Dry Eye Disease. Ophthalmology 2015, 122, 62–71.
- Wu, X.; Chen, X.; Ma, Y.; Lin, X.; Yu, X.; He, S.; Luo, C.; Xu, W. Analysis of tear inflammatory molecules and clinical correlations in evaporative dry eye disease caused by meibomian gland dysfunction. Int. Ophthalmol. 2020, 40, 3049–3058.
- Macri, A.; Scanarotti, C.; Bassi, A.M.; Giuffrida, S.; Sangalli, G.; Traverso, C.E.; Iester, M. Evaluation of oxidative stress levels in the conjunctival epithelium of patients with or without dry eye, and dry eye patients treated with preservative-free hyaluronic acid 0.15% and vitamin B12 eye drops. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 425–430.
- Choi, W.; Lian, C.; Ying, L.; Kim, G.E.; You, I.C.; Park, S.H.; Yoon, K.C. Expression of Lipid Peroxidation Markers in the Tear Film and Ocular Surface of Patients with Non-Sjogren Syndrome: Potential Biomarkers for Dry Eye Disease. Curr. Eye Res. 2016, 41, 1143–1149.
- Herbaut, A.; Liang, H.; Denoyer, A.; Baudouin, C.; Labbé, A. Tear film analysis and evaluation of optical quality: A review of the literature. J. Gynecol. Obstet. Biol. Reprod. 2019, 42, e21–e35.
- Su, Y.D.; Liang, Q.F.; Wang, N.L.; Antoine, L. A study on the diagnostic value of tear film objective scatter index in dry eye. Chin. J. Ophthalmol. 2017, 53, 668–674.
- Ma, J.; Wei, S.; Jiang, X.; Chou, Y.; Wang, Y.; Hao, R.; Yang, J.; Li, X. Evaluation of objective visual quality in dry eye disease and corneal nerve changes. Int. Ophthalmol. 2020, 40, 2995–3004.
- Herbaut, A.; Liang, H.; Rabut, G.; Trinh, L.; Kessal, K.; Baudouin, C.; Labbé, A. Impact of Dry Eye Disease on Vision Quality: An Optical Quality Analysis System Study. Transl. Vis. Sci. Technol. 2018, 7, 5.
- Gao, Y.; Liu, R.; Liu, Y.; Ma, B.; Yang, T.; Hu, C.; Qi, H. Optical quality in patients with dry eye before and after treatment. Clin. Exp. Optom. 2021, 104, 101–106.
- Lu, N.; Lin, F.; Huang, Z.; He, Q.; Han, W. Changes of Corneal Wavefront Aberrations in Dry Eye Patients after Treatment with Artificial Lubricant Drops. J. Ophthalmol. 2016, 2016, 1342056.
- Arikan, S.; Gökmen, F.; Comez, A.T.; Gencer, B.; Kara, S.; Akbal, A. Evaluation of possible factors affecting contrast sensitivity function in patients with primary Sjögren’s syndrome. Arq. Bras. Oftalmol. 2015, 78, 150–153.
- Chuang, J.; Shih, K.C.; Chan, T.C.; Wan, K.H.; Jhanji, V.; Tong, L. Preoperative optimization of ocular surface disease before cataract surgery. J. Cataract. Refract. Surg. 2017, 43, 1596–1607.
- Epitropoulos, A.T.; Matossian, C.; Berdy, G.J.; Malhotra, R.P.; Potvin, R. Effect of tear osmolarity on repeatability of keratometry for cataract surgery planning. J. Cataract. Refract. Surg. 2015, 41, 1672–1677.
- Röggla, V.; Leydolt, C.; Schartmüller, D.; Schwarzenbacher, L.; Meyer, E.; Abela-Formanek, C.; Menapace, R. Influence of Artificial Tears on Keratometric Measurements in Cataract Patients. Am. J. Ophthalmol. 2021, 221, 1–8.
- Jensen, M.N.; Søndergaard, A.P.; Pommerencke, C.; Møller, F. Variations in keratometric values (K-value) after administration of three different eye drops—Effects on the intraocular lens calculations in relation to cataract surgery. Acta Ophthalmol. 2020, 98, 613–617.
