Detailed AgNW synthesis methods, including template assisted histrorical improvements and modern wet chemical techniques. A detailed analysis of the various pre- and post-treatment methods used to improve the optoelectronic properties of the fabricated Transparent conducting electrode with future outlooks and challanges for practical applicabilty.
- nanowire
- transparent conducting electrodes
- optoelectronics
1. Introduction
One-dimensional (1D) metal nanomaterials have attracted widespread research interest due to their unique electrical, magnetic, optical, thermal and catalytic properties. Consequently, such nanomaterials have become promising candidates for a wide range of applications in optoelectronics, nanoelectronics, nanophotonics and micromechanics because of their exceptional density of states and high aspect ratio [1][2][3][4][5]. In particular, silver (Ag) has the highest electrical and thermal conductivities of all metals, and its 1D form, referred to as Ag nanowires (AgNWs), has huge potential for practical applicability in a range of technologies. Over the years, numerous AgNW synthesis protocols have been proposed, including the use of soft and hard templates and a range of wet-chemical techniques like hydrothermal, solvothermal and polyol-based synthesis. This has resulted in wide range of achievable dimensions (diameter 2–300 nm and length 1–500 µm). A relative comparison on the bases of simplicity, control, yield and cost demonstrated that the polyol process is the most promising method for AgNW synthesis. Research in the area of AgNWs has been extensive, with over 100,000 publications covering a vast range of applications including smart sensors [6][7][8][9][10], catalysis [11], energy harvesting devices [12], optoelectronic displays [13] and wearable electronics [14][15][16], among others. Furthermore, theoretical predictions and experimental demonstrations have highlighted the fact that AgNWs can significantly improve the properties of transparent conducting electrodes (TCE), which are an essential component of optoelectronic devices. Traditionally the material of choice for fabricating TCE has been indium tin oxide (ITO) due to its low sheet resistance, high optical transmittance, low surface roughness and ability to pattern electrodes using photolithography. However, ITO has various drawbacks, including its high fabrication cost and brittle nature, which hinders its applicability for use in next-generation flexible optoelectronic devices like smart windows [17][18], electronic skins [19], smart clothes [20], wearable heaters [21][22], foldable displays [23][24][25][26], compliant solar cells [27] and flexible display panels [28]. These devices require TCE that fulfill certain criteria, including solution-based low cost processing capability, excellent optoelectronic properties, mechanical compliance and chemical stability. A variety of alternatives to ITO, such as carbon nanotubes (CNTs), graphene oxide (GO), aluminum zinc oxide (AZO), metal nanowires, nanofibers and conductive polymers have been reported for TCE fabrication. In particular, AgNW based TCE have emerged as a perfect contender for modern optoelectronic and wearable devices due their low-cost and scalable solution based processing, high conductivity and transparency, smooth surface and mechanical flexibility [16][29][30][31][32][33][34][35][36][37]. AgNWs can be deposited on various substrates and form a network with openings between the nanowires that allow light transmittance while conductive pathways are formed at the nanowire junctions.
Herein, we have compiled and presented a detailed review of AgNW synthesis and strategies for AgNW based TCE fabrication. A schematically illustrated outline of the review is presented in Figure 1. To the best of our knowledge, this is the first review that discusses all solution based TCE fabrication methods in detail and provides a comprehensive outlook of the various treatment techniques utilized to improve TCE performance. Briefly, this review is structured in the following manner: Section 1 introduces AgNWs and their utilization for TCE fabrication. Section 2 discusses the numerous AgNW synthesis methods, including template assisted and wet chemical techniques. Section 3 discusses the various solution-based methods used for TCE fabrication. In addition, it also provides a detailed analysis of the various pre- and post-treatment methods used to improve the optoelectronic properties of the fabricated TCE. Finally, outlooks and challenges for the practical applicability of AgNWs are presented.

Figure 1. Schematic illustration of the review outline.
2. AgNW Synthesis
Over the years, several protocols for AgNW synthesis have been reported. The use of templates, such as hard and soft templates, were widely investigated in the early years of AgNW synthesis. While predefined dimensions of the template assist in uniform nanowire synthesis, purification methods are quite complex and result in low yields. To overcome these issues, various wet chemical methods such as hydrothermal, solvothermal and polyol were later introduced. Among these, the polyol method has gathered much attention, since it is facile, scalable and provides good control over nanowire morphology. In this section, first, we will discuss the template methods, followed by the wet chemical methods. A Table 1 summarizing the various synthesis protocols is also added in the end of this section to provide a concise comparison and improve readability.
Table 1. Summarized table of AgNW synthesis methods.
|
Method |
Template |
NW Length (µm) |
NW Diameter (nm) |
Ag Reduction Parameters |
Characterization |
Temperature and Time |
Key Findings |
Ref. |
|---|---|---|---|---|---|---|---|---|
|
Hard template |
λ -DNA |
(12–16) |
N/A |
HQ/NaCl |
FI (fluorescence imaging), AFM |
N/A |
a stretchable electrode in nanowire form |
[38] |
|
SBA-15 |
>1 |
7 |
DMAB |
TEM, EDS, XRD |
40 °C for four days |
nanowire length can be controlled by red. time |
[39] |
|
|
SBA-15 |
4 |
7 |
Modified SBA-15 |
TEM, XRD, SEM |
85 °C overnight |
dodecanthiol as surfactant to avoid agglomeration |
[40] |
|
|
AAO template |
>20 |
50 |
High temp. melting to get Ag ions |
XRD, SEM, EDS, BSE, TEM, DSC |
970 °C for 30 min |
novel hydrothermal purification step |
[41] |
|
|
Soft template |
CHQ nanotubes |
~1 |
~1 |
UV radiation, CHQ nanotube |
TEM, EDS |
NTP |
ultrathin nanowires with long term stability |
[42] |
|
β-CD |
<20 |
~65 |
β-CD, FeCl3 |
TEM, EDS, XRD, SEM |
170 °C for 1 h |
FeCl3 addition facilitates > 300 aspect ratio |
[43] |
|
|
J-aggregates of cyanine dyes |
>10 |
7 |
nanotubular J-aggregate |
UV-VIS, TEM |
NTP |
light enhanced growth of AgNW in dyes |
[44] |
|
|
DHBC (PEO-b-PMAA) |
>1 |
20–40 |
PMMA reducing agent |
UV-VIS, TEM |
NTP for 54 h |
double polymers help in reduction and solubility |
[45] |
|
|
Method |
Surfactant/ Red. Agent |
NW Length (µm) |
NW Diameter (nm) |
Salt Mediator |
Temperature and Time |
Note |
Ref. |
|
|
Hydrothermal |
PVP (40 k)/D+ glucose |
200–500 |
45–65 |
NaCl |
160 °C for 22 h |
PVP avoids precipitation |
[46] |
|
|
Gemini surfactant |
(10–90) |
∼30 |
No salts used |
100 °C for 24 h |
Simple procedure at low temp. without using salts |
[47] |
||
|
glucose |
<500 |
100 |
NaCl |
180 °C for 18 h |
Optimization of temp, time and reagent concentrations. |
[48] |
||
|
Solvothermal |
PVP (1300 k), EG |
220 |
50 |
FeCl3 |
130 °C for 8 h |
FeCl3 concentration optimization |
[49] |
|
|
PVP (1300 k), EG |
120 |
40 |
KBr/NaCl (1:4) |
170 °C for 2.5 h |
KBr and NaCl ratio optimization |
[50] |
||
|
PVP(1300 k), glycerol |
100–160 |
40–85 |
NaCl |
150 °C for 5 h |
AgNW synthesis without using transition metals and EG |
[51] |
||
|
PVP(1300 k), (propanediol) |
140 |
140 |
diluted HCl |
140 °C for 1 h |
propendiol as a substitute of EG for rapid synthesis |
[52] |
||
|
PVP (360 k), EG |
>100 |
100 |
CuCl2 |
130 °C for 3 h |
AgNW and kirigami pattern combined for skin electronics |
[53] |
||
|
PVP(1300 k), EG |
120 |
30 |
FeCl3, CuCl2, NaBr |
170 °C for <1 h |
Optimised Cu, Fe and Br conc. To attain 3000 aspect ratio |
[54] |
||
|
Conventional polyol |
PVP (360 k), EG |
80–110 |
50–70 |
KBr, CuCl2 |
170 °C for 1 h |
rapid synthesis with good control over dimensions |
[55] |
|
|
PVP (58 k), EG |
123 |
120 |
FeCl3 |
130 °C for 3.5 h |
high FoM of fabricated TCE |
[56] |
||
|
PVP(1300 k), EG |
75 |
40 |
CuCl2 |
160 °C for 1.42 h |
stirring and injection rate optimization |
[57] |
||
|
Millifluidic polyol |
PVP (55 k), EG |
(10–20) |
(50–100) |
CuCl2 |
170 °C for 1.5 h |
optimizing the millifluidic reactor parameter |
[58] |
|
FI: fluorescence imaging; PVP: polyvinylpyrrolidone; DHBC: double hydrophilic block copolymer; β-CD: β-cyclodextrin; PMMA: poly (methyl methacrylate); C-HQ: calix-hydroquinone nanotubes; SBA: mesoporous silica; AAO: anodic aluminium oxide; NTP: normal temperature and pressure; EG: ethylene glycol; FoM: figure of merit; DMAB: dimethylamine borane;Gemini surfactant: 1,3-bis(cetyldimethylammonium) propane dibromide; Amphiphilic cyanine dye: 3,3-bis(2-sulfopropyl)-5,5,6,6-tetrachloro-1,1-dioctylbenzimidacarbocyanine (C8S3).
2.1. Template Methods
Researchers have utilized a range of templates that enable the growth of 0-D nanoparticles into 1-D nanowires. These templates are generally used to provide a specific size and structure to the nanowire [59]. These templates can be broadly categorized as either hard or soft. Hard templates result in greater control over the shape, size and overall morphology of the nanowire because of their predesigned structure that shapes the Ag atoms into nanowires. Although simple and effective, there can be significant loss of generated nanowires during the purification step, leading to lower yields. On the other hand, soft template methods utilize chemicals which are dispersible/dissolvable in solvents. Consequently, the synthesized nanowires can be easily purified from the solvent phase, thus resulting in improved efficiency and scalability.
2.2. Chemical Methods
Most of the template methods are not efficient for scalable industrial production of AgNWs. This has encouraged researchers to think beyond existing templates and develop chemical synthesis methods that provide better control over the obtained morphology and avoid the template removal step. Chemical methods include solvents, surfactants, reducing and oxidizing agents to enable nanowires synthesis. The first stage of synthesis is nanocrystal formation in the solution, which later grows to form the nanorod (aspect ratio <10) or nanowire (aspect ratio ~101–104) structure. The process of synthesizing AgNWs and nanorods by Ag seed in controlled manner are quite similar which can be observed in Figure 2h,i. Ag seeds were synthesized by reducing AgNO3 in presence of Sodium borohydride (NaBH4) and trisodium citrate (Na3C6H5O7) where NaBH4 controls the particle size and Na3C6H5O7 stabilizes it. Later to synthesize nanowires and nanorods, ascorbic acid (AA) was used to reduce AgNO3 in a solution embedded with Ag seed, NaOH and the micelle template cetyltrimethylammoniun bromide (CTAB) [60]. The relative amount of NaOH in solution was the deciding factor for the synthesis of nanowires and nanorods. Since the optimized pH for nanorods and nanowires were 11.8 and 4.1 respectively, it suggests that the monoanion of ascorbic acid is the dominating factor in nanowire synthesis while the ascorbate dianion is an important factor in nanorod synthesis.
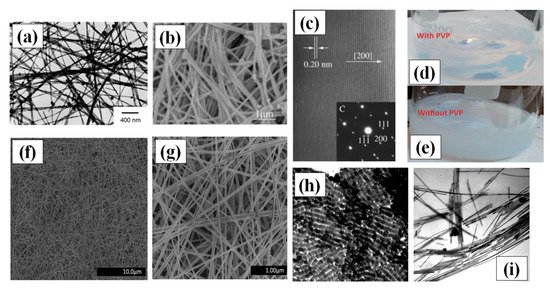
Figure 2. (a) TEM images synthesized AgNWs using 1,3-bis(cetyldimethylammonium) propane dibromide (adapted from [47] with permission from Elsevier, 2006) (b) SEM image of the prepared AgNWs using glucose as reducing agents (adapted from [48] with permission from Chemistry-A European Journal, 2005). (c) A HRTEM image and single crystal SAED pattern of AgNWs (d–e) Digital camera images of solutions prepared for hydrothermal synthesis (d) with PVP in the form of hydrosol and (e) without PVP having precipitates in solution (f,g) The SEM images of uniform, ultralong and thin AgNWs prepared by the hydrothermal method at 160 °C for 22 h of reaction (adapted from [46] with permission from Royal Society of Chemistry, 2013) (h) TEM image of shape controlled silver nanorods (using pH~11); scale bar = 100 nm. (i) TEM image of AgNWs (using pH~11); scale bar = 100 nm (adapted from [60] with permission from Royal Society of Chemistry, 1996).
Sonochemical methods have also been used for the synthesis of AgNWs [61][62]. J.-J. Zhu et al. proposed a novel and seedless method for synthesis of Ag nanowires by a sonoelectrochemical method [63]. The electrochemical cell was composed of two platinum (Pt) sheets and an electrolyte composed of AgNO3 and ethylenediamine tetraacetic acid (EDTA) in water. The whole cell was kept in ultrasonic bath under nitrogen (N2) atmosphere with a current and voltage controlled electrolysis process for 30–45 min to grow AgNWs at 30 °C temperature. Another wet chemical synthesis method of AgNW interlacing bunches on glass walls by mild chemical reduction in aqueous solutions of PMAA was demonstrated [64]. Single-crystalline AgNWs with diameters of about 30–40 nm were synthesized inside the glass tube after 24 h at ambient temperature in the presence of AA as a reducing agent. Liu et al. introduced the idea of seed and crystals, where they first synthesized the silver bromide (AgBr) crystal seed which later grows into AgNWs with a maximum length of 9 µm [65].
3. AgNW Based Transparent Conductive Electrodes
Transparent conducting electrodes (TCEs) with high optical transmittance and low sheet resistance are crucial for development and performance of optoelectronic devices [128]. ITO is the most commonly used transparent conductive electrode (TCE) for various applications such as solar cells [129], flat panel displays [130], LED [131], OLED [132] and touch screen [133], because of its excellent optoelectronic properties [134] (transparency~90% and sheet resistance~10 Ω/sq). However, there are some major limitations of using ITO which include its brittle nature [135] and high fabrication costs [136]. The growth in demands of modern optoelectronic devices needs to fulfil many crucial characteristics such as solution based processing, low cost, scalable fabrication, excellent optical transparency, low sheet resistance, good mechanical (flexible, stretchable and stress consumable) properties and improved chemical stability [13,16]. Hence, these properties are critically important for application and evaluation of TCF performance using the Figure of merit (FoM). FoM depends on transmittance (T) and sheet resistance Rs of the material. Transmittance follows lambert beers law and can be evaluated as

(where “a” is the absorption coefficient and “t” is the film thickness), while the sheet resistance can be simply calculated by keeping the electrodes at unit distance from each other such as the two or four probe method. The relation between T and Rs can be written as:

where T is transmittance, Z0 is impedance of free space (377 Ω) and the ratio of σDC and σop is known as (FoM), which determines the goodness of the fabricated TCF [137].
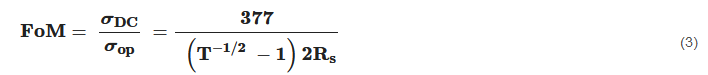
Researchers have proposed many alternatives to fulfil the above mentioned properties, such as graphene [66], CNT [67], AgNW [13] and (AZO) [68]. Graphene has high in plain conductivity with excellent optical transparency (~95) and low sheet resistance (50–1000 Ω) [69]. However solution processed graphene has high sheet resistance and chemical vapor deposition technique is too expensive for large scale applications due to the high temperature (>1000 °C) requirement [70]. CNT based transparent conducting electrodes are highly transparent (~90%), but the sheet resistance (200–1000 Ω) [67] of CNT is significantly higher as compared to ITO. Metallic nanostructures especially AgNWs (AgNWs) have arisen as a perfect alternative for transparent conducting electrodes due to their flexibility, low intrinsic resistance and excellent optical transmission [29]. The major requirement of transparency can be achieved by decreasing the density of the solution and minimizing the size of the material at the nanoscale [71]. Nanowires are the ideal material for such applications because of their crosslinking structure as they have two dimensions quantum mechanically confined to the nanoscale and one unconfined dimension that helps in conductivity and crosslinking to each other. Specific applications can be achieved by choosing a suitable nanowire size and diameter.
This entry is adapted from the peer-reviewed paper 10.3390/nano11030693
References
- Zhao, J.; Buia, C.; Han, J.; Lu, J.P. Quantum transport properties of ultrathin silver nanowires. Nanotechnology 2003, 14, 501.
- Dresselhaus, M.S.; Lin, Y.-M.; Rabin, O.; Black, M.R.; Kong, J.; Dresselhaus, G. Nanowires. In Springer Handbook of Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 119–167.
- Rodrigues, V.; Bettini, J.; Rocha, A.R.; Rego, L.G.C.; Ugarte, D. Quantum conductance in silver nanowires: Correlation between atomic structure and transport properties. Phys. Rev. B 2002, 65, 153402.
- Zhang, R.; Engholm, M. Recent progress on the fabrication and properties of silver nanowire-based transparent electrodes. Nanomaterials 2018, 8, 628.
- Yin, X.; Kumar, S. Flow visualization of the liquid emptying process in scaled-up gravure grooves and cells. Chem. Eng. Sci. 2006, 61, 1146–1156.
- Wang, L.; Gao, X.; Jin, L.; Wu, Q.; Chen, Z.; Lin, X. Amperometric glucose biosensor based on silver nanowires and glucose oxidase. Sens. Actuators B Chem. 2013, 176, 9–14.
- Jones, R.S.; Draheim, R.R.; Roldo, M. Silver nanowires: Synthesis, antibacterial activity and biomedical applications. Appl. Sci. 2018, 8, 673.
- Lee, H.; Kim, M.; Kim, I.; Lee, H. Flexible and stretchable optoelectronic devices using silver nanowires and graphene. Adv. Mater. 2016, 28, 4541–4548.
- Karthik, P.S.; Singh, S.P. Conductive silver inks and their applications in printed and flexible electronics. RSC Adv. 2015, 5, 77760–77790.
- Dahiya, R.; Yogeswaran, N.; Liu, F.; Manjakkal, L.; Burdet, E.; Hayward, V.; Jörntell, H. Large-area soft e-skin: The challenges beyond sensor designs. Proc. IEEE 2019, 107, 2016–2033.
- Kostowskyj, M.A.; Gilliam, R.J.; Kirk, D.W.; Thorpe, S.J. Silver nanowire catalysts for alkaline fuel cells. Int. J. Hydrog. Energy 2008, 33, 5773–5778.
- Liang, X.; Zhao, T.; Jiang, W.; Yu, X.; Hu, Y.; Zhu, P.; Zheng, H.; Sun, R.; Wong, C.-P. Highly transparent triboelectric nanogenerator utilizing in-situ chemically welded silver nanowire network as electrode for mechanical energy harvesting and body motion monitoring. Nano Energy 2019, 59, 508–516.
- Li, W.; Zhang, H.; Shi, S.; Xu, J.; Qin, X.; He, Q.; Yang, K.; Dai, W.; Liu, G.; Zhou, Q. Recent progress in silver nanowire networks for flexible organic electronics. J. Mater. Chem. C 2020, 8, 4636–4674.
- Huo, D.; Kim, M.J.; Lyu, Z.; Shi, Y.; Wiley, B.J.; Xia, Y. One-dimensional metal nanostructures: From colloidal syntheses to applications. Chem. Rev. 2019, 119, 8972–9073.
- Huang, Q.; Zhu, Y. Printing conductive nanomaterials for flexible and stretchable electronics: A review of materials, processes, and applications. Adv. Mater. Technol. 2019, 4, 1800546.
- Kwon, J.; Suh, Y.D.; Lee, J.; Lee, P.; Han, S.; Hong, S.; Yeo, J.; Lee, H.; Ko, S.H. Recent progress in silver nanowire based flexible/wearable optoelectronics. J. Mater. Chem. C 2018, 6, 7445–7461.
- Park, J.-Y.; Kim, H.-K. Highly stretchable polymer-dispersed liquid crystal-based smart windows with transparent and stretchable hybrid electrodes. RSC Adv. 2018, 8, 36549–36557.
- Mallikarjuna, K.; Kim, H. Highly transparent conductive reduced graphene oxide/silver nanowires/silver grid electrodes for low-voltage electrochromic smart windows. ACS Appl. Mater. Interfaces 2018, 11, 1969–1978.
- Lipomi, D.J.; Vosgueritchian, M.; Tee, B.C.; Hellstrom, S.L.; Lee, J.A.; Fox, C.H.; Bao, Z. Skin-like pressure and strain sensors based on transparent elastic films of carbon nanotubes. Nat. Nanotechnol. 2011, 6, 788–792.
- Hu, B.; Li, D.; Ala, O.; Manandhar, P.; Fan, Q.; Kasilingam, D.; Calvert, P.D. Textile-Based Flexible Electroluminescent Devices. Adv. Funct. Mater. 2011, 21, 305–311.
- Choi, S.; Park, J.; Hyun, W.; Kim, J.; Kim, J.; Lee, Y.B.; Song, C.; Hwang, H.J.; Kim, J.H.; Hyeon, T. Stretchable heater using ligand-exchanged silver nanowire nanocomposite for wearable articular thermotherapy. ACS Nano 2015, 9, 6626–6633.
- Hong, S.; Lee, H.; Lee, J.; Kwon, J.; Han, S.; Suh, Y.D.; Cho, H.; Shin, J.; Yeo, J.; Ko, S.H. Highly stretchable and transparent metal nanowire heater for wearable electronics applications. Adv. Mater. 2015, 27, 4744–4751.
- Liang, J.; Li, L.; Tong, K.; Ren, Z.; Hu, W.; Niu, X.; Chen, Y.; Pei, Q. Silver nanowire percolation network soldered with graphene oxide at room temperature and its application for fully stretchable polymer light-emitting diodes. ACS Nano 2014, 8, 1590–1600.
- Miller, M.S.; O’Kane, J.C.; Niec, A.; Carmichael, R.S.; Carmichael, T.B. Silver nanowire/optical adhesive coatings as transparent electrodes for flexible electronics. ACS Appl. Mater. Interfaces 2013, 5, 10165–10172.
- Wang, J.; Yan, C.; Cai, G.; Cui, M.; Lee-Sie Eh, A.; See Lee, P. Extremely stretchable electroluminescent devices with ionic conductors. Adv. Mater. 2016, 28, 4490–4496.
- Liang, J.; Li, L.; Niu, X.; Yu, Z.; Pei, Q. Elastomeric polymer light-emitting devices and displays. Nat. Photonics 2013, 7, 817–824.
- Lipomi, D.J.; Tee, B.C.K.; Vosgueritchian, M.; Bao, Z. Stretchable organic solar cells. Adv. Mater. 2011, 23, 1771–1775.
- Wang, J.; Liang, M.; Fang, Y.; Qiu, T.; Zhang, J.; Zhi, L. Rod-coating: Towards large-area fabrication of uniform reduced graphene oxide films for flexible touch screens. Adv. Mater. 2012, 24, 2874–2878.
- Langley, D.; Giusti, G.; Mayousse, C.; Celle, C.; Bellet, D.; Simonato, J.-P. Flexible transparent conductive materials based on silver nanowire networks: A review. Nanotechnology 2013, 24, 452001.
- Basarir, F.; Irani, F.S.; Kosemen, A.; Camic, B.T.; Oytun, F.; Tunaboylu, B.; Shin, H.J.; Nam, K.Y.; Choi, H. Recent progresses on solution-processed silver nanowire based transparent conducting electrodes for organic solar cells. Mater. Today Chem. 2017, 3, 60–72.
- Kumar, A.; Zhou, C. The race to replace tin-doped indium oxide: Which material will win? ACS Nano 2010, 4, 11–14.
- Zhang, P.; Wyman, I.; Hu, J.; Lin, S.; Zhong, Z.; Tu, Y.; Huang, Z.; Wei, Y. Silver nanowires: Synthesis technologies, growth mechanism and multifunctional applications. Mater. Sci. Eng. B 2017, 223, 1–23.
- Hecht, D.S.; Hu, L.; Irvin, G. Emerging transparent electrodes based on thin films of carbon nanotubes, graphene, and metallic nanostructures. Adv. Mater. 2011, 23, 1482–1513.
- Xiang, X.-Z.; Gong, W.-Y.; Kuang, M.-S.; Wang, L. Progress in application and preparation of silver nanowires. Rare Met. 2016, 35, 289–298.
- Abbasi, N.M.; Yu, H.; Wang, L.; Amer, W.A.; Akram, M.; Khalid, H.; Chen, Y.; Saleem, M.; Sun, R.; Shan, J. Preparation of silver nanowires and their application in conducting polymer nanocomposites. Mater. Chem. Phys. 2015, 166, 1–15.
- Sun, Y. Silver nanowires–unique templates for functional nanostructures. Nanoscale 2010, 2, 1626–1642.
- He, W.; Ye, C. Flexible transparent conductive films on the basis of Ag nanowires: Design and applications: A review. J. Mater. Sci. Technol. 2015, 31, 581–588.
- Braun, E.; Eichen, Y.; Sivan, U.; Ben-Yoseph, G. DNA-templated assembly and electrode attachment of a conducting silver wire. Nature 1998, 391, 775–778.
- Takai, A.; Doi, Y.; Yamauchi, Y.; Kuroda, K. Soft-chemical approach of noble metal nanowires templated from mesoporous silica (SBA-15) through vapor infiltration of a reducing agent. J. Phys. Chem. C 2010, 114, 7586–7593.
- Kim, K.-J.; Lee, E.-S.; Kwon, Y.-U. Syntheses of micrometer-long Pt and Ag nanowires through SBA-15 templating. J. Nanoparticle Res. 2012, 14, 1270.
- Zhang, C.; Li, C.; Si, X.; He, Z.; Qi, J.; Feng, J.; Cao, J. Single-crystalline silver nanowire arrays directly synthesized onto substrates by template-assisted chemical wetting. Materialia 2020, 9, 100529.
- Hong, B.H.; Bae, S.C.; Lee, C.-W.; Jeong, S.; Kim, K.S. Ultrathin single-crystalline silver nanowire arrays formed in an ambient solution phase. Science 2001, 294, 348–351.
- Liu, C.Y.; Zhang, Y.S.; Kao, C.K.; Liu, J.-H. Fabrication of silver nanowires via a β-cyclodextrin-derived soft template. Express Polym. Lett. 2018, 12, 591–599.
- Eisele, D.M.; Berlepsch, H.V.; Bottcher, C.; Stevenson, K.J.; Vanden Bout, D.A.; Kirstein, S.; Rabe, J.R.P. Photoinitiated growth of sub-7 nm silver nanowires within a chemically active organic nanotubular template. J. Am. Chem. Soc. 2010, 132, 2104–2105.
- Zhang, D.; Qi, L.; Ma, J.; Cheng, H. Formation of silver nanowires in aqueous solutions of a double-hydrophilic block copolymer. Chem. Mater. 2001, 13, 2753–2755.
- Bari, B.; Lee, J.; Jang, T.; Won, P.; Ko, S.H.; Alamgir, K.; Arshad, M.; Guo, L.J. Simple hydrothermal synthesis of very-long and thin silver nanowires and their application in high quality transparent electrodes. J. Mater. Chem. A 2016, 4, 11365–11371.
- Xu, J.; Hu, J.; Peng, C.; Liu, H.; Hu, Y. A simple approach to the synthesis of silver nanowires by hydrothermal process in the presence of gemini surfactant. J. Colloid Interface Sci. 2006, 298, 689–693.
- Wang, Z.; Liu, J.; Chen, X.; Wan, J.; Qian, Y. A Simple Hydrothermal Route to Large-Scale Synthesis of Uniform Silver Nanowires. Chem. Eur. J. 2005, 11, 160–163.
- Zhang, Y.; Guo, J.; Xu, D.; Sun, Y.; Yan, F. One-Pot Synthesis and Purification of Ultralong Silver Nanowires for Flexible Transparent Conductive Electrodes. ACS Appl. Mater. Interfaces 2017, 9, 25465–25473.
- Li, Y.; Yuan, X.; Yang, H.; Chao, Y.; Guo, S.; Wang, C. One-step synthesis of silver nanowires with ultra-long length and thin diameter to make flexible transparent conductive films. Materials 2019, 12, 401.
- Li, Y.; Guo, S.; Yang, H.; Chao, Y.; Jiang, S.; Wang, C. One-step synthesis of ultra-long silver nanowires of over 100 μm and their application in flexible transparent conductive films. RSC Adv. 2018, 8, 8057–8063.
- Madeira, A.; Papanastasiou, D.T.; Toupance, T.; Servant, L.; Tréguer-Delapierre, M.; Bellet, D.; Goldthorpe, I.A. Rapid synthesis of ultra-long silver nanowires for high performance transparent electrodes. Nanoscale Adv. 2020, 2, 3804–3808.
- Won, P.; Park, J.J.; Lee, T.; Ha, I.; Han, S.; Choi, M.; Lee, J.; Hong, S.; Cho, K.-J.; Ko, S.H. Stretchable and transparent kirigami conductor of nanowire percolation network for electronic skin applications. Nano Lett. 2019, 19, 6087–6096.
- Mao, Y.; Yang, H.; Hu, C.; Guo, J.; Meng, X.; Yang, Y. Large-scale synthesis of AgNWs with ultra-high aspect ratio above 4000 and their application in conductive thin film. J. Mater. Sci. Mater. Electron. 2017, 28, 5308–5314.
- Andrés, L.J.; Menéndez, M.F.; Gómez, D.; Martínez, A.L.; Bristow, N.; Kettle, J.P.; Menéndez, A.; Ruiz, B. Rapid synthesis of ultra-long silver nanowires for tailor-made transparent conductive electrodes: Proof of concept in organic solar cells. Nanotechnology 2015, 26, 265201.
- Xu, F.; Xu, W.; Mao, B.; Shen, W.; Yu, Y.; Tan, R.; Song, W. Preparation and cold welding of silver nanowire based transparent electrodes with optical transmittancesn > 90% and sheet resistances <10 ohm/sq. J. Colloid Interface Sci. 2018, 512, 208–218.
- Liu, X.; Li, D.; Chen, X.; Lai, W.-Y.; Huang, W. Highly transparent and flexible all-solid-state supercapacitors based on ultralong silver nanowire conductive networks. ACS Appl. Mater. Interfaces 2018, 10, 32536–32542.
- Hemmati, S.; Barkey, D.P.; Eggleston, L.; Zukas, B.; Gupta, N.; Harris, M. Silver Nanowire Synthesis in a Continuous Millifluidic Reactor. ECS J. Solid State Sci. Technol. 2017, 6, P144–P149.
- Huczko, A. Template-based synthesis of nanomaterials. Appl. Phys. A 2000, 70, 365–376.
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Wet chemical synthesis of silver nanorods and nanowires of controllable aspect ratioElectronic supplementary information (ESI) available: UV–VIS spectra of silver nanorods. Chem. Commun. 2001, 7, 617–618. Available online: (accessed on 9 March 2021).
- Mastai, Y.; Polsky, R.; Koltypin, Y.; Gedanken, A.; Hodes, G. Pulsed sonoelectrochemical synthesis of cadmium selenide nanoparticles. J. Am. Chem. Soc. 1999, 121, 10047–10052.
- Mastai, Y.; Homyonfer, M.; Gedanken, A.; Hodes, G. Room Temperature Sonoelectrochemical Synthesis of Molybdenum Sulfide Fullerene-Like Nanoparticles. Adv. Mater. 1999, 11, 1010–1013.
- Zhu, J.-J.; Qiu, Q.-F.; Wang, H.; Zhang, J.-R.; Zhu, J.-M.; Chen, Z.-Q. Synthesis of silver nanowires by a sonoelectrochemical method. Inorg. Chem. Commun. 2002, 5, 242–244.
- Zhang, D.; Qi, L.; Yang, J.; Ma, J.; Cheng, H.; Huang, L. Wet chemical synthesis of silver nanowire thin films at ambient temperature. Chem. Mater. 2004, 16, 872–876.
- Liu, S.; Yue, J.; Gedanken, A. Synthesis of long silver nanowires from AgBr nanocrystals. Adv. Mater. 2001, 13, 656–658.
- Wu, J.; Agrawal, M.; Becerril, H.A.; Bao, Z.; Liu, Z.; Chen, Y.; Peumans, P. Organic light-emitting diodes on solution-processed graphene transparent electrodes. ACS Nano 2010, 4, 43–48.
- Sharma, S.; Shriwastava, S.; Kumar, S.; Bhatt, K.; Tripathi, C.C. Alternative transparent conducting electrode materials for flexible optoelectronic devices. Opto-Electron. Rev. 2018, 26, 223–235.
- Jiang, X.; Wong, F.L.; Fung, M.K.; Lee, S.T. Aluminum-doped zinc oxide films as transparent conductive electrode for organic light-emitting devices. Appl. Phys. Lett. 2003, 83, 1875–1877.
- Pang, S.; Hernandez, Y.; Feng, X.; Müllen, K. Graphene as transparent electrode material for organic electronics. Adv. Mater. 2011, 23, 2779–2795.
- Park, H.; Brown, P.R.; Bulović, V.; Kong, J. Graphene as transparent conducting electrodes in organic photovoltaics: Studies in graphene morphology, hole transporting layers, and counter electrodes. Nano Lett. 2012, 12, 133–140.
- Guo, C.F.; Ren, Z. Flexible transparent conductors based on metal nanowire networks. Mater. Today 2015, 18, 143–154.
