The objective of this project is to take into account the mechanical constraints formed by diffusion of hydrogen or isotope and the cathodic corrosion produced by formation of superficial hydride transients, both responsible of destruction of palladium or alloyed cathode. To know the origin of these, it was necessary to discriminating the damaging effects encountered.
- hydrogen isotope
- electrolyzer
- cathode
- diffusion
- palladium alloy
- stress corrosion cracking
- cathodic corrosion
Hydrogen is an alternative fuel reducing dependence on fossil fuels and a resource that respects the environment with zero-emission of greenhouse gases. One attractive way of producing high purity hydrogen is electrolysis using watertight palladium diffusion cathode at double face for adsorption, diffusion and desorption in the back side as seen in [1]. In another application concerning nuclear fusion energy systems, tritium can be recycled by this innovating electrolysis process using the same palladium cathode.
Hydrogen or isotope taking place during diffusion distends the palladium by production of three solid phases not miscible of different size and in three regions of cathode requiring more space for metal, leading to deformation and embrittlement [2]. The presence of these phases modifies the mechanical behavior and induces stress corrosion cracking [3] . This mechanism is a source of modification in the lattice, thickening, irreversible process of loss of permeability, and over concentration of proton or hydrogen in the cathode. The drastic structural changes lead to crumpled cathode and mechanical constraints. This destructive effect is more and more progressing limiting the lifetime of electrolyzer. Palladium can be alloyed with silver or yttrium to favorably increase diffusion and reduce these constraints. Using palladium-silver alloy, the cathode has not the sufficient mechanical quality, and it requires porous support that has to contain lattice expansion so that to limit deformation and damage. Support has to possess similar thermal expansion to palladium alloy to ensure good adhesion. Flow in the support and permeation in the palladium have to be equilibrated to avoid over concentration of hydrogen at the interface cathode-support [4].
Cathodic corrosion occurs at a sufficiently negative potential to be in presence of superficial absorbed hydrogen promoting transient hydride palladium formation leading to severe loss of cohesion and weathering of metal [5]. Cathodic corrosion would weaken the superficial structure of palladium, leading to degradation of surface. Cathodic corrosion and stress corrosion cracking act in concert since they form in the same range of potential in presence of hydride as seen in the Figure.
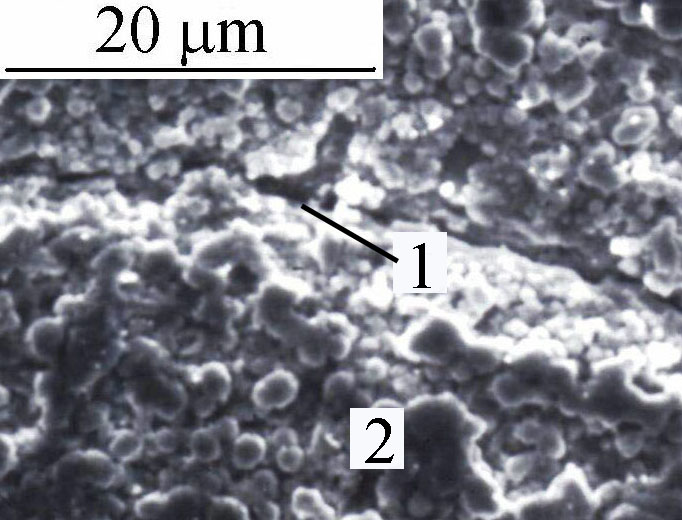
Figure - Scanning Electron Microscopy of Pd-Ag cathode showing cracking (1) and loss of cohesion (2) by hydrogen charging.
Palladium-yttrium alloy makes possible to omit the porous support and permits a gain in permeation value with lower capacity for cracking [6]. It permits more easily to disregard the drawbacks encountered for the palladium and palladium-silver alloy used for the diffusion cathode. Solving the difficulties in detail is given in the reference [7].
References
- Bellanger, G. Corrosion Induced by Low-Energy Radionuclides - Modeling of Tritium and Its Radiolytic and Decay Products Formed in Nuclear Installations; Elsevier Science: Amsterdam, 2004; pp. 1-524.
- Tosti, S.; Overview of Pd-based membranes for producing pure hydrogen and state of art at ENEA laboratories. Int. J. Hydrogen Energy 2010, 35, 12650-12659, doi: 10.1016/j.ijhydene.2010.07.116.
- Grant, J.L. Palladium, Yttrium, Copper Ternary Alloys for Hydrogen Purification; Ph.D. Thesis, University of Birmingham: Birmingham, 2009; pp. 1-98.
- Fletcher, S. . Thin Film Palladium-Yttrium Membranes for Hydrogen Separation; Ph.D. Thesis, University of Birmingham: Birmingham, 2009; pp. 1-268.
- Yanson, A.I.; Antonov, P.V.; Rodriguez, P.; Koper, M.T.M; Influence of the electrolyte concentration on the size and shape of platinum nanoparticles synthesized by cathodic corrosion. Electrochim. Acta 2013, 112, 913–918, doi: 10.1016/j.electacta.2013.01.056.
- Wang, D.; Flanagan, T.B.; Shanahan, K; Diffusion of H through Pd-Y alloy membranes. J. Membr. Sci. 2016, 499, 452–461, doi: 10.1016/j.memsci.2015.10.020.
- Bellanger, G.; Prospecting Stress Formed by Hydrogen or Isotope Diffused in Palladium Alloy Cathode. Corros. Mater. Degrad. 2018, /, /-/, article accepted.
