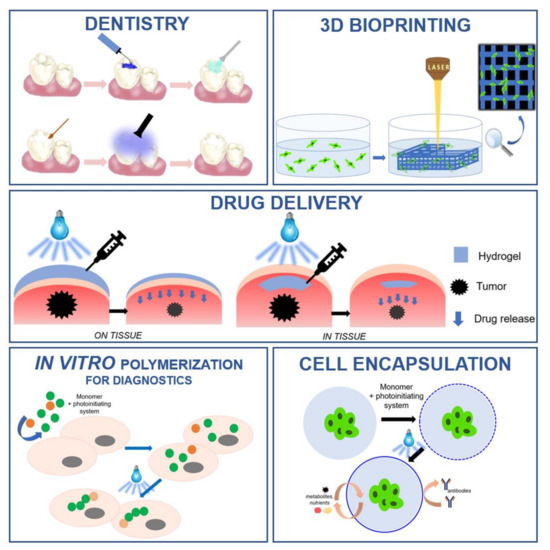
Light-initiated polymerization processes are currently an important tool in various industrial fields. The advancement of technology has resulted in the use of photopolymerization in various biomedical applications, such as the production of 3D hydrogel structures, the encapsulation of cells, and in drug delivery systems. The use of photopolymerization processes requires an appropriate initiating system which, in biomedical applications, must meet additional criteria: high water solubility, non-toxicity to cells, and compatibility with visible low-power light sources. This article is a literature review on those compounds that act as photoinitiators of photopolymerization processes in biomedical applications. The division of initiators according to the method of photoinitiation was described and the related mechanisms were discussed. Examples from each group of photoinitiators are presented, and their benefits, limitations and applications are outlined.
Currently, polymerization processes are one of the most widely used chemical processes in various fields of industry [
1,
2]. One of the most modern and rapidly developing methods of obtaining polymers is light-induced polymerization, i.e., photopolymerization [
3,
4,
5,
6]. The technique of converting liquid monomers to solid polymers under the influence of applied light is widely developed in the polymer materials sector in the industry of solvent-free paints [
7], varnishes [
8], and adhesives [
9], in optoelectronics [
10], in the printing industry for 3D printing materials [
11,
12,
13,
14,
15,
16,
17], and many others. Numerous advantages of photopolymerization, such as performing reactions at ambient temperature, lack of solvents, and extremely short processing times, made light-initiated polymerization perfectly suited for biomedical applications () [
18,
19].
Figure 1. Examples of light-induced polymerization processes in biomedical applications.
The global market for photopolymerization in biomedical applications can be divided into various groups based on the area of application in the medical sector. The main segments are: dentistry [
20,
21,
22,
23], tissue engineering [
24,
25,
26,
27,
28,
29], bioimaging [
30,
31], drug delivery systems [
32,
33,
34,
35], and medical devices. In dentistry, photochemical-initiated processes are used for the filling of hard dental tissue cavities with photocured polymer composites [
36,
37,
38,
39]. An interesting application of photopolymerization processes is the production of photo-crosslinked polymeric biomaterials especially those based on totally or partially degradable materials [
40,
41,
42,
43,
44], scaffolds for tissue culture [
45,
46,
47,
48,
49], and diagnostic genetic or cellular matrixes [
50,
51,
52,
53,
54,
55,
56,
57,
58].
The unquestionable advantages of the photopolymerization technique in the context of applications in tissue engineering and biomedical science are primarily its ability to form structures of any geometry as well as the deposition of such materials on various carriers. Lack of these possibilities is often a limitation of the functionality of biomaterials obtained through conventional polymerization processes.
Due to the mechanisms of polymerization as well as the type of used monomers and initiating systems, there is a distinction between radical photopolymerization and cationic photopolymerization, which are the basic processes used in light-initiated polymerization technologies. Radical photopolymerization is a chain reaction consisting of three main stages: initiation, propagation, chain growth, and termination (which may be accompanied by side reactions) [
59]. Free-radical photopolymerization is mainly used for acrylate and methacrylate monomers. The factor that limits the usefulness of radical photopolymerization is the occurrence of oxygen inhibition caused by the presence of atmospheric oxygen during the polymerization process. The negative influence of oxygen on polymerization is reflected, for example, by extinguishing the excited states of the initiator, which, in turn, affects the efficiency of the whole process. It is the free-radical polymerization, however, that is mostly used in biomedical applications, as proven by numerous literature reports [
60,
61,
62,
63,
64].
The second type of polymerization is cationic photopolymerization, which is particularly interesting and relatively widespread in industrial applications, since it has a number of major advantages that make this process practical [
65]. The living nature of cationic photopolymerization guarantees that the reaction continues to be effective even after shutting down the radiation source [
66]. This enables a high degree of conversion to be achieved, which plays an extremely important role in the industrial practice. For this reason, photoinitiated cationic polymerization is becoming increasingly prevalent in global markets as an easy and energy-saving method for obtaining cross-linked polymers [
67,
68]. Despite its numerous advantages, cationic polymerization is very unlikely to be used in biomedical applications. One of the reasons is that cationic initiators generate strong protonic acids during initiation, whose acidic character negatively affects cell cultures [
69]. The second reason is the sensitivity of cationic photopolymerization to moisture and water. Numerous scientific articles prove that the presence of water slows down or inhibits the polymerization reaction [
70]. In addition, water can act as a chain transfer agent and promote the growth of new chains, which reduces the average molecular weight of the obtained polymer [
71].
One of the basic requirements of photocuring systems used in biomedical sciences is their total or partial solubility in water. Water-based photocuring systems have already garnered interest since the late 1970s. Even then, it was well known that the use of water as a non-toxic, green, and cheap solvent was the solution to many problems related to the classical, organic compositions [
72]. In addition, aqueous formulations can, in many cases, provide a reaction efficiency that cannot be achieved with conventional organic systems. Interestingly, the oxygen concentration in aqueous systems is an inch lower than in organic preparations, which significantly reduces oxygen inhibition for radical photopolymerization processes. Therefore, the use of water-soluble photoinitiators in aqueous systems for light-initiated polymerization is of great importance in the rapidly growing medical industry, and this article provides an overview of the literature related to the development of water-soluble initiators and their use in biomedical applications.
This entry is adapted from the peer-reviewed paper 10.3390/polym12051073