Prostate cancer is the second most leading and prevalent malignancy around the world, following lung cancer. Prostate cancer is characterized by the uncontrolled growth of cells in the prostate gland. Prostate cancer morbidity and mortality have grown drastically, and intensive prostate cancer care is unlikely to produce adequate outcomes. The synthetic drugs for the treatment of prostate cancer in clinical practice face several challenges. Quercetin is a natural flavonoid found in fruits and vegetables. Apart from its beneficial effects, its plays a key role as an anti-cancer agent. Quercetin has shown anticancer potential, both alone and in combination.
- quercetin
- chemotherapeutic
- chemo resistance
- mechanisms
- nano scale delivery
1. Quercetin in Oncology
In recent decades, the scientific community has uncovered the enormous potential role for natural compounds in the therapy and management of terrible diseases such as cancer. Despite the availability of a wide range of natural therapeutic agents, the creation of a definitive treatment for cancer is still pending. Therefore, it is important to understand the relationships between natural molecules and their respective cellular targets to devise an efficient cancer treatment strategy. This would involve numerous intracellular targets, which include apoptosis, cell cycle, detoxification, replication of antioxidants, and angiogenesis. The scope of the synergistic studies available strongly reinforces the use of quercetin as a medication for chemoprevention [1].
Apoptosis is characterized by specific cellular events such as blebbing, failure of cell adhesion, cytoplasmic expansion, fragmentation of DNA, and caspase activation via external and internal pathways. Research indicates that quercetin can induce apoptosis through the mitochondrial pathway involving activation of caspase-3 and 9, accompanied by liberation of cytochrome C and poly-ADP-ribose polymerase cleavage [2][3][4]. This induction of apoptosis by quercetin through mitochondrial pathways and the caspase cascade has been documented in various cancer cell lines including MCF-7 cells of breast cancer, HK1 cells of nasopharyngeal carcinoma, HL60 cells of leukemia, and SCC-9 cells of oral squamous cell carcinoma [5][6][7][8]. Induction of apoptosis via cellular signaling protein modulation, upregulation of Bax (Bcl2 associated X protein), Cox-2, and downregulation of Bcl-2 proteins is also triggered by quercetin [9][10].
Normally, cyclin and cyclin dependent kinases regulate the cell cycle. Conversely, cyclin dependent kinase inhibiter regulates cyclin dependent kinases [11][12]. Quercetin induces S phase cell cycle arrest and subsequently leads to the inhibition of DNA synthesis in SCC-9 cells [6]. During the S-phase of MCF-7 breast cancer cells, quercetin induces cell arrest, which leads to downregulation of cyclin dependent kinases-2 and p53, and p57 upregulation in a dose-time dependent manner [7]. By inhibiting cell cycle progression, quercetin prevents the proliferation of ovarian cancer cells and promotes cell apoptosis [13]. Quercetin anticancer potential evaluated in multiple cancers is briefly shown in Table 1.
Table 1. Effect of quercetin on multiple cancers.
| Cancer Type | Cell Line | Observed Effects | References |
|---|---|---|---|
| Breast cancer | MCF-7 cells | Apoptosis induction, cell cycle arrest | [5][7] |
| Nasopharyngeal carcinoma | HK-1 cells | Cell cycle arrest and apoptosis induction | [7] |
| Leukemia | HL-60 cells | Apoptosis induction, detoxification | [8] |
| oral squamous cell carcinoma | SCC-9 cells | Necrosis and apoptosis induction, cell cycle arrest during S-phase | [6] |
| Ovarian cancer | SKOV-3 cells | Promotes cell apoptosis, prevents cancer cells proliferation | [13] |
| Lung cancer | A549 cells | inhibition of CYP1A2 activity | [14] |
| Gastric cancer | GC 1401 | Suppression of gastric cancer cell growth, apoptosis modulation | [15] |
| Colorectal cancer | HT-229 | Apoptosis promotion, provoke cell cycle arrest, proliferation inhibition | [16][17] |
| Oral cancer | SAS cells | Repression of invasion, migration and cell viability, decrease tumor rate and enhanced apoptosis | [18][19] |
| Liver cancer | SMMC7721, QGY7701 | Antitumor effect via apoptosis induction | [20] |
| Thyroid cancer | B-CPAP, K1 | Promote apoptosis, reduce cell proliferation. | [21][22][23] |
| Pancreatic cancer | MIA PaCa-2 | Apoptosis induction, reduced cell proliferation, apoptosis induction | [24][25] |
Evidence indicates that quercetin deregulates multiple CYP enzyme isoforms in tumor cells [26]. In vitro studies have demonstrated quercetin induced inhibition of CYP1A2 activity in human lung carcinoma A549 cells, HepG2 cells, and human hepatocytes [14]. Apart from CYP enzyme modulation, quercetin follows the mechanism of Nrf2 (nuclear erythroid factor 2-cognate factor 2) mediated enzyme induction, contributing to its anti-cancer potential. When phase II enzymes like heme oxygenase-1, UDP-glucuronosyl, and glutathione S transferases pose any carcinogens, quercetin causes their suppression. The genes of these enzymes involve antioxidant replication components that are rigorously regulated by nuclear erythroid factor 2-cognate factor 2. This, as a consequence, is correlated with another protein known as the Kelch-like ECH-associated protein-1, a Nrf2 repressor, and further reinforces its deterioration via the ubiquitin-dependent proteasome pathway [27][28][29].
In the treatment of ARE-mediated inducer cells, quercetin facilitates the detachment of the Nrf2-Keap1 complex, resulting in the translocation of Nrf2 to the nucleus, where it composes heterodimers with other transcription factors, binds to ARE, and activates phase II enzyme gene transcription [30]. The molecular target pathway is shown in Figure 4.
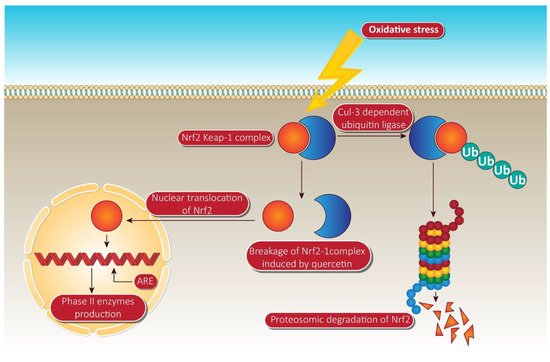
Figure 4. Quercetin targeting via phase II enzyme pathway mediated through Nrf-2. When the cells are unstimulated under stress conditions, Keap-1 isolates Nrf-2 and exposes it to cul-e dependent ubiquitin ligase. This enzyme leads to the proteasomal cleavage of Nrf-2. In the meantime, quercetin degrades the Nrf-2 keap-1 complex and shifts the Nrf2 to the nucleus. Inside the nucleus, Nrf-2 binds to ARE, resulting in the production of phase II enzymes via Nrf-2 associated expression. NrF-2, nuclear factor erythroid 2 related factor-2; Ub, ubiquitin; ARE, anti-oxidant response element. Human colorectal adenocarcinoma cells and duodenal adenocarcinoma HuTu 80 cells were further identified as receiving a quercetin triggered boost in phase II oxidative stress (detoxification enzymes). Additionally, the time-dependent influence of quercetin on the transcriptional regulation of Nrf2 and its increased mRNA and protein expression was consistently observed in HepG2 and malignant mesothelioma cells [31].
2. Quercetin and Prostate Cancer
Recently, morbidity and mortality of prostate cancer have risen, and systematic cures for prostate are unable to produce sufficient results. Quercetin is a naturally occurring flavonoid compound that has gained immense attention and focus because of its effectiveness against cancer. Both in vitro and in vivo studies have confirmed that quercetin effectively inhibits prostate cancer via different pathways.
2.1. Quercetin and Cell Death
Despite the dismal situation in prostate cancer care, the findings of the anticancer effects of quercetin are promising, having been used in a variety of human prostate cancer trials with beneficial effects. During the progression of prostate cancer, quercetin suppresses the epithelial-to-mesenchymal transition process, promoting apoptosis via deactivation of the PI3K/Akt signaling pathway [32]. Additionally, quercetin has been shown to decrease the ratio of Bcl-xL to Bcl-xS and in contrast, maximize the efflux of Bax to the mitochondrial matrix in human prostate cancer cells [10]. Apart from this, quercetin promotes apoptosis of cancer cells by downregulation of heat shock protein-90 levels. Quercetin depletion of heat shock protein-90 results in reduced cell viability, inhibition of surrogate markers, mediated apoptosis, and activation of caspases [4].
A research study on the correlation between quercetin and prostate cancer indicates that quercetin reduces the viability of androgen-independent prostate cancer cells by regulating the expression of system components of insulin-like growth factors (IGF), signal transduction, and inducing apoptosis, which could be very beneficial for the treatment of androgen-independent prostate cancer [33]. There is no study to discuss the role of endoplasmic reticulum stress in quercetin-induced apoptosis in prostate cancer cells. Multiple pieces of evidence indicate several potential signaling pathways for quercetin in apoptosis. In this regard, Liu et al. demonstrated that quercetin decreases the expression of Bcl-2 protein and activates the caspase cascade via mitochondrial and endoplasmic reticulum stress, subsequently leading to apoptosis in prostate cancer cells [34].
Quercetin downregulated the Notch/AKT/mTOR, a fundamental signaling pathway in tumor progression, which leads significantly to apoptosis of U937 leukemia cells [11]. Targeting extrinsic domains, quercetin has been found to boost tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) mediated apoptosis in DU-145 cells (human prostate cancer cell line) via overexpression of death receptor-5 (DR5) [35]. Downregulation of survivin through histone (H-3 regulated) deacetylation and AKT dephosphorylation in prostate cancer-3 and DU-145 cell line also leads to apoptosis by quercetin due to its anti-prostate cancer potential [36][37]. Apart from apoptosis induced by the caspase cascade, quercetin also triggers other apoptosis pathways, which are schematically shown in Figure 5. Apoptosis induction by quercetin, which could be the significant parameter for its anti-prostate cancer effectiveness, has been extensively explored in numerous types of prostate cancer cell and is attracting ever more attention.
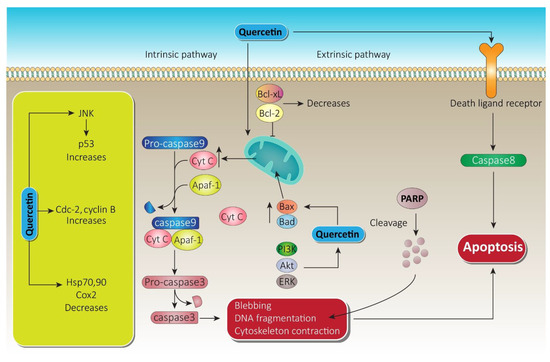
Figure 5. Quercetin apoptotic mechanism via death ligand and mitochondrial membrane. Following the intrinsic pathway, quercetin causes massive release of Cyt-c from the mitochondrial intramembranous space and induces apoptosomes. Furthermore, via blebbing, DNA fragmentation, and cytoskeleton contraction, it paves the way to apoptosis. On the other hand, through the extrinsic pathway, quercetin initiates caspase-8, which leads to apoptosis. A substantial increase in JNK and cdc cyclin B while decreasing heat shock proteins by quercetin also promotes therapy of prostate cancer. Bcl-xL, B-cell lymphoma extra-large; Bcl-2, B-cell lymphoma-2; Cyt-c, cytochrome c; JNK, c-jun N terminal kinase; ERK, extracellular signal regulated kinase; PARP, poly ADP-ribose polymerase; PI3K, phosphoinositide 3 kinase; Akt, serine/threonine specific protein kinase.
2.2. Quercetin and Metastasis
The epithelial–mesenchymal transition (EMT) is a flexible transition in the progression of tumors, during which cancer cells undergo drastic changes to develop highly invasive properties. Transforming growth factor-β (TGF-β) is an epithelial–mesenchymal transition inducer within epithelial cells, required for the development of the invasive carcinoma phenotype. Transforming growth factor-β plays a critical role in prostate cancer metastasis and tumorigenesis, with mutations in the Wnt signaling pathway being linked to a further variety of cancer types. Quercetin interferes with the Wnt signaling pathway, leading to inhibition of migration and invasion [38].
Urokinase plasminogen activator (uPA) is a serine protease that is associated with the progression of prostate cancer, especially the invasion and metastasis stages. In the prostate cell proliferation stage, urokinase plasminogen activator is regulated by uPA and transactivation of the epidermal growth factor receptor. Cells of prostate cancer (PC-3) are highly invasive when expressing the uPA and uPAR genes. Quercetin downregulates mRNA expressions for uPA, uPAR, and EGF. In addition, quercetin also inhibits β-catenin, NF-ceB, and even proliferative signaling molecules such as p-EGF-R, N-Ras, Raf-1, c. Fos c. Jun, and p-c. Jun protein expressions of the cell survival factor. This whole process leads to the inhibition of invasion and migration phenomena, resulting in inhibition of prostate cancer metastasis [39]. Quercetin also blocks angiogenesis and metastasis by upregulating thrombospondin-1 to suppress in vitro and in vivo growth of PC-3 cells in human prostate cancer [40].
Angiogenesis is a vital step in the invasion and progression of cancer as it helps the expanding tumor to acquire oxygen and nutrients. At non-toxic concentrations, quercetin significantly inhibits the protrusion of micro vessels and shows substantial inhibition of the proliferation, migration, invasion, and tube forming of endothelial cells, which are essential events in the angiogenesis process. The findings of an associated study revealed that quercetin inhibits angiogenesis and cell growth targeting the VEGF-R2 regulated AKT/mTOR/P70S6K signaling pathway, leading to inhibition of prostate cancer metastasis [41]. Another target for quercetin is miR-21, where it significantly suppresses the proliferation and metastasis of prostate cancer cells and decreases the expression of multiple miRNA associated with prostate tumors, particularly miR-21. Such an inhibition of the miR-21 signaling pathway results in the prevention of prostate cancer metastasis [42]. The comparative detail of quercetin on multiple prostate cancer cell lines, along with the observed effects, are shown in Table 2.
| Molecular Mechanism | Signaling Pathway | Cell Lines | Observed Effects | References |
|---|---|---|---|---|
| Apoptosis | PI3K/Akt signaling pathway | PC-3 and its xenograft tumor | Suppression of epithelial to mesenchymal transition | [32] |
| Caspase activation, regulation of Bcl-2, | PC-3 | Decrease the ratio of Bcl-xL to Bcl-xS and in contrast maximize the efflux of Bax to the mitochondrial matrix | [10] | |
| Downregulation of heat shock protein-90 | PC-3, LNCaP, DU-145 | Reduced cell viability, inhibition of surrogate markers, mediated apoptosis and activation of caspases |
[4] | |
| Insulin-like growth factors (IGF), signal transduction both internal and external | PC-3 | Reduces the viability of androgen-independent prostate cancer cells | [33] | |
| Notch/AKT/mTOR, caspase-3, and caspase-9 | DU-145 | Boost tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), sensitization cancer cells to apoptosis | [35] | |
| Metastasis | Wnt signaling pathway, | PC-3 | Inhibition of migration and invasion | [38] |
| Inhibition of β-catenin, NF-κB, p-EGF-R, N-Ras, Raf-1, c. Fos c. Jun and p-c. Jun | PC-3 | Inhibition of migration and invasion of prostate cancer cell lines | [39] | |
| Thrombospondin-1 | PC-3 | Suppress in vitro and in vivo growth of PC-3 cells in human prostate cancer | [40] | |
| Angiogenesis and proliferation | VEGF regulated AKT/mTOR/P70S6K | HUVECs (Human umbilical vein endothelial cells), PC-3 | Inhibition of angiogenesis and tumor growth | [41] |
| VEGF/PI3k/Akt | LNCap, PC-3 | Synergistic inhibition of cell invasion and proliferation | [43] | |
| capase-3/7, nuclear β-catenin, and TCF-1/LEF | LNCap, PC-3 | Inhibition of invasion and proliferation | [44] | |
| Bcl-2/Bax | LNCap, PC-3 | Antiproliferative effect, growing the Stage G2/M | [45] | |
| PI3K/Akt | LAPC-4 and LNCaP | Inhibition of cell migration, antiprostate cancer potency at lower dose, antiproliferative effect | [46] |
Quercetin in combination with metformin targets the VEGF/PI3k/Akt signaling pathway, which synergistically inhibits cell invasion and proliferation in prostate cancer cell lines [43]. In addition, quercetin in combination with epigallocatechin gallate inhibits the invasion and progression of prostate cancer stem cells via activation of X-linked inhibitor of apoptosis protein (XIAP) and survivin, leading to its metastasis inhibition potential in prostate cancer [44]. With regard to this synergy, in PC-3—the cell lines of human prostate cancer—quercetin and 2-methoxyestradiol display antiproliferative and proapoptotic activity by growing the Stage G2/M of the cell population and decreasing Bcl-2/Bax. Thus, promoting the G2/M stage leads to the anti-metastatic potential of prostate cancer [45]. At low physiological doses, the combination of arctigenin and quercetin targeting related pathways (androgen receptor and PI3K/Akt) offers a novel protocol for accelerated chemoprevention in prostate cancer [46].
2.3. Quercetin in Reversing Chemoresistance
Chemotherapy is indeed an indispensable therapy for prostate cancer. The development of chemoresistance, however, is a widespread and crucial issue that requires urgent remedies to be dealt with.
Advanced drug studies have shown that quercetin serves as a potential anti-cancer agent in several types of cancer by regulating multiple pathways. However, current therapies are limited by resistance, which might be reversed by quercetin. In this regard, doxorubicin induced resistance was successfully recovered via quercetin in a research study. A cell line-PC3/R of prostate cancer with acquired doxorubicin resistance was identified. In comparison with normal PC3 cells, a strong drug-resistance to doxorubicin and significant activation of the phosphoinositide 3-kinase/protein kinase-B (PI3K/AKT) pathway was shown in PC3/R cells. Doxorubicin combination therapy with quercetin greatly facilitated the apoptosis induced by doxorubicin in PC3/R cells via the mitochondrial/reaction oxygen species pathway. A major upregulation of tyrosine-protein kinase-met was observed in PC3/R cells as opposed to normal PC3 cells. Furthermore, c-met mediated expression rescued quercetin-promoted apoptosis in doxorubicin treated PC3/R cells [47]. This clearly indicates that quercetin can reverse the resistance of prostate cancer cells to doxorubicin by downregulating the expression of c-met. This might provide a potential strategy to reverse prostate cancer chemoresistance.
Docetaxel is a first line therapeutic drug that is used in the treatment of prostate cancer metastasis. Unfortunately, the advent of resistance reduces its effectiveness and restricts its benefits to survival. In prostate cancer cells and xenograft models, quercetin can reverse docetaxel resistance on proliferation, colony formation, migration, invasion, and apoptosis. Combination therapy of quercetin with docetaxel can sufficiently inhibit the PI3K/Akt pathway and promote apoptosis. Subclones susceptible to docetaxel and prone subclones have been treated with quercetin, which showed that docetaxel-resistant subclones had greater androgen receptor and PI3K/Akt pathway activation, more remarkable phenotypes of mesenchymal and stem-like cells, and more expression of P-gp than that of parental cells. All these transformations were interestingly reversed by quercetin [48]. This offers in-depth evidence for the clinical use of quercetin in docetaxel-resistant prostate cancer.
The effect of cancer treatment and ATP-dependent drug efflux pumps may be significantly affected by multidrug resistance to chemotherapy, P-glycoprotein, and midkine (MK) contribute to the resistance of different chemotherapeutic agents. Z—polypeptide 1 is one of the midkine receptors and, in PI3K and MAPK pathways, has been found to be synergistically active in midkine-mediated cell migration. Consequently, modulation of the PI3K and MAPK signaling pathways by quercetin can cause amplification of gene expression associated with endothelial–mesenchymal transition. Thus, quercetin modulation of the endothelial–mesenchymal transition and drug resistance genes might contribute to the inhibition of CD44+/CD133+ proliferation and migration [49][50][51]. In summary, these findings show that MK siRNA coupled with quercetin can inhibit the therapeutic resistance of CD44+/CD133+ cells. Treatment with quercetin combined with the midkine knockdown strategy could effectively target and facilitate removal of CD44+/CD133+ cells, thereby preventing chemoresistance.
The splice variant AR-V7 is implicated in resistance not only to enzalutamide, but also to abiraterone and other traditional therapeutics. Clinical evidence indicates that resistance toward the next-generation antiandrogen, enzalutamide, can be largely induced by alternative androgen receptor splicing to establish constitutively active splice variants (AR-V7). Recent studies indicate that fusing factors such as hnRNPA1 promote the production of AR-V7 and thus contribute to the resistance of enzalutamide in cells of prostate cancer. Quercetin decreases hnRNPA1, and subsequently AR-V7 expression. Quercetin suppression of AR-V7 desensitizes enzalutamide-resistant prostate cancer cells to enzalutamide therapy. Altogether, the underlying mechanism involves downregulation of hnRNPA1 expression, downregulation of AR-V7 expression, antagonizes the signaling pathway of androgen receptors, and desensitizes enzalutamide-resistant prostate cancer cells to in vivo treatment with enzalutamide in mouse xenografts [52]. These findings indicate that blocking the alternative splicing of the androgen receptor can have major consequences in overwhelming resistance to antiandrogen therapy of the next generation.
Metastatic or locally induced prostate cancer is usually managed with androgen deprivation therapy. Prostate cancer initially reacts to the medication, and then its response begins to revert, gaining tolerance to androgen deprivation and developing toward castrate-resistant prostate cancer-an incurable form. Research using transgenic mouse models shows that modulation of the Wnt/β-Catenin signaling pathway in the prostate cancer is cancerous, allowing for castration-resistant growth of prostate cancer, inducing an epithelial-to-mesenchymal transformation, promoting differentiation of neuroendocrine and giving stem cell-like characteristics to prostate cancer cells [53]. These major Wnt/β-Catenin signaling functions in prostate cancer development emphasize the need to establish drugs targeting this pathway for dealing with resistance to prostate cancer therapy.
This entry is adapted from the peer-reviewed paper 10.3390/cancers13071602
References
- Ferreira, V.F.; Pinto, A.C. A fitoterapia no mundo atual. Química Nova 2010, 33, 1829. [Google Scholar] [CrossRef]
- Enioutina, E.Y.; Salis, E.R.; Job, K.M.; Gubarev, M.I.; Krepkova, L.V.; Sherwin, C.M.T. Herbal Medicines: Challenges in the Modern World. Part 5. Status and current directions of complementary and alternative herbal medicine worldwide. Expert Rev. Clin. Pharmacol. 2016, 10, 327–338. [Google Scholar] [CrossRef]
- Kamboj, V.P. Herbal medicine. Curr. Sci. 2000, 78, 35–39. [Google Scholar]
- Silva, P.; Bonifácio, B.; Ramos, M.; Negri, K.; Bauab, T.M.; Chorilli, M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2013, 9, 1–15. [Google Scholar] [CrossRef]
- Pandey, M.; Debnath, M.; Gupta, S.; Chikara, S.K. Phytomedicine: An ancient approach turning into future potential source of therapeutics. J. Pharmacogn. Phytother. 2011, 3, 113–117. [Google Scholar]
- Dhama, K.; Karthik, K.; Khandia, R.; Munjal, A.; Tiwari, R.; Rana, R.; Khurana, S.K.; Ullah, S.; Khan, R.U.; Alagawany, M.; et al. Medicinal and therapeutic potential of herbs and plant metabolites/extracts countering viral pathogens-current knowledge and future prospects. Curr. Drug Metab. 2018, 19, 236–263. [Google Scholar] [CrossRef] [PubMed]
- Sarangi, M.; Padhi, S. Novel herbal drug delivery system: An overview. Arch. Med. Health Sci. 2018, 6, 171. [Google Scholar] [CrossRef]
- Singh, S.; Tripathi, J.S.; Rai, N.P. An appraisal of the bioavailability enhancers in Ayurveda in the light of recent pharmacological advances. AYU Int. Q. J. Res. Ayurveda 2016, 37, 3–10. [Google Scholar] [CrossRef]
- D’Andrea, G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef]
- Larson, A.; Witman, M.A.; Guo, Y.; Ives, S.; Richardson, R.S.; Bruno, R.S.; Jalili, T.; Symons, J.D. Acute, quercetin-induced reductions in blood pressure in hypertensive individuals are not secondary to lower plasma angiotensin-converting enzyme activity or endothelin-1: Nitric oxide. Nutr. Res. 2012, 32, 557–564. [Google Scholar] [CrossRef]
- Sharma, A.; Kashyap, D.; Sak, K.; Tuli, H.S.; Sharma, A.K. Therapeutic charm of quercetin and its derivatives: A review of research and patents. Pharm. Pat. Anal. 2018, 7, 15–32. [Google Scholar] [CrossRef]
- Ahmad, U.; Ali, A.; Khan, M.M.; Siddiqui, M.A.; Akhtar, J.; Ahmad, F.J. Nanotechnology-Based Strategies for Nutraceuticals: A Review of Current Research Development. Nanosci. Technol. Int. J. 2019, 10, 133–155. [Google Scholar] [CrossRef]
- Alexander, A.; Patel, R.J.; Saraf, S.; Saraf, S. Recent expansion of pharmaceutical nanotechnologies and targeting strategies in the field of phytopharmaceuticals for the delivery of herbal extracts and bioactives. J. Controll. Release 2016, 241, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pandey, A.N.; Jain, S.K. Nasal-nanotechnology: Revolution for efficient therapeutics delivery. Drug Deliv. 2016, 23, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Naeimirad, M.; Zadhoush, A.; Kotek, R.; Neisiany, R.E.; Khorasani, S.N.; Ramakrishna, S. Recent advances in core/shell bicomponent fibers and nanofibers: A review. J. Appl. Polym. Sci. 2018, 135. [Google Scholar] [CrossRef]
- Mamillapalli, V. Nanoparticles for herbal extracts. Asian J. Pharm. 2016, 10. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, H.; Xu, C.; Gu, L. A review: Using nanoparticles to enhance absorption and bioavailability of phenolic phytochemicals. Food Hydrocoll. 2015, 43, 153–164. [Google Scholar] [CrossRef]
- Coleman, W.B. Molecular Pathogenesis of Prostate Cancer. In Molecular Pathology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 555–568. [Google Scholar]
- Goodarzi, E.; Khazaei, Z.; Sohrabivafa, M.; Momenabadi, V.; Moayed, L. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide prostate cancers and their relationship with the human development index. Adv. Hum. Biol. 2019, 9, 245. [Google Scholar] [CrossRef]
- Panigrahi, G.K.; Praharaj, P.P.; Kittaka, H.; Mridha, A.R.; Black, O.M.; Singh, R.; Mercer, R.; Van Bokhoven, A.; Torkko, K.C.; Agarwal, C.; et al. Exosome proteomic analyses identify inflammatory phenotype and novel biomarkers in African American prostate cancer patients. Cancer Med. 2019, 8, 1110–1123. [Google Scholar] [CrossRef] [PubMed]
- Pernar, C.H.; Ebot, E.M.; Wilson, K.M.; Mucci, L.A. The Epidemiology of Prostate Cancer. Cold Spring Harb. Perspect. Med. 2018, 8, a030361. [Google Scholar] [CrossRef]
- Ferrís-I-Tortajada, J.; García-I-Castell, J.; Berbel-Tornero, O.; Ortega-García, J. Constitutional risk factors in prostate cancer. Actas Urológicas Españolas 2011, 35, 282–288. [Google Scholar] [CrossRef]
- Zuo, L.; Ren, K.-W.; Bai, Y.; Zhang, L.-F.; Zou, J.-G.; Qin, X.-H.; Mi, Y.-Y.; Okada, A.; Yasui, T. Association of a common genetic variant in RNASEL and prostate cancer susceptibility. Oncotarget 2017, 8, 75141–75150. [Google Scholar] [CrossRef]
- Malathi, K.; Dong, B.; Gale, M.; Silverman, R.H. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nat. Cell Biol. 2007, 448, 816–819. [Google Scholar] [CrossRef]
- Bjartell, A.S. Re: Identification of a Novel Gammaretrovirus in Prostate Tumors of Patients Homozygous for R462Q RNASEL Variant. Eur. Urol. 2006, 50, 613. [Google Scholar] [CrossRef]
- Manivannan, P.; Reddy, V.; Mukherjee, S.; Clark, K.N.; Malathi, K. RNase L Induces Expression of a Novel Serine/Threonine Protein Kinase, DRAK1, to Promote Apoptosis. Int. J. Mol. Sci. 2019, 20, 3535. [Google Scholar] [CrossRef] [PubMed]
- Bergthorsson, J.; Johannesdottir, G.; Arason, A.; Benediktsdottir, K.; Agnarsson, B.; Bailey-Wilson, J.; Gillanders, E.; Smith, J.; Trent, J.; Barkardottir, R. Analysis of HPC1, HPCX, and PCaP in Icelandic hereditary prostate cancer. Qual. Life Res. 2000, 107, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Nandeesha, H. Insulin: A novel agent in the pathogenesis of prostate cancer. Int. Urol. Nephrol. 2008, 41, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Komura, K.; Sweeney, C.J.; Inamoto, T.; Ibuki, N.; Azuma, H.; Kantoff, P.W. Current treatment strategies for advanced prostate cancer. Int. J. Urol. 2018, 25, 220–231. [Google Scholar] [CrossRef]
- Hotte, S.J.; Saad, F. Current Management of Castrate-Resistant Prostate Cancer. Curr. Oncol. 2010, 17, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, G.; Ferro, M.; Buonerba, C. Sipuleucel-T (Provenge®) for castration-resistant prostate cancer. BJU Int. 2012, 110, E99–E104. [Google Scholar] [CrossRef]
- Drake, C.G.; Antonarakis, E.S. Update: Immunological Strategies for Prostate Cancer. Curr. Urol. Rep. 2010, 11, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Cattrini, C.; Zanardi, E.; Vallome, G.; Cavo, A.; Cerbone, L.; Di Meglio, A.; Fabbroni, C.; Latocca, M.; Rizzo, F.; Messina, C.; et al. Targeting androgen-independent pathways: New chances for patients with prostate cancer? Crit. Rev. Oncol. 2017, 118, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Chikuma, S. CTLA-4, an Essential Immune-Checkpoint for T-Cell Activation. In Emerging Concepts Targeting Immune Checkpoints in Cancer and Autoimmunity; Current Topics in Microbiology and, Immunology; Yoshimura, A., Ed.; Springer: Cham, Switzerland, 2017; Volume 410, pp. 99–126. [Google Scholar]
- Stohl, W.; Yu, N.; Chalmers, S.A.; Putterman, C.; Jacob, C.O. Constitutive reduction in the checkpoint inhibitor, CTLA-4, does not accelerate SLE in NZM 2328 mice. Lupus Sci. Med. 2019, 6, e000313. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Bendell, J.; Calvo, E.; Kim, J.W.; Ascierto, P.A.; Sharma, P.; Ott, P.A.; Peltola, K.; Jaeger, D.; Evans, J.; et al. CheckMate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients with Metastatic Esophagogastric Cancer. J. Clin. Oncol. 2018, 36, 2836–2844. [Google Scholar] [CrossRef]
- Pérez-Ruiz, E.; Etxeberria, I.; Rodriguez-Ruiz, M.E.; Melero, I. Anti-CD137 and PD-1/PD-L1 Antibodies En Route toward Clinical Synergy. Clin. Cancer Res. 2017, 23, 5326–5328. [Google Scholar] [CrossRef] [PubMed]
- Eckert, F.; Schaedle, P.; Zips, D.; Schmid-Horch, B.; Rammensee, H.-G.; Gani, C.; Gouttefangeas, C. Impact of curative radiotherapy on the immune status of patients with localized prostate cancer. OncoImmunology 2018, 7, e1496881. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Sailo, B.L.; Banik, K.; Harsha, C.; Prasad, S.; Gupta, S.C.; Bharti, A.C.; Aggarwal, B.B. Chronic diseases, inflammation, and spices: How are they linked? J. Transl. Med. 2018, 16, 1–25. [Google Scholar] [CrossRef]
- Zubair, H.; Azim, S.; Ahmad, A.; Khan, M.A.; Patel, G.K.; Singh, S.; Singh, A.P. Cancer Chemoprevention by Phytochemicals: Nature’s Healing Touch. Molecules 2017, 22, 395. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Shanmugam, M.K.; Kumar, A.P.; Yap, C.T.; Sethi, G.; Bishayee, A. Targeting autophagy using natural compounds for cancer prevention and therapy. Cancer 2019, 125, 1228–1246. [Google Scholar] [CrossRef]
- Tang, S.-M.; Deng, X.-T.; Zhou, J.; Li, Q.-P.; Ge, X.-X.; Miao, L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharmacother. 2020, 121, 109604. [Google Scholar] [CrossRef]
- Kim, D.H.; Khan, H.; Ullah, H.; Hassan, S.T.; Šmejkal, K.; Efferth, T.; Mahomoodally, M.F.; Xu, S.; Habtemariam, S.; Filosa, R.; et al. MicroRNA targeting by quercetin in cancer treatment and chemoprotection. Pharmacol. Res. 2019, 147, 104346. [Google Scholar] [CrossRef] [PubMed]
- Pezzuto, J.M.; Vang, O. Natural Products for Cancer Chemoprevention; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Taylor, W.F.; Jabbarzadeh, E. The use of natural products to target cancer stem cells. Am. J. Cancer Res. 2017, 7, 1588–1605. [Google Scholar]
- Salehi, B.; Fokou, P.V.T.; Yamthe, L.R.T.; Tali, B.T.; Adetunji, C.O.; Rahavian, A.; Mudau, F.N.; Martorell, M.; Setzer, W.N.; Rodrigues, C.F.; et al. Phytochemicals in Prostate Cancer: From Bioactive Molecules to Upcoming Therapeutic Agents. Nutrients 2019, 11, 1483. [Google Scholar] [CrossRef] [PubMed]
- Kallifatidis, G.; Hoy, J.J.; Lokeshwar, B.L. Bioactive natural products for chemoprevention and treatment of castration-resistant prostate cancer. Semin. Cancer Biol. 2016, 40-41, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Roell, D.; Baniahmad, A. The natural compounds atraric acid and N-butylbenzene-sulfonamide as antagonists of the human androgen receptor and inhibitors of prostate cancer cell growth. Mol. Cell. Endocrinol. 2011, 332, 1–8. [Google Scholar] [CrossRef]
- Saeed, A.F.; Su, J.; Ouyang, S. Marine-derived drugs: Recent advances in cancer therapy and immune signaling. Biomed. Pharmacother. 2021, 134, 111091. [Google Scholar] [CrossRef] [PubMed]
- Mudit, M.; Khanfar, M.; Muralidharan, A.; Thomas, S.; Shah, G.V.; Van Soest, R.W.; El Sayed, K.A. Discovery, design, and synthesis of anti-metastatic lead phenylmethylene hydantoins inspired by marine natural products. Bioorg. Med. Chem. 2009, 17, 1731–1738. [Google Scholar] [CrossRef] [PubMed]
- Lindequist, U. Marine-Derived Pharmaceuticals—Challenges and Opportunities. Biomol. Ther. 2016, 24, 561–571. [Google Scholar] [CrossRef]
- Tao, L.-Y.; Zhang, J.-Y.; Liang, Y.-J.; Chen, L.-M.; Zheng, L.-S.; Wang, F.; Mi, Y.-J.; She, Z.-G.; To, K.K.W.; Lin, Y.-C.; et al. Anticancer Effect and Structure-Activity Analysis of Marine Products Isolated from Metabolites of Mangrove Fungi in the South China Sea. Mar. Drugs 2010, 8, 1094–1105. [Google Scholar] [CrossRef]
- Dyshlovoy, S.A.; Otte, K.; Tabakmakher, K.M.; Hauschild, J.; Makarieva, T.N.; Shubina, L.K.; Fedorov, S.N.; Bokemeyer, C.; Stonik, V.A.; von Amsberget, G. Synthesis and anticancer activity of the derivatives of marine compound rhizochalin in castration resistant prostate cancer. Oncotarget 2018, 9, 16962. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Adamczak, A. Fucoxanthin—An antibacterial carotenoid. Antioxidants 2019, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-Z.; Ding, G.-F.; Huang, F.-F.; Yang, Z.-S.; Yu, F.-M.; Tang, Y.-P.; Jia, Y.-L.; Zheng, Y.-Y.; Chen, R. Anticancer Activity of Anthopleura anjunae Oligopeptides in Prostate Cancer DU-145 Cells. Mar. Drugs 2018, 16, 125. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zeng, X.; Li, S.; Sun, Z.; Yu, J.; Chen, C.; Shen, X.; Pan, W.; Luo, H. A Novel Tanshinone Analog Exerts Anti-Cancer Effects in Prostate Cancer by Inducing Cell Apoptosis, Arresting Cell Cycle at G2 Phase and Blocking Metastatic Ability. Int. J. Mol. Sci. 2019, 20, 4459. [Google Scholar] [CrossRef] [PubMed]
- Trump, D.L.; Aragon-Ching, J.B. Vitamin D in prostate cancer. Asian J. Androl. 2018, 20, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Van Royen, M.E.; van Cappellen, W.A.; de Vos, C.; Houtsmuller, A.B.; Trapman, J. Stepwise androgen receptor dimerization. J. Cell Sci. 2012, 125, 1970–1979. [Google Scholar] [CrossRef]
- Dai, C.; Heemers, H.; Sharifi, N. Androgen Signaling in Prostate Cancer. Cold Spring Harb. Perspect. Med. 2017, 7, a030452. [Google Scholar] [CrossRef]
- Nadal, M.; Prekovic, S.; Gallastegui, N.; Helsen, C.; Abella, M.; Zielinska, K.; Gay, M.; Vilaseca, M.; Taulès, M.; Houtsmuller, A.B.; et al. Structure of the homodimeric androgen receptor ligand-binding domain. Nat. Commun. 2017, 8, 14388. [Google Scholar] [CrossRef]
- Lall, R.K.; Adhami, V.M.; Mukhtar, H. Dietary flavonoid fisetin for cancer prevention and treatment. Mol. Nutr. Food Res. 2016, 60, 1396–1405. [Google Scholar] [CrossRef]
- Khan, N.; Asim, M.; Afaq, F.; Abu Zaid, M.; Mukhtar, H. A Novel Dietary Flavonoid Fisetin Inhibits Androgen Receptor Signaling and Tumor Growth in Athymic Nude Mice. Cancer Res. 2008, 68, 8555–8563. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef]
- Chiu, F.-L.; Lin, J.-K. Downregulation of androgen receptor expression by luteolin causes inhibition of cell proliferation and induction of apoptosis in human prostate cancer cells and xenografts. Prostate 2007, 68, 61–71. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef]
- Ide, H.; Lu, Y.; Noguchi, T.; Muto, S.; Okada, H.; Kawato, S.; Horie, S. Modulation of AKR 1C2 by curcumin decreases testosterone production in prostate cancer. Cancer Sci. 2018, 109, 1230–1238. [Google Scholar] [CrossRef]
- Guo, H.; Xu, Y.M.; Ye, Z.Q.; Yu, J.H.; Hu, X.Y. Curcumin induces cell cycle arrest and apoptosis of prostate cancer cells by regulating the expression of IκBα, c-Jun and androgen receptor. Die Pharmazie Int. J. Pharm. Sci. 2013, 68, 431–434. [Google Scholar]
- Wang, T.T.Y.; Hudson, T.S.; Remsberg, C.M.; Davies, N.M.; Takahashi, Y.; Kim, Y.S.; Seifried, H.; Vinyard, B.T.; Perkins, S.N.; Hursting, S.D. Differential effects of resveratrol on androgen-responsive LNCaP human prostate cancer cells in vitro and in vivo. Carcinogenesis 2008, 29, 2001–2010. [Google Scholar] [CrossRef] [PubMed]
- Mitani, T.; Harada, N.; Tanimori, S.; Nakano, Y.; Inui, H.; Yamaji, R. Resveratrol inhibits hypoxia-inducible factor-1α-mediated androgen receptor signaling and represses tumor progression in castration-resistant prostate cancer. J. Nutr. Sci. Vitamin 2014, 60, 276–282. [Google Scholar] [CrossRef]
- Wilson, S.; Cavero, L.; Tong, D.; Liu, Q.; Geary, K.; Talamonti, N.; Xu, J.; Fu, J.; Jiang, J.; Zhang, D. Resveratrol enhances polyubiquitination-mediated ARV7 degradation in prostate cancer cells. Oncotarget 2017, 8, 54683–54693. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Zhou, Z.; Cai, Y.; Castro, P.; Dakhov, O.; Shi, P.; Bai, Y.; Ji, H.; Shen, W.; Wang, J. Celastrol suppresses tumor cell growth through targeting an AR-ERG-NF-κB pathway in TMPRSS2/ERG fusion gene expressing prostate cancer. PLoS ONE 2013, 8, e58391. [Google Scholar] [CrossRef] [PubMed]
- Hahm, E.-R.; Karlsson, A.I.; Bonner, M.Y.; Arbiser, J.L.; Singh, S.V. Honokiol inhibits androgen receptor activity in prostate cancer cells. Prostate 2013, 74, 408–420. [Google Scholar] [CrossRef]
- Li, J.; Cao, B.; Liu, X.; Fu, X.; Xiong, Z.; Chen, L.; Sartor, O.; Dong, Y.; Zhang, H. Berberine Suppresses Androgen Receptor Signaling in Prostate Cancer. Mol. Cancer Ther. 2011, 10, 1346–1356. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, J.-S.; Young, C.Y. Silymarin inhibits function of the androgen receptor by reducing nuclear localization of the receptor in the human prostate cancer cell line LNCaP. Carcinogenesis 2001, 22, 1399–1403. [Google Scholar] [CrossRef]
- Nanao-Hamai, M.; Son, B.-K.; Komuro, A.; Asari, Y.; Hashizume, T.; Takayama, K.-I.; Ogawa, S.; Akishita, M. Ginsenoside Rb1 inhibits vascular calcification as a selective androgen receptor modulator. Eur. J. Pharmacol. 2019, 859, 172546. [Google Scholar] [CrossRef]
- Basak, S.; Pookot, D.; Noonan, E.J.; Dahiya, R. Genistein down-regulates androgen receptor by modulating HDAC6-Hsp90 chaperone function. Mol. Cancer Ther. 2008, 7, 3195–3202. [Google Scholar] [CrossRef]
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52. [Google Scholar] [CrossRef]
- Huang, Z.-H.; Zheng, H.-F.; Wang, W.-L.; Wang, Y.; Zhong, L.-F.; Wu, J.-L.; Li, Q.-X. Berberine targets epidermal growth factor receptor signaling to suppress prostate cancer proliferation in vitro. Mol. Med. Rep. 2014, 11, 2125–2128. [Google Scholar] [CrossRef]
- Huynh, H.; Nguyen, T.; Chan, E.; Tran, E. Inhibition of ErbB-2 and ErbB-3 expression by quercetin prevents transforming growth factor alpha (TGF-α)- and epidermal growth factor (EGF)-induced human PC-3 prostate cancer cell proliferation. Int. J. Oncol. 2003, 23. [Google Scholar] [CrossRef]
- Markaverich, B.M.; Vijjeswarapu, M.; Shoulars, K.; Rodriguez, M. Luteolin and gefitinib regulation of EGF signaling pathway and cell cycle pathway genes in PC-3 human prostate cancer cells. J. Steroid Biochem. Mol. Biol. 2010, 122, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.Y.; Leem, J.; Yoon, S.J.; Yoon, S.; Hong, S.J. Lipid raft cholesterol and genistein inhibit the cell viability of prostate cancer cells via the partial contribution of EGFR-Akt/p70S6k pathway and down-regulation of androgen receptor. Biochem. Biophys. Res. Commun. 2010, 393, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.R.; O’Brian, C.A. Resveratrol antagonizes EGFR-dependent Erk1/2 activation in human androgen-independent prostate cancer cells with associated isozyme-selective PKCα inhibition. Investig. New Drugs 2004, 22, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhang, Y.-L.; Xue, B.; Xu, G.-Y. Association of Caveolin-1 Expression with Prostate Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 10, 2964. [Google Scholar] [CrossRef] [PubMed]
- Ganai, S.A. Histone deacetylase inhibitor sulforaphane: The phytochemical with vibrant activity against prostate cancer. Biomed. Pharmacother. 2016, 81, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Bilani, N.; Bahmad, H.; Abou-Kheir, W. Prostate Cancer and Aspirin Use: Synopsis of the Proposed Molecular Mechanisms. Front. Pharmacol. 2017, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-C.; Chang, C.-H.; Ke, H.-L.; Chang, W.-S.; Cheng, H.-N.; Lin, H.-H.; Wu, C.-Y.; Tsai, C.-W.; Tsai, R.-Y.; Lo, W.-C.; et al. Association of cyclooxygenase 2 polymorphic genotypes with prostate cancer in Taiwan. Anticancer Res. 2011, 31, 221–225. [Google Scholar]
- Heidegger, I.; Kern, J.; Ofer, P.; Klocker, H.; Massoner, P. Oncogenic functions of IGF1R and INSR in prostate cancer include enhanced tumor growth, cell migration and angiogenesis. Oncotarget 2014, 5, 2723–2735. [Google Scholar] [CrossRef] [PubMed]
- Wing Ying Cheung, C.; Gibbons, N.; Wayne Johnson, D.; Lawrence Nicol, D. Silibinin-a promising new treatment for cancer. Anticancer Agents Med. Chem. 2010, 10, 186–195. [Google Scholar] [CrossRef]
- Zi, X.; Zhang, J.; Agarwal, R.; Pollak, M. Silibinin up-regulates insulin-like growth factor-binding protein 3 expression and inhibits proliferation of androgen-independent prostate cancer cells. Cancer Res. 2000, 60, 5617–5620. [Google Scholar]
- Fang, J.; Zhou, Q.; Shi, X.-L.; Jiang, B.-H. Luteolin inhibits insulin-like growth factor 1 receptor signaling in prostate cancer cells. Carcinogenesis 2006, 28, 713–723. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Wu, Y.-L.; Gao, X.-H.; Guo, F. Mitochondrial protein cyclophilin-D-mediated programmed necrosis attributes to berberine-induced cytotoxicity in cultured prostate cancer cells. Biochem. Biophys. Res. Commun. 2014, 450, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Delmulle, L.; Berghe, T.V.; Keukeleire, D.D.; Vandenabeele, P. Treatment of PC-3 and DU145 prostate cancer cells by prenylflavonoids from hop (Humulus lupulus L.) induces a caspase-independent form of cell death. Phytother. Res. 2008, 22, 197–203. [Google Scholar] [CrossRef]
- Shin, S.W.; Kim, S.Y.; Park, J.W. Autophagy inhibition enhances ursolic acid-induced apoptosis in PC3 cells. Biochim. Biophys. Acta BBA Mol. Cell Res. 2012, 1823, 451–457. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, K.-Y.; Yu, S.-N.; Park, S.-K.; Choi, H.-D.; Ji, J.-H.; Ahn, S.-C. Autophagy inhibition enhances silibinin-induced apoptosis by regulating reactive oxygen species production in human prostate cancer PC-3 cells. Biochem. Biophys. Res. Commun. 2015, 468, 151–156. [Google Scholar] [CrossRef]
- Yang, C.; Ma, X.; Wang, Z.; Zeng, X.; Hu, Z.; Ye, Z.; Shen, G. Curcumin induces apoptosis and protective autophagy in castration-resistant prostate cancer cells through iron chelation. Drug Des. Dev. Ther. 2017, ume11, 431–439. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Huysentruyt, L.C. On the Origin of Cancer Metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Zhang, L.L.; Li, L.; Wu, D.D.; Fan, J.H.; Li, X.; Wu, K.J.; Wang, X.Y.; He, D.L. A novel anti-cancer effect of genistein: Reversal of epithelial mesenchymal transition in prostate cancer cells 1. Acta Pharmacologica Sinica. 2008, 29, 1060–1068. [Google Scholar] [CrossRef]
- Lee, J.; Ju, J.; Park, S.; Hong, S.J.; Yoon, S. Inhibition of IGF-1 signaling by genistein: Modulation of E-cadherin expression and downregulation of β-catenin signaling in hormone refractory PC-3 prostate cancer cells. Nutr. Cancer 2012, 64, 153–162. [Google Scholar] [CrossRef]
- Khan, M.I.; Adhami, V.M.; Lall, R.K.; Sechi, M.; Joshi, D.C.; Haidar, O.M.; Syed, D.N.; Siddiqui, I.A.; Chiu, S.-Y.; Mukhtar, H. YB-1 expression promotes epithelial-to-mesenchymal transition in prostate cancer that is inhibited by a small molecule fisetin. Oncotarget 2014, 5, 2462–2474. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, K.; Arunkumar, R.; Elumalai, P.; Sharmila, G.; Gunadharini, D.N.; Banudevi, S.; Krishnamoorthy, G.; Benson, C.S.; Arunakaran, J. Quercetin inhibits invasion, migration and signalling molecules involved in cell survival and proliferation of prostate cancer cell line (PC-3). Cell Biochem. Funct. 2011, 29, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.-H.; Lin, Y.-W.; Wen, Y.-C.; Yang, Y.-C.; Hsiao, M.; Chang, J.-L.; Huang, H.-C.; Lee, W.-J. Targeting the SPOCK1-snail/slug axis-mediated epithelial-to-mesenchymal transition by apigenin contributes to repression of prostate cancer metastasis. J. Exp. Clin. Cancer Res. 2019, 38, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Dorai, T.; Diouri, J.; O’Shea, O.; Doty, S.B. Curcumin Inhibits Prostate Cancer Bone Metastasis by Up-Regulating Bone Morphogenic Protein-7 in Vivo. J. Cancer Ther. 2014, 5, 369–386. [Google Scholar] [CrossRef]
- Li, Y.; Che, M.; Bhagat, S.; Ellis, K.-L.; Kucuk, O.; Doerge, D.R.; Abrams, J.; Cher, M.L.; Sarkar, F.H. Regulation of Gene Expression and Inhibition of Experimental Prostate Cancer Bone Metastasis by Dietary Genistein. Neoplasia 2004, 6, 354–363. [Google Scholar] [CrossRef]
- Kuchta, K.; Xiang, Y.; Huang, S.; Tang, Y.; Peng, X.; Wang, X.; Zhu, Y.; Li, J.; Xu, J.; Lin, Z.; et al. Celastrol, an active constituent of the TCM plant Tripterygium wilfordii Hook.f., inhibits prostate cancer bone metastasis. Prostate Cancer Prostatic Dis. 2017, 20, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Mittal, S.; Sak, K.; Singhal, P.; Tuli, H.S. Molecular mechanisms of action of quercetin in cancer: Recent advances. Tumor Biol. 2016, 37, 12927–12939. [Google Scholar] [CrossRef]
- Tuli, H.S.; Sandhu, S.S.; Sharma, A.K. Pharmacological and therapeutic potential of Cordyceps with special reference to Cordycepin. 3 Biotech 2014, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Palumbo, R.; Tedesco, I.; Mazzarella, G.; Russo, P.; Iacomino, G.; Russo, G.L. Quercetin and anti-CD95(Fas/Apo1) enhance apoptosis in HPB-ALL cell line. FEBS Lett. 1999, 462, 322–328. [Google Scholar] [CrossRef]
- Aalinkeel, R.; Bindukumar, B.; Reynolds, J.L.; Sykes, D.E.; Mahajan, S.D.; Chadha, K.C.; Schwartz, S.A. The dietary bioflavonoid, quercetin, selectively induces apoptosis of prostate cancer cells by down-regulating the expression of heat shock protein 90. Prostate 2008, 68, 1773–1789. [Google Scholar] [CrossRef]
- Lautraite, S.; Musonda, A.; Doehmer, J.; Edwards, G.; Chipman, J. Flavonoids inhibit genetic toxicity produced by carcinogens in cells expressing CYP1A2 and CYP1A1. Mutagen. 2002, 17, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Haghiac, M.; Walle, T. Quercetin Induces Necrosis and Apoptosis in SCC-9 Oral Cancer Cells. Nutr. Cancer 2005, 53, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-C.; Yang, J.-S.; Lu, H.-F.; Ip, S.-W.; Lo, C.; Wu, C.-C.; Lin, J.-P.; Tang, N.-Y.; Chung, J.-G.; Chou, M.-J.; et al. Quercetin-mediated cell cycle arrest and apoptosis involving activation of a caspase cascade through the mitochondrial pathway in human breast cancer MCF-7 cells. Arch. Pharm. Res. 2010, 33, 1181–1191. [Google Scholar] [CrossRef]
- Niu, G.; Yin, S.; Xie, S.; Li, Y.; Nie, D.; Ma, L.; Wang, X.; Wu, Y. Quercetin induces apoptosis by activating caspase-3 and regulating Bcl-2 and cyclooxygenase-2 pathways in human HL-60 cells. Acta Biochim. Biophys. Sin. 2011, 43, 30–37. [Google Scholar] [CrossRef]
- Banerjee, T.; Van Der Vliet, A.; Ziboh, V. Downregulation of COX-2 and iNOS by amentoflavone and quercetin in A549 human lung adenocarcinoma cell line. Prostaglandins Leukot. Essent. Fat Acids 2002, 66, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Granado-Serrano, A.B.; Martín, M.A.; Bravo, L.; Goya, L.; Ramos, S. Quercetin Induces Apoptosis via Caspase Activation, Regulation of Bcl-2, and Inhibition of PI-3-Kinase/Akt and ERK Pathways in a Human Hepatoma Cell Line (HepG2). J. Nutr. 2006, 136, 2715–2721. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Dong, X.-S.; Gao, H.-Y.; Jiang, Y.-F.; Jin, Y.-L.; Chang, Y.-Y.; Chen, L.-Y.; Wang, J.-H. Suppression of HSP27 increases the anti-tumor effects of quercetin in human leukemia U937 cells. Mol. Med. Rep. 2015, 13, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.J.; Tuli, H.S.; Mittal, S.; Shandilya, J.K.; Tiwari, A.; Sandhu, S.S. Isothiocyanates: A class of bioactive metabolites with chemopreventive potential. Tumor Biol. 2015, 36, 4005–4016. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.-X.; Deng, X.-H.; Ai, F.; Yuan, G.-Y.; Song, H.-Y. Effect of quercetin on the proliferation of the human ovarian cancer cell line SKOV-3 in vitro. Exp. Ther. Med. 2015, 10, 579–583. [Google Scholar] [CrossRef]
- Yeh, S.-L.; Wu, S.-H. Effects of quercetin on β-apo-8′-carotenal-induced DNA damage and cytochrome P1A2 expression in A549 cells. Chem. Inter. 2006, 163, 199–206. [Google Scholar] [CrossRef]
- Nebert, D.W.; Dalton, T.P. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat. Rev. Cancer 2006, 6, 947–960. [Google Scholar] [CrossRef]
- Tan, X.-L.; Spivack, S.D. Dietary chemoprevention strategies for induction of phase II xenobiotic-metabolizing enzymes in lung carcinogenesis: A review. Lung Cancer 2009, 65, 129–137. [Google Scholar] [CrossRef]
- Kansanen, E.; Jyrkkänen, H.K.; Volger, O.L.; Leinonen, H.; Kivelä, A.M.; Häkkinen, S.K.; Woodcock, S.R.; Schopfer, F.J.; Horrevoets, A.J.; Ylä-Herttuala, S. Nrf2-dependent and-independent responses to nitro-fatty acids in human endothelial cells identification of heat shock response as the major pathway activated by nitro-oleic acid. J. Biol. Chem. 2009, 284, 33233–33241. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Motohashi, H.; Yamamoto, M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells 2011, 16, 123–140. [Google Scholar] [CrossRef]
- Tanigawa, S.; Fujii, M.; Hou, D.-X. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic. Biol. Med. 2007, 42, 1690–1703. [Google Scholar] [CrossRef]
- Ramyaa, P.; Padma, V.V. Quercetin modulates OTA-induced oxidative stress and redox signalling in HepG2 cells—up regulation of Nrf2 expression and down regulation of NF-κB and COX-2. Biochim. Biophys. Acta BBA Gen. Subj. 2014, 1840, 681–692. [Google Scholar] [CrossRef]
- Lu, X.; Chen, D.; Yang, F.; Xing, N. Quercetin Inhibits Epithelial-to-Mesenchymal Transition (EMT) Process and Promotes Apoptosis in Prostate Cancer via Downregulating lncRNA MALAT1. Cancer Manag. Res. 2020, 12, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, K.; Elumalai, P.; Arunkumar, R.; Banudevi, S.; Gunadharini, N.D.; Sharmila, G.; Selvakumar, K.; Arunakaran, J. Quercetin regulates insulin like growth factor signaling and induces intrinsic and extrinsic pathway mediated apoptosis in androgen independent prostate cancer cells (PC-3). Mol. Cell. Biochem. 2010, 344, 173–184. [Google Scholar] [CrossRef]
- Liu, K.-C.; Yen, C.-Y.; Wu, R.S.-C.; Yang, J.-S.; Lu, H.-F.; Lu, K.-W.; Lo, C.; Chen, H.-Y.; Tang, N.-Y.; Wu, C.-C.; et al. The roles of endoplasmic reticulum stress and mitochondrial apoptotic signaling pathway in quercetin-mediated cell death of human prostate cancer PC-3 cells. Environ. Toxicol. 2014, 29, 428–439. [Google Scholar] [CrossRef]
- Jung, Y.-H.; Heo, J.; Lee, Y.J.; Kwon, T.K.; Kim, Y.-H. Quercetin enhances TRAIL-induced apoptosis in prostate cancer cells via increased protein stability of death receptor 5. Life Sci. 2010, 86, 351–357. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Lee, D.-H.; Jeong, J.-H.; Guo, Z.S.; Lee, Y.J. Quercetin augments TRAIL-induced apoptotic death: Involvement of the ERK signal transduction pathway. Biochem. Pharmacol. 2008, 75, 1946–1958. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Lee, Y.J. TRAIL apoptosis is enhanced by quercetin through Akt dephosphorylation. J. Cell. Biochem. 2007, 100, 998–1009. [Google Scholar] [CrossRef]
- Baruah, M.M.; Khandwekar, A.P.; Sharma, N. Quercetin modulates Wnt signaling components in prostate cancer cell line by inhibiting cell viability, migration, and metastases. Tumor Biol. 2016, 37, 14025–14034. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Jiang, X.; Song, L.; Wang, H.; Mei, Z.; Xu, Z.; Xing, N. Quercetin inhibits angiogenesis through thrombospondin-1 upregulation to antagonize human prostate cancer PC-3 cell growth in vitro and in vivo. Oncol. Rep. 2016, 35, 1602–1610. [Google Scholar] [CrossRef] [PubMed]
- Pratheeshkumar, P.; Budhraja, A.; Son, Y.-O.; Wang, X.; Zhang, Z.; Ding, S.; Wang, L.; Hitron, A.; Lee, J.-C.; Xu, M.; et al. Quercetin Inhibits Angiogenesis Mediated Human Prostate Tumor Growth by Targeting VEGFR- 2 Regulated AKT/mTOR/P70S6K Signaling Pathways. PLoS ONE 2012, 7, e47516. [Google Scholar] [CrossRef]
- Yang, F.-Q.; Liu, M.; Li, W.; Che, J.-P.; Wang, G.-C.; Zheng, J.-H. Combination of quercetin and hyperoside inhibits prostate cancer cell growth and metastasis via regulation of microRNA-21. Mol. Med. Rep. 2015, 11, 1085–1092. [Google Scholar] [CrossRef]
- Sun, S.; Gong, F.; Liu, P.; Miao, Q. Metformin combined with quercetin synergistically repressed prostate cancer cells via inhibition of VEGF/PI3K/Akt signaling pathway. Gene 2018, 664, 50–57. [Google Scholar] [CrossRef]
- Tang, S.-N.; Singh, C.; Nall, D.; Meeker, D.; Shankar, S.; Srivastava, R.K. The dietary bioflavonoid quercetin synergizes with epigallocathechin gallate (EGCG) to inhibit prostate cancer stem cell characteristics, invasion, migration and epithelial-mesenchymal transition. J. Mol. Signal. 2010, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Song, L.; Wang, H.; Xing, N. Quercetin synergizes with 2-methoxyestradiol inhibiting cell growth and inducing apoptosis in human prostate cancer cells. Oncol. Rep. 2013, 30, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Phan, T.; Gordon, D.; Chung, S.; Henning, S.M.; Vadgama, J.V. Arctigenin in combination with quercetin synergistically enhances the antiproliferative effect in prostate cancer cells. Mol. Nutr. Food Res. 2014, 59, 250–261. [Google Scholar] [CrossRef]
- Shu, Y.; Xie, B.; Liang, Z.; Chen, J. Quercetin reverses the doxorubicin resistance of prostate cancer cells by downregulating the expression of c-met. Oncol. Lett. 2017, 15, 2252–2258. [Google Scholar] [CrossRef]
- Lu, X.; Yang, F.; Chen, D.; Zhao, Q.; Chen, D.; Ping, H.; Xing, N. Quercetin reverses docetaxel resistance in prostate cancer via androgen receptor and PI3K/Akt signaling pathways. Int. J. Biol. Sci. 2020, 16, 1121–1134. [Google Scholar] [CrossRef] [PubMed]
- Aynacıoğlu, A.Ş.; Bilir, A.; Kadomatsu, K. Dual inhibition of P-glycoprotein and midkine may increase therapeutic effects of anticancer drugs. Med. Hypotheses 2017, 107, 26–28. [Google Scholar] [CrossRef]
- Qi, M.; Ikematsu, S.; Maeda, N.; Ichihara-Tanaka, K.; Sakuma, S.; Noda, M.; Muramatsu, T.; Kadomatsu, K. Haptotactic Migration Induced by Midkine Involvement of Protein-Tyrosine Phosphatase Ζ, Mitogen-Activated Protein Kinase, And Phosphatidylinositol 3-Kinase. J. Biol. Chem. 2001, 276, 15868–15875. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Abrams, S.L.; Stadelman, K.; Chappell, W.H.; LaHair, M.; Ferland, R.A.; Steelman, L.S. Targeting signal transduction pathways to eliminate chemotherapeutic drug resistance and cancer stem cells. Adv. Enzym. Regul. 2010, 50, 285–307. [Google Scholar] [CrossRef]
- Tummala, R.; Lou, W.; Gao, A.C.; Nadiminty, N. Quercetin Targets hnRNPA1 to Overcome Enzalutamide Resistance in Prostate Cancer Cells. Mol. Cancer Ther. 2017, 16, 2770–2779. [Google Scholar] [CrossRef]
- Yeh, Y.; Guo, Q.; Connelly, Z.; Cheng, S.; Yang, S.; Prieto-Dominguez, N.; Yu, X. Wnt/Beta-Catenin Signaling and Prostate Cancer Therapy Resistance. In Prostate Cancer; Advances in Experimental Medicine and Biology; Dehm, S., Tindall, D., Eds.; Springer: Cham, Switzerland, 2019; Volume 1210, pp. 351–378. [Google Scholar]
- Kumari, A.; Kumar, V.; Yadav, S.K. Plant Extract Synthesized PLA Nanoparticles for Controlled and Sustained Release of Quercetin: A Green Approach. PLoS ONE 2012, 7, e41230. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, K.; King, J.W.; Howard, L.R.; Monrad, J.K. Solubility and solution thermodynamic properties of quercetin and quercetin dihydrate in subcritical water. J. Food Eng. 2010, 100, 208–218. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, J.; Wei, T.; Ma, X.; Cheng, Q.; Huo, S.; Zhang, C.; Zhang, Y.; Duan, X.; Liang, X.-J. Quercetin-loaded nanomicelles to circumvent human castration-resistant prostate cancer in vitro and in vivo. Nanoscale 2016, 8, 5126–5138. [Google Scholar] [CrossRef] [PubMed]
- Shitole, A.A.; Sharma, N.; Giram, P.; Khandwekar, A.; Baruah, M.; Garnaik, B.; Koratkar, S. LHRH-conjugated, PEGylated, poly-lactide-co-glycolide nanocapsules for targeted delivery of combinational chemotherapeutic drugs Docetaxel and Quercetin for prostate cancer. Mater. Sci. Eng. C 2020, 114, 111035. [Google Scholar] [CrossRef]
- Hemati, M.; Haghiralsadat, F.; Yazdian, F.; Jafari, F.; Moradi, A.; Malekpour-Dehkordi, Z. Development and characterization of a novel cationic PEGylated niosome-encapsulated forms of doxorubicin, quercetin and siRNA for the treatment of cancer by using combination therapy. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1295–1311. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Miao, L.; Goodwin, T.J.; Li, J.; Liu, Q.; Huang, L. Quercetin Remodels the Tumor Microenvironment To Improve the Permeation, Retention, and Antitumor Effects of Nanoparticles. ACS Nano 2017, 11, 4916–4925. [Google Scholar] [CrossRef] [PubMed]
- Van Brussel, J.P.; Mickisch, G.H. Multidrug resistance in prostate cancer. Oncol. Res. Treat. 2003, 26, 175–181. [Google Scholar] [CrossRef]
- Aras, A.; Khokhar, A.R.; Qureshi, M.Z.; Silva, M.F.; Sobczak-Kupiec, A.; Pineda, E.A.G.; Hechenleitner, A.A.W.; Farooqi, A.A. Targeting Cancer with Nano-Bullets: Curcumin, EGCG, Resveratrol and Quercetin on Flying Carpets. Asian Pac. J. Cancer Prev. 2014, 15, 3865–3871. [Google Scholar] [CrossRef]
