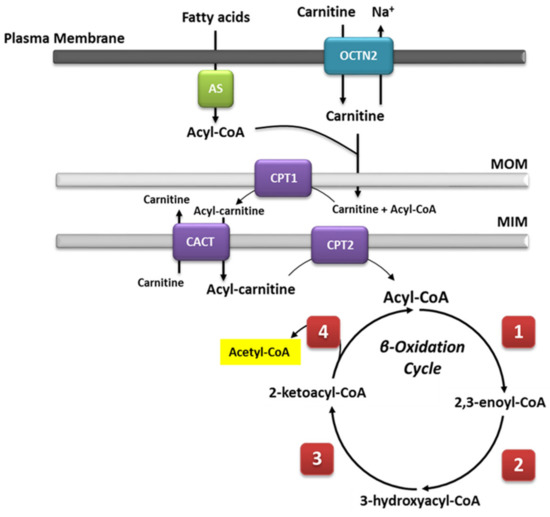
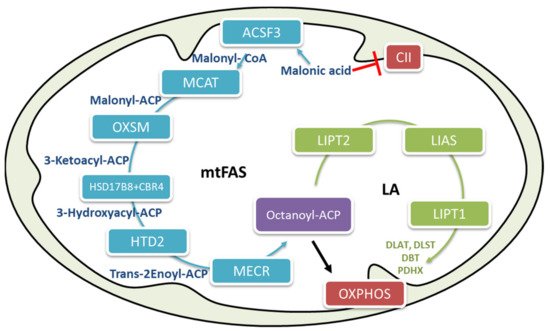
Diet has a considerable role in metabolic flexibility, depending on the type of nutrients and the period of fasting [
77]. It is well known that a decrease in circulating dietary carbohydrates and lipids and a decline in insulin/glucagon ratio during fasting induce a switch toward fatty acid oxidation [
77]. In line with these studies, other reports have shown that subjects under a high-fat diet were able to increase fatty acid oxidation at the expense of the glycolytic rate, though this effect was not observed in obese individuals. Very likely these individuals are unable to up-regulate PDK4, which inhibits the pyruvate dehydrogenase complex, and they therefore did not show a fast response to the enhancement of circulating lipids [
78]. Skeletal muscle is particular sensitive to nutrient availability; the stimulation of insulin production increases glucose transport into the cell and the uptake of triglycerides from the blood into muscle, and at the same time, decreases the rate of fatty acid oxidation and increases the rate of protein synthesis [
79]. In the white skeletal muscle of VLCAD
−/− mice, adaptive compensatory up-regulation mediated by mtFAS, toward glycolysis in type II muscle fibers, was reversed by prolonged supplementation with an MCT diet [
38], likely in response to nutrient availability. In fact, MCTs are rapidly hydrolyzed after ingestion, and the circulating medium-chain fatty acids can be easily uptaken up by peripheral tissue and undergo β-oxidation as they can cross the mitochondrial membrane without the need for an active transport system [
80].
The involvement of mFAS in up-regulating glucose oxidation has not been previously described and underlines its relevance in the maintenance of metabolic flexibility. In a similar manner, sex represents an important variable due to the significant influence of hormonal changes and even lifestyle [
76]. In the literature, there are several reports that describe sex-specific differences in basal metabolism; however, the data are very controversial [
81,
82,
83]. Basal metabolism represents the minimum energetic rate required by an organism at rest [
84]. On the one hand, some reports have shown higher efficiency in mitochondria isolated from the heart, skeletal muscles, and liver of male B6(C57Bl/6J) mouse strain [
82]. On the other hand, it has been shown that mitochondria isolated from the cardiac muscle of female rats are fewer in number but morphologically more differentiated than those of male rats, indicative of a more efficient mitochondrial electron transport chain and lower H
2O
2 generation [
83]. Similarly, mitochondria from tissues other than heart have also displayed higher energetic efficiency and mitochondrial oxygen consumption [
81,
85]. These studies are in line with the higher respiration rate observed in fibroblasts from wildtype (WT) female mice, reported by Seahorse experiments [
42]. However, when cells were incubated with octanoate (C8), one of the major components of MCTs, the nutrient availability changed and mitochondrial respiration was more efficiently enhanced in WT male mice, suggesting that an overload of available substrate may, in part, hamper the process involved in the metabolic flexibility in female cells or that females have a higher mitochondrial oxygen consumption rate when using non-fatty acid substrates [
42]. These findings are in accordance with previous observations on the development of severe metabolic syndrome in female VLCAD
−/− mice on a long-term MCT diet, whereas male mice were protected [
35]. Moreover, incubation with C8 has also shown a completely different response and sex-specific activation of signaling pathways, in particular, sex-specific activation of mTORc1 was observed in female mice. The central role of mTOR signaling in regulating both lipogenesis and lipolysis has been acknowledged for a long time [
86]. In this regard, incubation of female VLCAD
−/− mouse fibroblasts with C8 has led to the activation of the ERK/mTORc1 pathway and, subsequently, of lipogenesis [
42], corroborating the previously reported sex-specific development of a metabolic syndrome in female VLCAD
−/− mice after prolonged MCT supplementation [
35]. These findings were also confirmed by gene expression analysis and SILAC-based proteomic analysis in fibroblasts, which showed clear, subsequent stimulation of de novo fatty acid biosynthesis. Although lipogenesis was also up-regulated in cells of male mice, mTORc1 was not activated by C8. Under control conditions, cells from male VLCAD
−/− mice already show up-regulated levels of ERK1/ERK2 factors; C8 had no further effects. In addition, it has been shown that medium-chain fatty acids are able to occupy the ligand binding pocket (LBP) of the ligand binding domain (LBD) of the peroxisome proliferator-activated receptor (PPAR)γ and are therefore considered to be selective PPARγ activators [
87]. The data are in line with the up-regulation of PPARγ after incubation with C8, with the subsequent increment in peroxisomal activity reflected by higher peroxisomal β-oxidation and biogenesis [
42]. These observations clearly support the fact that sex and diet play a key role in the regulation of energy homeostasis and metabolic flexibility in fatty acid oxidation disorders, and that these two variables should likely be taken into consideration in the long-term treatment of FAOD patients.