Interleukins were discovered as small secreted signaling proteins that mediate communication between immune cells, in particular leukocyte communication (reviewed in great detail in [14]). Indeed, the majority of ILs are produced by immune cells [15]. However, several other cell types including bone cells and tumor cells are now known to produce ILs and to respond to both, autocrine and paracrine IL-signaling [16,17,18,19,20]. By binding to their cognate cell surface receptor, ILs modify several physiological cell processes including proliferation, differentiation and apoptosis. Since the discovery of IL-1 in 1977, in total 38 ILs have been identified with distinct signaling and biological properties (). Several classification systems exist but briefly ILs are grouped into families according to biological function, sequence homology and/or receptor chain similarities [21,22]. With regards to the receptors this includes for example type I and type II cytokine receptor families as well as the interleukin-1 receptor/Toll-like receptor (IL-1R/TLR) [23]. Signaling via type I and type II receptors including for example IL-6 or IL-11 involves intracellular signaling cascades such as mitogen-activated protein kinase (MAPK), extracellular-signal-regulated kinase (ERK), Janus kinase/signal transducer and activator of transcription (JAK/STAT), and the phosphoinositide 3-kinase (PI3-K) [23]. Signaling downstream the IL-1R/TLR axis involves TNF receptor associated factor 6 (TRAF6), nuclear factor-kappa B (NFkB), c-Jun N-terminal kinase (JNK) and p38 [23]. Of note, whereas IL-1, IL-6 and IL-11 belong to the interleukin family of cytokines, IL-8 belongs to the CXC-motive chemokine family [24].
2. The Role of ILs during the Establishment and Progression of Bone Metastasis
2.1. Cancer Stem Cells, Circulating Tumor Cells and IL-Mediated Attraction to the Bone Metastatic Niche
Only a small subset of cancer cells is thought to be able to initiate metastatic growth. Emerging evidence has demonstrated that within tumors a subpopulation of cancer cells with stem cell-like features exists. This subpopulation is referred to as cancer stem cells (CSCs) and has the ability to self-renew, differentiate and initiate metastatic outgrowth (colonization) [
56]. In addition, CSCs are also critical in regulating resistance to anti-cancer therapy [
57]. Recently, bone marrow-derived factors, including ILs, have been suggested to specifically enhance breast CSCs to form colonies upon their arrival at the metastatic site [
58].
Circulating tumor cells (CTCs) are often found in the blood stream of patients with solid tumors. These cells are shed into the circulation and are thought to function as seeds for metastases. Similar characteristics of CTCs compared to CSC including a high plasticity and the capability to form distant metastases have been reported previously [
70]. Recently, Koch et al. established a CTC line (CTC-ITB-01) from the blood of a patient with advanced ER
+ breast cancer [
71]. CTC-ITB-01 cells were capable of forming metastases at different organs including the bone in an in vivo xenograft mouse model after intraductal injection. Further characterization of common stemness markers in breast cancer revealed a high expression of ALDH1, a more recent breast cancer stemness marker [
72] in CTC-ITB-01 cells. CTCs are often associated with poor prognosis and used as clinical biomarkers or as novel therapeutic targets in oncology [
73,
74,
75]. Dynamic changes in CTC levels in combination with corresponding alterations in serum IL levels might serve as prognostic markers for the progression of breast cancer () [
50,
76,
77,
78]. A study investigated if certain serum cytokine profiles (e.g., ILs, tumor necrosis factor alpha, interferon gamma) could be associated with the presence of CTCs in breast cancer patients [
77]. Interestingly, in the CTC-positive group, elevated levels of serum IL-1α were observed in patients without lymph metastases when compared to patients with lymph involvement, suggesting that IL-1α is associated with the release of CTCs into the circulation rather than into the lymphatic system [
77]. In mouse models of breast cancer bone metastasis elevated expression of IL-1β has been determined in CTCs when compared to breast cancer cells isolated from mammary tumors [
50]. Others have associated CTC counts in patients suffering from progressing metastatic breast cancer with elevated IL-6 and IL-8 levels [
76]. Others report elevated serum levels of IL-8 and IL-13 in patients with no CTCs and progesterone receptor-negative breast cancer when compared to patients with progesterone receptor-positive breast cancer [
78]. In contrast, no differences in IL-levels were observed in breast cancer patients with CTCs [
78]. Separate studies have found a reverse correlation of CTCs and IL-2 levels in patients with advanced breast cancer [
79]. Together, these studies underscore the challenge of establishing a correlation between the levels of ILs and CTCs as prognostic markers, in particular as it might depend on the cancer subtype (e.g., receptor status).
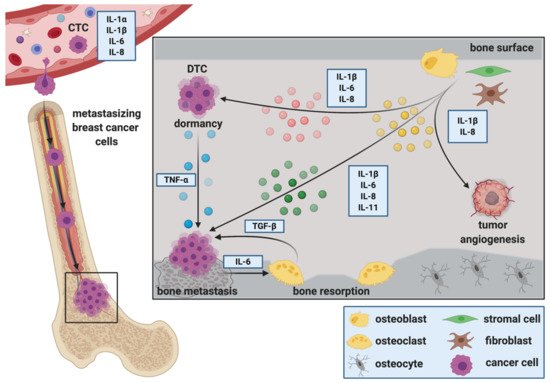
Figure 1. Interleukins during the progression of breast cancer bone metastasis. Interleukins (ILs) play pivotal roles in mediating several steps of the metastatic cascade in breast cancer. Dynamic changes in circulating tumor cell (CTC) levels in combination with corresponding alterations in serum IL levels might serve as prognostic markers for the progression of breast cancer. Furthermore, ILs are suggested to regulate the attraction of disseminated tumor cells (DTCs) to the metastatic site, facilitate extravasation from the circulation, adhesion and migration in the secondary organ. Once in the secondary organ, DTCs receive interleukin signals from adjacent, tissue-resident cells that regulate their fate in the secondary organ. Cells of the bone microenvironment secrete ILs that control tumor cell dormancy. In turn, proliferating tumor cells secrete ILs that stimulate osteoclastic bone resorption and consequently osteolysis. Factors released during osteolysis (e.g., Transforming growth factor β (TGF-β)) then fuel the vicious cycle of bone metastasis. Stromal-derived ILs can also regulate tumor growth via affecting vascular endothelial cells, thus promoting vascularization and metastatic growth.
2.2. The Role of ILs during the Extravasation of DTCs at the Metastatic Site
The extravasation of DTCs from the circulation into distant organs requires interaction of DTCs with endothelial and stromal cells via adhesion molecules. Interestingly, IL-1ß and IL-6 have been shown to increase focal adhesion kinase (FAK) in breast cancer cells [
64,
81]. Further in vitro studies have shown that osteoblasts can stimulate the adhesion of breast cancer cells to a monolayer of bone marrow endothelial cells [
82]. In these experiments osteoblasts produced increased levels of inflammatory cytokines, including IL-1ß, which increased the expression of adhesion molecules on endothelial cells and consequently facilitated breast cancer cell adhesion [
82]. Similarly, macrophage derived IL-1ß has been shown to support breast cancer cell adhesion to endothelial cell monolayers [
83]. Also adipocytes have been reported to alter adhesion molecules in breast cancer cells via IL-8 [
84]. Stimulation with recombinant human IL-8 increased mucin-1 in MCF-7 and T47D breast cancer cells and decreased intercellular adhesion molecule 1 (ICAM-1) as well as vascular cell adhesion molecule 1 (VCAM-1) in T47D cells [
84]. Bendre and colleagues compared characteristics of highly metastatic MDA-MET breast cancer cells against non-metastatic MDA-231 cells in vitro [
53]. Although these studies did not report differences in proliferation between the two cell lines, an increased early adhesion to type IV collagen matrix and increased invasion through matrigel was observed [
53]. Microarray and gene expression analysis revealed that only a limited number of genes was differentially expressed between the two cell lines, with one of them being IL-8 [
53]. Together, these studies highlight the importance of ILs in breast cancer cell adhesion, a key process in cancer progression and metastasis.
2.3. ILs during Migration, Invasion and Epithelial to Mesenchymal Transition (EMT) at the Metastatic Site
ILs are also implicated in the migration of tumor cells to the site of metastatic growth. Both, stromal cells as well as tumor cells can be the source of ILs, resulting in autocrine as well as paracrine signaling between bone and cancer cells. For example, overexpression of IL-1β in tumor cells has been shown to increase their migration as well as their invasion through Matrigel towards osteoblasts [
50]. Others have reported that human bone tissue—conditioned medium increased the migration of MDA-MB-231-fluc-EGFP breast cancer cells when compared to control medium, which was associated with increased levels of adipokines and cytokines including IL-1ß [
12]. Additionally, IL-1β enhances tumor cell invasion through supporting the production of matrix-degrading enzymes (e.g., matrix metalloprotease 9 (MMP-9)) as well as through activation of the Src/FAK pathway [
81]. Similarly, osteoblast-derived cytokines including IL-6 and IL-8 have been reported to stimulate the migration of breast cancer cells in vitro [
85].
Isolated fibroblasts from common sites of breast cancer metastasis, including lung and bone, produce elevated levels of IL-6 when compared to normal skin fibroblasts [
86]. Fibroblast-derived IL-6 stimulated growth and invasion of breast cancer cells in vitro through activation of STAT-3 signaling [
86]. Oncostatin M (OSM), an IL-6 family cytokine that is produced by tumor stromal cells such as neutrophils [
87], increased breast cancer cell invasion in vitro [
87,
88]. IL-6 expressing breast cancer cells have also been shown to recruit myeloid-derived suppressor cells to infiltrate sites of primary as well as distant, metastatic tumor growth [
89]. Metastasizing cancer cells stimulated myeloid-derived suppressor cells to secrete elevated levels of IL-6 and IL-6 receptor-α, which in turn stimulated breast cancer cell aggressiveness and metastasis [
89]. Thus, an IL-6 signaling loop between breast cancer cells and myeloid derived suppressor cells that drives cancer cell invasiveness and distant metastasis has been proposed [
89].
Epithelial to mesenchymal transition (EMT) is well established to be involved in breast cancer cell invasion and metastasis [
90]. Vimentin, smooth-muscle actin, N-cadherin and cadherin-11 are commonly referred as markers for EMT [
91]. CD44
+ T47D breast cancer stem cells induced by IL-6 have been shown to undergo EMT in vitro, which was characterized by an increased presence of vimentin [
62]. Others have shown that several breast cancer cell lines over expressing IL-1ß demonstrated elevated EMT due to higher expression of N-cadherin and decreased levels of E-cadherin [
50]. Additionally, the IL-8/IL-8R signaling axis is induced in Brachyury-overexpressing breast cancer cells during EMT, which further promotes tumor progression by altering the tumor microenvironment and adjacent tumor cells [
92].
2.4. ILs as Regulators of DTC Dormancy at the Metastatic Site
Once in the secondary organ, DTCs receive signals from adjacent, tissue-resident cells that regulate their fate in the distant site. It has been suggested that inflammatory cytokines associated with pathological bone remodeling (e.g., IL-1β and tumor necrosis factor-α (TNF-α)) may trigger escape from dormancy and the occurrence of bone metastases [
93]. Others propose that stromal-derived IL-6, IL-8 and TGF-β1 stimulate breast cancer cell escape from dormancy in vitro [
94] (). However, precise mechanisms regulating tumor cell dormancy are poorly understood [
95]. As breast cancer bone metastases remains incurable once progressing in bone, preventing dormant DTCs from reawakening could be a potential key to avoid cancer recurrence at secondary sites.
Bone marrow stromal cells, including osteoblasts, interact with DTCs to regulate tumor cell dormancy. Interestingly, some reports shown that osteoblasts promote breast cancer cell dormancy in bone, whereas others suggest that they support metastatic growth [
93,
96,
97,
98,
99]. Recently, a special subpopulation of osteoblasts that supports breast cancer cell dormancy has been reported [
100]. This subpopulation showed a reduced expression of IL-6 and α-smooth muscle actin (α-SMA) [
100]. On the other hand, osteoblasts have been shown to express increased levels of inflammatory cytokines including IL-6 and IL-8 upon the presence of breast cancer cells, which facilitates metastatic breast cancer growth in bone [
98,
101]. Additionally, co-culture of otherwise weakly metastatic (dormant) breast cancer cells with MC3T3-E1 osteoblasts increased breast cancer growth in vitro when stimulated with IL-1ß and TNF-α [
93]. Using ex-vivo co-culture systems of breast cancer cells and mouse bones, others have also shown that a CXCL5/CXCR2 (the IL-8 receptor-β) signaling axis might be involved in regulating the balance between cancer cell quiescence/dormancy and subsequent breast cancer outgrowth in bone [
102]. Additionally, breast cancer cells that remain dormant within the bone are suggested to be sensitive to the leukemia inhibitory factor (LIF), which belongs to the IL-6 family of cytokines [
103]. LIF binds to LIF receptor (LIFR), which has been shown to be downregulated in patients with breast cancer bone metastasis [
103].Of note, several key bone cells including osteoblasts, osteoclasts, chondrocytes and adipocytes secrete LIF and also express corresponding receptors [
104]. Mechanistically, loss of LIF/STAT3 signaling was associated with re-awakening of cells from dormancy. Indeed, loss of LIFR down-regulated dormancy-related genes (e.g., thrombospondin-1 (TSP1), tropomyosin-1 (TPM1), TGF-ß2) and resulted in tumor cell dissemination to bone and osteolytic disease in vivo [
103]. In the liver, another common site of breast cancer metastasis, activated hepatic stellate cells have been proposed to aid MDA-MB-231 breast cancer cell escape from dormancy via elevated secretion of IL-8 [
105]. In summary, IL-signaling between bone cells and DTCs could be used as a therapeutic target to prevent DTC escape from dormancy ().
2.5. ILs and Breast Cancer Colonization in Bone, Metastatic Outgrowth and the Creation of a Metastasis—Supporting Environment
Upon their presence in bone, DTCs alter the microenvironment to provide suitable growth conditions. Mesenchymal derived cells including osteoblasts and fibroblasts make up a large proportion of the cellular entities that DTCs encounter upon their arrival in bone. Stimulated by DTCs, osteoblasts are suggested to produce soluble factors with chemo attractive and growth regulating functions for both, tumor cells and osteoclasts [96,98]. Thereby, they are thought to regulate the initiation and activation of the vicious cycle of bone metastasis and consequently metastatic bone destruction. Metastatic colonization is defined as the ability of cancer cells to “form a clinically relevant metastasis at a secondary cancer site(s)” [106]. DTCs have to overcome many obstacles to successfully colonize distant organs. This includes the infiltration of the distant site, evading immune attack, adapting to the new environment and surviving to eventually initiate metastasis.