Genomics comprises a set of current and valuable technologies implemented as selection tools in dairy cattle commercial breeding programs. The intensive progeny testing for production and reproductive traits based on genomic breeding values (GEBVs) has been crucial to increasing dairy cattle productivity. The knowledge of key genes and haplotypes, including their regulation mechanisms, as markers for productivity traits, may improve the strategies on the present and future for dairy cattle selection. Genome-wide association studies (GWAS) such as quantitative trait loci (QTL), single nucleotide polymorphisms (SNPs), or single-step genomic best linear unbiased prediction (ssGBLUP) methods have already been included in global dairy programs for the estimation of marker-assisted selection-derived effects. The increase in genetic progress based on genomic predicting accuracy has also contributed to the understanding of genetic effects in dairy cattle offspring. However, the crossing within inbred-lines critically increased homozygosis with accumulated negative effects of inbreeding like a decline in reproductive performance. Thus, inaccurate-biased estimations based on empirical-conventional models of dairy production systems face an increased risk of providing suboptimal results derived from errors in the selection of candidates of high genetic merit-based just on low-heritability phenotypic traits. This extends the generation intervals and increases costs due to the significant reduction of genetic gains.
- genomic analysis
- gene edition
- production
- reproduction
- health
- welfare
- environment
- nutrition
- linear types
- dairy cattle
1. Introduction
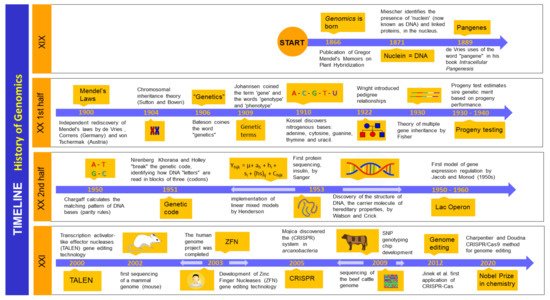
2. Genomics: History and Background
2.1. Genomic Factors Related to Production
2.2. Genomic Factors Related to Reproduction
2.3. Genomic Factors Related to Health and Animal Welfare
2.4. Genomic Factors Related to the Environment
2.5. Genomic Factors Related to Linear Type Traits
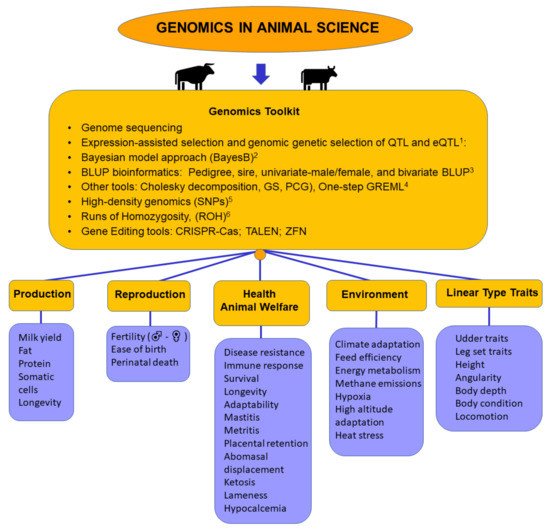
2.6. Genomics Toolkit
2.7. Methods in Genomics
2.8. Emerging Genomics: Nutritional, Metabolic, and Environmental Genomics
This entry is adapted from the peer-reviewed paper 10.3390/ani11030599
References
- Suravajhala, P.; Kogelman, L.J.A.; Kadarmideen, H.N. Multi-omic data integration and analysis using systems genomics approaches: Methods and applications in animal production, health and welfare. Genet. Sel. Evol. 2016, 48, 38.
- Schöpke, K.; Swalve, H.H. Review: Opportunities and challenges for small populations of dairy cattle in the era of genomics. Animal 2016, 10, 1050–1060.
- Egger-Danner, C.; Cole, J.B.; Pryce, J.E.; Gengler, N.; Heringstad, B.; Bradley, A.; Stock, K.F. Invited review: Overview of new traits and phenotyping strategies in dairy cattle with a focus on functional traits. Animal 2015, 9, 191–207.
- Miglior, F.; Fleming, A.; Malchiodi, F.; Brito, L.F.; Martin, P.; Baes, C.F. A 100-Year Review: Identification and genetic selection of economically important traits in dairy cattle. J. Dairy Sci. 2017, 100, 10251–10271.
- Sun, H.Z.; Plastow, G.; Guan, L.L. Invited review: Advances and challenges in application of feedomics to improve dairy cow production and health. J. Dairy Sci. 2019, 102, 5853–5870.
- García-Ruiz, A.; Cole, J.B.; VanRaden, P.M.; Wiggans, G.R.; Ruiz-López, F.J.; Van Tassell, C.P. Changes in genetic selection differentials and generation intervals in US Holstein dairy cattle as a result of genomic selection. Proc. Natl. Acad. Sci. USA 2016, 113, E3995–E4004.
- Berry, D.P.; Wall, E.; Pryce, J.E. Genetics and genomics of reproductive performance in dairy and beef cattle. Animal 2014, 8, 105–121.
- Guarini, A.R.; Lourenco, D.A.L.; Brito, L.F.; Sargolzaei, M.; Baes, C.F.; Miglior, F.; Tsuruta, S.; Misztal, I.; Schenkel, F.S. Use of a single-step approach for integrating foreign information into national genomic evaluation in Holstein cattle. J. Dairy Sci. 2019, 102, 8175–8183.
- Weller, J.I.; Ezra, E.; Ron, M. Invited review: A perspective on the future of genomic selection in dairy cattle. J. Dairy Sci. 2017, 100, 8633–8644.
- Kadarmideen, H.N. Genomics to systems biology in animal and veterinary sciences: Progress, lessons and opportunities. Livest. Sci. 2014, 166, 232–248.
- Boichard, D.; Chung, H.; Dassonneville, R.; David, X.; Eggen, A.; Fritz, S.; Gietzen, K.J.; Hayes, B.J.; Lawley, C.T.; Sonstegard, T.S.; et al. Design of a bovine low-density snp array optimized for imputation. PLoS ONE 2012, 7, e34130.
- Elsik, C.G.; Tellam, R.L.; Worley, K.C.; Gibbs, R.A.; Muzny, D.M.; Weinstock, G.M.; Adelson, D.L.; Eichler, E.E.; Elnitski, L.; Guigó, R.; et al. The genome sequence of taurine cattle: A window to ruminant biology and evolution. Science 2009, 324, 522–528.
- Kõks, S.; Lilleoja, R.; Reimann, E.; Salumets, A.; Reemann, P.; Jaakma, Ü. Sequencing and annotated analysis of the Holstein cow genome. Mamm. Genome 2013, 24, 309–321.
- Stafuzza, N.B.; Zerlotini, A.; Lobo, F.P.; Yamagishi, M.E.B.; Chud, T.C.S.; Caetano, A.R.; Munari, D.P.; Garrick, D.J.; Machado, M.A.; Martins, M.F.; et al. Single nucleotide variants and InDels identified from whole-genome re-sequencing of Guzerat, Gyr, Girolando and Holstein cattle breeds. PLoS ONE 2017, 12, e0173954.
- Wiggans, G.R.; Cole, J.B.; Hubbard, S.M.; Sonstegard, T.S. Genomic Selection in Dairy Cattle: The USDA Experience. Annu. Rev. Anim. Biosci. 2017, 5, 309–327.
- Gayon, J. De Mendel à l’épigénétique: Histoire de la génétique. C. R. Biol. 2016, 339, 225–230.
- Shendure, J.; Balasubramanian, S.; Church, G.M.; Gilbert, W.; Rogers, J.; Schloss, J.A.; Waterston, R.H. DNA sequencing at 40: Past, present and future. Nature 2017, 550, 345–353.
- Lenay, C. Hugo De Vries: From the theory of intracellular pangenesis to the rediscovery of Mendel. C. R. Acad. Sci. Ser. III 2000, 323, 1053–1060.
- Bateson, W. Mendel’s Principles of Heredity: A Defence with a Translation of Mendel’s Original Papers on Hybridisation; Cambridge University Press: Cambridge, UK, 1902.
- Johannsen, W. Elemente der Exakten Erblichkeitslehre; Verlag Von Gustav Fischer: Jena, Germany, 1926.
- Gayon, J.; Burian, R.M. France in the era of mendelism (1900–1930). C. R. Acad. Sci. Ser. III 2000, 323, 1097–1106.
- Moore, S.G.; Hasler, J.F. A 100-Year Review: Reproductive technologies in dairy science. J. Dairy Sci. 2017, 100, 10314–10331.
- Schrödinger, E. What is Life?: And Other Scientific Essays; Anchor; Doubleday Anchor Books: New York, NY, USA, 1956.
- Watson, J.D.; Crick, F.H.C. Molecular structure of nucleic acids: A structure for deoxyribose nucleic acid. Nature 1953, 171, 737–738.
- Sanger, F.; Thompson, E.O. The amino-acid sequence in the glycyl chain of insulin. Biochem. J. 1952, 52, 3.
- Jacob, F.; Monod, J. Genetic regulatory mechanisms in the synthesis of proteins. J. Mol. Biol. 1961, 3, 318–356.
- Fleischmann, R.D.; Adams, M.D.; White, O.; Clayton, R.A.; Kirkness, E.F.; Kerlavage, A.R.; Bult, C.J.; Tomb, J.F.; Dougherty, B.A.; Merrick, J.M.; et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science 1995, 269, 496–512.
- Ricroch, A. Global developments of genome editing in agriculture. Transgenic Res. 2019, 28, 45–52.
- Meuwissen, T.H.E.; Hayes, B.J.; Goddard, M.E. Prediction of total genetic value using genome-wide dense marker maps. Genetics 2001, 157, 1819–1829.
- Abdellah, Z.; Ahmadi, A.; Ahmed, S.; Aimable, M.; Ainscough, R.; Almeida, J.; Almond, C.; Ambler, A.; Ambrose, K.; Ambrose, K.; et al. Finishing the euchromatic sequence of the human genome. Nature 2004, 431, 931–945.
- Hu, Y.; Xia, H.; Li, M.; Xu, C.; Ye, X.; Su, R.; Zhang, M.; Nash, O.; Sonstegard, T.S.; Yang, L.; et al. Comparative analyses of copy number variations between Bos taurus and Bos indicus. BMC Genom. 2020, 21, 682.
- Matukumalli, L.K.; Lawley, C.T.; Schnabel, R.D.; Taylor, J.F.; Allan, M.F.; Heaton, M.P.; O’Connell, J.; Moore, S.S.; Smith, T.P.L.; Sonstegard, T.S.; et al. Development and Characterization of a High Density SNP Genotyping Assay for Cattle. PLoS ONE 2009, 4, e5350.
- Cain, C. CRISPR genome editing. Sci. Bus. Exch. 2013, 6, 77.
- Maltecca, C.; Tiezzi, F.; Cole, J.B.; Baes, C. Symposium review: Exploiting homozygosity in the era of genomics—Selection, inbreeding, and mating programs. J. Dairy Sci. 2020, 103, 5302–5313.
- Carthy, T.R.; McCarthy, J.; Berry, D.P. A mating advice system in dairy cattle incorporating genomic information. J. Dairy Sci. 2019, 102, 8210–8220.
- Aguilar, I.; Misztal, I.; Johnson, D.L.; Legarra, A.; Tsuruta, S.; Lawlor, T.J. Hot topic: A unified approach to utilize phenotypic, full pedigree, and genomic information for genetic evaluation of Holstein final score. J. Dairy Sci. 2010, 93, 743–752.
- Goddard, M. Genomic selection: Prediction of accuracy and maximisation of long term response. Genetica 2009, 136, 245–257.
- Doublet, A.C.; Croiseau, P.; Fritz, S.; Michenet, A.; Hozé, C.; Danchin-Burge, C.; Laloë, D.; Restoux, G. The impact of genomic selection on genetic diversity and genetic gain in three French dairy cattle breeds. Genet. Sel. Evol. 2019, 51, 52.
- Cesarani, A.; Pocrnic, I.; Macciotta, N.P.P.; Fragomeni, B.O.; Misztal, I.; Lourenco, D.A.L. Bias in heritability estimates from genomic restricted maximum likelihood methods under different genotyping strategies. J. Anim. Breed. Genet. 2019, 136, 40–50.
- Gao, H.; Madsen, P.; Aamand, G.P.; Thomasen, J.R.; Sørensen, A.C.; Jensen, J. Bias in estimates of variance components in populations undergoing genomic selection: A simulation study. BMC Genom. 2019, 20, 956.
- Aldridge, M.N.; Vandenplas, J.; Bergsma, R.; Calus, M.P.L. Variance estimates are similar using pedigree or genomic relationships with or without the use of metafounders or the algorithm for proven and young animals. J. Anim. Sci. 2020, 98, skaa019.
- De Haas, Y.; Pszczola, M.; Soyeurt, H.; Wall, E.; Lassen, J. Invited review: Phenotypes to genetically reduce greenhouse gas emissions in dairying. J. Dairy Sci. 2017, 100, 855–870.
- Taylor, J.F.; Schnabel, R.D.; Sutovsky, P. Review: Genomics of bull fertility. Animal 2018, 12, s172–s183.
- Cole, J.B.; Null, D.J. Short communication: Phenotypic and genetic effects of the polled haplotype on yield, longevity, and fertility in US Brown Swiss, Holstein, and Jersey cattle. J. Dairy Sci. 2019, 102, 8247–8250.
- Lee, Y.-M.; Dang, C.-G.; Alam, M.Z.; Kim, Y.-S.; Cho, K.-H.; Park, K.-D.; Kim, J.-J. The effectiveness of genomic selection for milk production traits of Holstein dairy cattle. Asian Australas. J. Anim. Sci. 2020, 33, 382–389.
- Schmitt, M.R.; VanRaden, P.M.; De Vries, A. Ranking sires using genetic selection indices based on financial investment methods versus lifetime net merit. J. Dairy Sci. 2019, 102, 9060–9075.
- Lu, H.; Wang, Y.; Bovenhuis, H. Genome-wide association study for genotype by lactation stage interaction of milk production traits in dairy cattle. J. Dairy Sci. 2020, 103, 5234–5245.
- Lu, H.; Bovenhuis, H. Genome-wide association studies for genetic effects that change during lactation in dairy cattle. J. Dairy Sci. 2019, 102, 7263–7276.
- Iso-Touru, T.; Sahana, G.; Guldbrandtsen, B.; Lund, M.S.; Vilkki, J. Genome-wide association analysis of milk yield traits in Nordic Red Cattle using imputed whole genome sequence variants. BMC Genet. 2016, 17, 55.
- Zielke, L.G.; Bortfeldt, R.H.; Reissmann, M.; Tetens, J.; Thaller, G.; Brockmann, G.A. Impact of Variation at the FTO Locus on Milk Fat Yield in Holstein Dairy Cattle. PLoS ONE 2013, 8, e63406.
- Rohmeier, L.; Petzl, W.; Koy, M.; Eickhoff, T.; Hülsebusch, A.; Jander, S.; MacIas, L.; Heimes, A.; Engelmann, S.; Hoedemaker, M.; et al. In vivo model to study the impact of genetic variation on clinical outcome of mastitis in uniparous dairy cows. BMC Vet. Res. 2020, 16, 33.
- Zambrano, J.C.; Rincón, J.C.; Echeverri, J.J. Parámetros genéticos para caracteres productivos y reproductivos en Holstein y Jersey colombiano. Arch. Zootec. 2014, 63, 495–506.
- Pinedo, P.; Santos, J.E.P.; Chebel, R.C.; Galvão, K.N.; Schuenemann, G.M.; Bicalho, R.C.; Gilbert, R.O.; Rodrigez-Zas, S.L.; Seabury, C.M.; Rosa, G.; et al. Associations of reproductive indices with fertility outcomes, milk yield, and survival in Holstein cows. J. Dairy Sci. 2020, 103, 6647–6660.
- Lima, F.S.; Silvestre, F.T.; Peñagaricano, F.; Thatcher, W.W. Early genomic prediction of daughter pregnancy rate is associated with improved reproductive performance in Holstein dairy cows. J. Dairy Sci. 2020, 103, 3312–3324.
- Santos, D.J.A.; Cole, J.B.; Lawlor, T.J.; VanRaden, P.M.; Tonhati, H.; Ma, L. Variance of gametic diversity and its application in selection programs. J. Dairy Sci. 2019, 102, 5279–5294.
- Purfield, D.C.; Evans, R.D.; Carthy, T.R.; Berry, D.P. Genomic Regions Associated with Gestation Length Detected Using Whole-Genome Sequence Data Differ Between Dairy and Beef Cattle. Front. Genet. 2019, 10, 1068.
- Purfield, D.C.; Bradley, D.G.; Evans, R.D.; Kearney, F.J.; Berry, D.P. Genome-wide association study for calving performance using high-density genotypes in dairy and beef cattle. Genet. Sel. Evol. 2015, 47, 47.
- König, S.; May, K. Invited review: Phenotyping strategies and quantitative-genetic background of resistance, tolerance and resilience associated traits in dairy cattle. Animal 2019, 13, 897–908.
- Yang, J.; Jiang, J.; Liu, X.; Wang, H.; Guo, G.; Zhang, Q.; Jiang, L. Differential expression of genes in milk of dairy cattle during lactation. Anim. Genet. 2016, 47, 174–180.
- Ilska-Warner, J.J.; Psifidi, A.; Seeker, L.A.; Wilbourn, R.V.; Underwood, S.L.; Fairlie, J.; Whitelaw, B.; Nussey, D.H.; Coffey, M.P.; Banos, G. The Genetic Architecture of Bovine Telomere Length in Early Life and Association with Animal Fitness. Front. Genet. 2019, 10, 1048.
- Lopes, F.; Rosa, G.; Pinedo, P.; Santos, J.E.P.; Chebel, R.C.; Galvao, K.N.; Schuenemann, G.M.; Bicalho, R.C.; Gilbert, R.O.; Rodrigez-Zas, S.; et al. Genome-enable prediction for health traits using high-density SNP panel in US Holstein cattle. Anim. Genet. 2020, 51, 192–199.
- McNeel, A.K.; Reiter, B.C.; Weigel, D.; Osterstock, J.; Di Croce, F.A. Validation of genomic predictions for wellness traits in US Holstein cows. J. Dairy Sci. 2017, 100, 9115–9124.
- Gonzalez-Peña, D.; Vukasinovic, N.; Brooker, J.J.; Przybyla, C.A.; DeNise, S.K. Genomic evaluation for calf wellness traits in Holstein cattle. J. Dairy Sci. 2019, 102, 2319–2329.
- Gonzalez-Peña, D.; Vukasinovic, N.; Brooker, J.J.; Przybyla, C.A.; Baktula, A.; DeNise, S.K. Genomic evaluation for wellness traits in US Jersey cattle. J. Dairy Sci. 2020, 103, 1735–1748.
- Vukasinovic, N.; Bacciu, N.; Przybyla, C.A.; Boddhireddy, P.; DeNise, S.K. Development of genetic and genomic evaluation for wellness traits in US Holstein cows. J. Dairy Sci. 2017, 100, 428–438.
- Sirard, M.A. 40 years of bovine IVF in the new genomic selection context. Reproduction 2018, 156, R1–R7.
- Tsuruta, S.; Lourenco, D.A.L.; Misztal, I.; Lawlor, T.J. Genotype by environment interactions on culling rates and 305-day milk yield of Holstein cows in 3 US regions. J. Dairy Sci. 2015, 98, 5796–5805.
- Stronen, A.V.; Pertoldi, C.; Iacolina, L.; Kadarmideen, H.N.; Kristensen, T.N. Genomic analyses suggest adaptive differentiation of northern European native cattle breeds. Evol. Appl. 2019, 12, 1096–1113.
- Connor, E.E. Invited review: Improving feed efficiency in dairy production: Challenges and possibilities. Animal 2015, 9, 395–408.
- Strandén, I.; Kantanen, J.; Russo, I.R.M.; Orozco-Terwengel, P.; Bruford, M.W. Genomic selection strategies for breeding adaptation and production in dairy cattle under climate change. Heredity (Edinb.) 2019, 123, 307–317.
- Verdugo, M.P.; Mullin, V.E.; Scheu, A.; Mattiangeli, V.; Daly, K.G.; Delser, P.M.; Hare, A.J.; Burger, J.; Collins, M.J.; Kehati, R.; et al. Ancient cattle genomics, origins, and rapid turnover in the Fertile Crescent. Science 2019, 365, 173–176.
- Scheper, C.; Wensch-Dorendorf, M.; Yin, T.; Dressel, H.; Swalve, H.; König, S. Evaluation of breeding strategies for polledness in dairy cattle using a newly developed simulation framework for quantitative and Mendelian traits. Genet. Sel. Evol. 2016, 48, 50.
- Hayes, B.J.; Lewin, H.A.; Goddard, M.E. The future of livestock breeding: Genomic selection for efficiency, reduced emissions intensity, and adaptation. Trends Genet. 2013, 29, 206–214.
- Qiu, Q.; Zhang, G.; Ma, T.; Qian, W.; Wang, J.; Ye, Z.; Coa, C.; Hu, Q.; Kim, J.; Larkin, D.M.; et al. The Yak Genome and Adaptation to Life at High Altitude. Nat. Genet. 2012, 44, 946–949.
- Becker, C.A.; Collier, R.J.; Stone, A.E. Invited review: Physiological and behavioral effects of heat stress in dairy cows. J. Dairy Sci. 2020, 103, 6751–6770.
- Liu, X.; Wang, Y.; Guo, W.; Chang, B.; Liu, J.; Guo, Z.; Quan, F.; Zhang, Y. Zinc-finger nickase-mediated insertion of the lysostaphin gene into the beta-casein locus in cloned cows. Nat. Commun. 2013, 4, 2565.
- Negrón-Pérez, V.M.; Fausnacht, D.W.; Rhoads, M.L. Invited review: Management strategies capable of improving the reproductive performance of heat-stressed dairy cattle. J. Dairy Sci. 2019, 102, 10695–10710.
- Sigdel, A.; Liu, L.; Abdollahi-Arpanahi, R.; Aguilar, I.; Peñagaricano, F. Genetic dissection of reproductive performance of dairy cows under heat stress. Anim. Genet. 2020, 51.
- Sigdel, A.; Abdollahi-Arpanahi, R.; Aguilar, I.; Peñagaricano, F. Whole Genome Mapping Reveals Novel Genes and Pathways Involved in Milk Production Under Heat Stress in US Holstein Cows. Front. Genet. 2019, 10, 928.
- Pryce, J.E.; Haile-Mariam, M. Symposium review: Genomic selection for reducing environmental impact and adapting to climate change. J. Dairy Sci. 2020, 103, 5366–5375.
- Dekkers, J.C.M.; Jairath, L.K.; Lawrence, B.H. Relationships between Sire Genetic Evaluations for Conformation and Functional Herd Life of Daughters. J. Dairy Sci. 1994, 77, 844–854.
- Olasege, B.S.; Zhang, S.; Zhao, Q.; Liu, D.; Sun, H.; Wang, Q.; Ma, P.; Pan, Y. Genetic parameter estimates for body conformation traits using composite index, principal component, and factor analysis. J. Dairy Sci. 2019, 102, 5219–5229.
- Guo, J.; Jorjani, H.; Carlborg, Ö. A genome-wide association study using international breeding-evaluation data identifies major loci affecting production traits and stature in the Brown Swiss cattle breed. BMC Genet. 2012, 13, 82.
- Jardim, J.G.; Guldbrandtsen, B.; Lund, M.S.; Sahana, G. Association analysis for udder index and milking speed with imputed whole-genome sequence variants in Nordic Holstein cattle. J. Dairy Sci. 2018, 101, 2199–2212.
- Cooper, T.A.; Eaglen, S.A.E.; Wiggans, G.R.; Jenko, J.; Huson, H.J.; Morrice, D.R.; Bichard, M.; Luff, D.L.W.G.; Woolliams, J.A. Genomic evaluation, breed identification, and population structure of Guernsey cattle in North America, Great Britain, and the Isle of Guernsey. J. Dairy Sci. 2016, 99, 5508–5515.
- Battagin, M.; Sartori, C.; Biffani, S.; Penasa, M.; Cassandro, M. Genetic parameters for body condition score, locomotion, angularity, and production traits in Italian Holstein cattle. J. Dairy Sci. 2013, 96, 5344–5351.
- Cassandro, M.; Battagin, M.; Penasa, M.; De Marchi, M. Short communication: Genetic relationships of milk coagulation properties with body condition score and linear type traits in Holstein-Friesian cows. J. Dairy Sci. 2015, 98, 685–691.
- Kougioumtzis, A.; Valergakis, G.E.; Oikonomou, G.; Arsenos, G.; Banos, G. Profile and genetic parameters of dairy cattle locomotion score and lameness across lactation. Animal 2014, 8, 20–27.
- Tiezzi, F.; Maltecca, C.; Cecchinato, A.; Penasa, M.; Bittante, G. Thin and fat cows, and the nonlinear genetic relationship between body condition score and fertility. J. Dairy Sci. 2013, 96, 6730–6741.
- Manafiazar, G.; Goonewardene, L.; Miglior, F.; Crews, D.H.; Basarab, J.A.; Okine, E.; Wang, Z. Genetic and phenotypic correlations among feed efficiency, production and selected conformation traits in dairy cows. Animal 2015, 10, 381–389.
- Zetouni, L.; Kargo, M.; Norberg, E.; Lassen, J. Genetic correlations between methane production and fertility, health, and body type traits in Danish Holstein cows. J. Dairy Sci. 2018, 101, 2273–2280.
- Mouresan, E.F.; Selle, M.; Rönnegård, L. Genomic prediction including SNP-specific variance predictors. G3 Genes Genomes Genet. 2019, 9, 3333–3343.
- Kadarmideen, H.N.; Von Rohr, P.; Janss, L.L.G. From genetical genomics to systems genetics: Potential applications in quantitative genomics and animal breeding. Mamm. Genome 2006, 17, 548–564.
- VanRaden, P.M. Symposium review: How to implement genomic selection. J. Dairy Sci. 2020, 103, 5291–5301.
- Shamimuzzaman, M.; Le Tourneau, J.J.; Unni, D.R.; Diesh, C.M.; Triant, D.A.; Walsh, A.T.; Tayal, A.; Conant, G.C.; Hagen, D.E.; Elsik, C.G. Bovine Genome Database: New annotation tools for a new reference genome. Nucleic Acids Res. 2020, 48, D676–D681.
- Seno, D.O.L.; Guidolin, D.G.F.; Aspilcueta-Borquis, R.R.; Do Nascimento, G.B.; Da Silva, T.B.R.; De Oliveira, H.N.; Munari, D.P. Genomic selection in dairy cattle simulated populations. J. Dairy Res. 2018, 85, 125–132.
- Uemoto, Y.; Osawa, T.; Saburi, J. Effect of genotyped cows in the reference population on the genomic evaluation of Holstein cattle. Animal 2017, 11, 382–393.
- Aliloo, H.; Pryce, J.E.; González-Recio, O.; Cocks, B.G.; Hayes, B.J. Accounting for dominance to improve genomic evaluations of dairy cows for fertility and milk production traits. Genet. Sel. Evol. 2016, 48, 8.
- Habier, D.; Tetens, J.; Seefried, F.R.; Lichtner, P.; Thaller, G. The impact of genetic relationship information on genomic breeding values in German Holstein cattle. Genet. Sel. Evol. 2010, 42, 5.
- VanRaden, P.M.; Tooker, M.E.; Wright, J.R.; Sun, C.; Hutchison, J.L. Comparison of single-trait to multi-trait national evaluations for yield, health, and fertility. J. Dairy Sci. 2014, 97, 7952–7962.
- Strandén, I.; Matilainen, K.; Aamand, G.P.; Mäntysaari, E.A. Solving efficiently large single-step genomic best linear unbiased prediction models. J. Anim. Breed. Genet. 2017, 134, 264–274.
- Kang, H.; Ning, C.; Zhou, L.; Zhang, S.; Yan, Q.; Liu, J.F. Short communication: Single-step genomic evaluation of milk production traits using multiple-trait random regression model in Chinese Holsteins. J. Dairy Sci. 2018, 101, 11143–11149.
- Misztal, I.; Legarra, A. Invited review: Efficient computation strategies in genomic selection. Animal 2017, 11, 731–736.
- Lourenco, D.A.L.; Misztal, I.; Tsuruta, S.; Aguilar, I.; Ezra, E.; Ron, M.; Shirak, A.; Weller, J.I. Methods for genomic evaluation of a relatively small genotyped dairy population and effect of genotyped cow information in multiparity analyses. J. Dairy Sci. 2014, 97, 1742–1752.
- Ferdosi, M.H.; Connors, N.K.; Tier, B. An efficient method to calculate genomic prediction accuracy for new individuals. Front. Genet. 2019, 10, 596.
- Daetwyler, H.D.; Pong-Wong, R.; Villanueva, B.; Woolliams, J.A. The impact of genetic architecture on genome-wide evaluation methods. Genetics 2010, 185, 1021–1031.
- Mäki-Tanila, A.; Webster, L. Heritability, SNP, inbreeding, dairy cattle, genomic selection-and other keywords. J. Anim. Breed. Genet. 2019, 136, 1–2.
- Seidel, G.E. Brief introduction to whole-genome selection in cattle using single nucleotide polymorphisms. Reprod. Fertil. Dev. 2010, 22, 138–144.
- Schultz, N.E.; Weigel, K.A. Inclusion of herdmate data improves genomic prediction for milk-production and feed-efficiency traits within North American dairy herds. J. Dairy Sci. 2019, 102, 11081–11091.
- De Vries, A. Symposium review: Why revisit dairy cattle productive lifespan? J. Dairy Sci. 2020, 103, 3838–3845.
- Bickhart, D.M.; McClure, J.C.; Schnabel, R.D.; Rosen, B.D.; Medrano, J.F.; Smith, T.P.L. Symposium review: Advances in sequencing technology herald a new frontier in cattle genomics and genome-enabled selection. J. Dairy Sci. 2020, 103, 5278–5290.
- Kelleher, M.M.; Berry, D.P.; Kearney, J.F.; McParland, S.; Buckley, F.; Purfield, D.C. Inference of population structure of purebred dairy and beef cattle using high-density genotype data. Animal 2017, 11, 15–23.
- Martikainen, K.; Tyrisevä, A.M.; Matilainen, K.; Pösö, J.; Uimari, P. Estimation of inbreeding depression on female fertility in the Finnish Ayrshire population. J. Anim. Breed. Genet. 2017, 134, 383–392.
- Ma, L.; Cole, J.B.; Da, Y.; VanRaden, P.M. Symposium review: Genetics, genome-wide association study, and genetic improvement of dairy fertility traits. J. Dairy Sci. 2019, 102, 3735–3743.
- Fonseca, P.A.D.S.; Dos Santos, F.C.; Lam, S.; Suárez-Vega, A.; Miglior, F.; Schenkel, F.S.; Diniz, L.D.A.F.; Id-Lahoucine, S.; Carvalho, M.R.S.; Cánovas, A. Genetic mechanisms underlying spermatic and testicular traits within and among cattle breeds: Systematic review and prioritization of GWAS results. J. Anim. Sci. 2018, 96, 4978–4999.
- Toghiani, S.; Hay, E.; Sumreddee, P.; Geary, T.W.; Rekaya, R.; Roberts, A.J. Genomic prediction of continuous and binary fertility traits of females in a composite beef cattle breed. J. Anim. Sci. 2017, 95, 4787–4795.
- Olson, K.M.; VanRaden, P.M.; Tooker, M.E. Multibreed genomic evaluations using purebred Holsteins, Jerseys, and Brown Swiss. J. Dairy Sci. 2012, 95, 5378–5383.
- Obšteter, J.; Jenko, J.; Hickey, J.M.; Gorjanc, G. Efficient use of genomic information for sustainable genetic improvement in small cattle populations. J. Dairy Sci. 2019, 102, 9971–9982.
- Hozé, C.; Fritz, S.; Phocas, F.; Boichard, D.; Ducrocq, V.; Croiseau, P. Efficiency of multi-breed genomic selection for dairy cattle breeds with different sizes of reference population. J. Dairy Sci. 2014, 97, 3918–3929.
- Thomasen, J.R.; Liu, H.; Sørensen, A.C. Genotyping more cows increases genetic gain and reduces rate of true inbreeding in a dairy cattle breeding scheme using female reproductive technologies. J. Dairy Sci. 2020, 103, 597–606.
- Rowan, T.N.; Hoff, J.L.; Crum, T.E.; Taylor, J.F.; Schnabel, R.D.; Decker, J.E. A multi-breed reference panel and additional rare variants maximize imputation accuracy in cattle. Genet. Sel. Evol. 2019, 51, 77.
- Koivula, M.; Strandén, I.; Aamand, G.P.; Mäntysaari, E.A. Reducing bias in the dairy cattle single-step genomic evaluation by ignoring bulls without progeny. J. Anim. Breed. Genet. 2018, 135, 107–115.
- Martikainen, K.; Sironen, A.; Uimari, P. Estimation of intrachromosomal inbreeding depression on female fertility using runs of homozygosity in Finnish Ayrshire cattle. J. Dairy Sci. 2018, 101, 11097–11107.
- Jenko, J.; Wiggans, G.R.; Cooper, T.A.; Eaglen, S.A.E.; Luff, W.G.D.L.; Bichard, M.; Pong-Wong, R.; Woolliams, J.A. Cow genotyping strategies for genomic selection in a small dairy cattle population. J. Dairy Sci. 2017, 100, 439–452.
- Sun, C.; VanRaden, P.M.; O’Connell, J.R.; Weigel, K.A.; Gianola, D. Mating programs including genomic relationships and dominance effects. J. Dairy Sci. 2013, 96, 8014–8023.
- Kiser, J.N.; Clancey, E.; Moraes, J.G.N.; Dalton, J.; Burns, G.W.; Spencer, T.E.; Neibergs, H.L. Identification of loci associated with conception rate in primiparous Holstein cows. BMC Genom. 2019, 20, 840.
- Do, N.D.; Bissonnette, N.; Lacasse, P.; Miglior, F.; Zhao, X.; Ibeagha-Awemu, E.M. A Targeted Genotyping Approach to Enhance the Identification of Variants for Lactation Persistency in Dairy Cows. J. Anim. Sci. 2019, 97, 4066–4075.
- Xu, L.; Bickhart, D.M.; Cole, J.B.; Schroeder, S.G.; Song, J.; Van Tassell, C.P.; Sonstegard, T.S.; Liu, G.E. Genomic signatures reveal new evidences for selection of important traits in domestic cattle. Mol. Biol. Evol. 2015, 32, 711–725.
- Buttchereit, N.; Stamer, E.; Junge, W.; Thaller, G. Genetic parameters for energy balance, fat/protein ratio, body condition score and disease traits in German Holstein cows. J. Anim. Breed. Genet. 2012, 129, 280–288.
- Huson, H.J.; Sonstegard, T.S.; Godfrey, J.; Hambrook, D.; Wolfe, C.; Wiggans, G.; Blackburn, H.; VanTassell, C.P. A Genetic Investigation of Island Jersey Cattle, the Foundation of the Jersey Breed: Comparing Population Structure and Selection to Guernsey, Holstein, and United States Jersey Cattle. Front. Genet. 2020, 11, 366.
- Gaddis, P.K.L.; Megonigal, J.H.; Clay, J.S.; Wolfe, C.W. Genome-wide association study for ketosis in US Jerseys using producer-recorded data. J. Dairy Sci. 2018, 101, 413–424.
- Pacheco, H.A.; Da Silva, S.; Sigdel, A.; Mak, C.K.; Galvão, K.N.; Texeira, R.A.; Dias, L.T.; Peñagaricano, F. Gene Mapping and Gene-Set Analysis for Milk Fever Incidence in Holstein Dairy Cattle. Front. Genet. 2018, 9, 465.
- Rzewuska, K.; Strabel, T. Genetic parameters for milk urea concentration and milk traits in Polish Holstein-Friesian cows. J. Appl. Genet. 2013, 54, 473–482.
- Hossein-Zadeh, N.G.; Ardalan, M. Genetic relationship between milk urea nitrogen and reproductive performance in Holstein dairy cows. Animal 2011, 5, 26–32.
- Oikonomou, G.; Arsenos, G.; Valergakis, G.E.; Tsiaras, A.; Zygoyiannis, D.; Banos, G. Genetic relationship of body energy and blood metabolites with reproduction in Holstein cows. J. Dairy Sci. 2008, 91, 4323–4332.
- Kowsar, R.; Izadi, F.; Sadeghi, N.; Riasi, A.; Zadegan, F.G.; Hajian, M.; Nasr-Esfahani, M.H.; Farrokhpour, H.; Miyamoto, A. Urea changes oocyte competence and gene expression in resultant bovine embryo in vitro. Zygote 2018, 26, 207–219.
- Pryce, J.E.; Nguyen, T.T.T.; Axford, M.; Nieuwhof, G.; Shaffer, M. Symposium review: Building a better cow—The Australian experience and future perspectives. J. Dairy Sci. 2018, 101, 3702–3713.
- Higgins, M.G.; Fitzsimons, C.; McClure, M.C.; McKenna, C.; Conroy, S.; Kenny, D.A.; McGee, M.; Waters, S.M.; Morris, D.W. GWAS and eQTL analysis identifies a SNP associated with both residual feed intake and GFRA2 expression in beef cattle. Sci. Rep. 2018, 8.
- Li, B.; VanRaden, P.M.; Guduk, E.; O’Connell, J.R.; Null, D.J.; Connor, E.E.; VandeHaar, M.J.; Tempelman, R.J.; Weigel, K.A.; Cole, J.B. Genomic prediction of residual feed intake in US Holstein dairy cattle. J. Dairy Sci. 2020, 103, 2477–2486.
