Steroids constitute a unique class of chemical compounds, playing an important role in physiopathological processes, and have high pharmacological interest. Due to their straightforward preparation and intrinsic chemical reactivity, steroidal arylidene derivatives are important synthetic intermediates for the preparation of other compounds, particularly bearing heterocyclic systems, in addition to their relevant bioactivity with potential pharmacological interest.
- steroids
- arylidenesteroids
- aldol condensation
- bioactivity
- heterocycles
1. Introduction
Steroids are natural products that share a 17-carbon-atom skeleton and are composed of four fused rings: three cyclohexanes (A, B, and C rings) and one cyclopentane (D ring). These compounds vary on the attached functional groups, their position, and configuration [1]. In addition, steroids represent a unique class of chemical products, playing an important role in several biological processes, being the most important group of regulatory and signaling molecules [2][3]. Usually, steroids are lipophilic and readily enter cells, being able to interact with nuclear receptors as well as with membrane proteins. Therefore, they are associated with most physiological functions and pathological conditions. In addition, due to their low toxicity, less vulnerability to multidrug resistance, and high bioavailability [4][5][6][7][8], steroid-based therapeutic drugs have called attention to the scientific academia and industry for a long time.
Due to their relevance, several modified steroids have been synthesized and biologically evaluated, being verified that their relevant pharmacological properties depend on the structural features of the steroidal four-ring skeleton and side-chain [5][9]. In fact, even a minor structural variation on the steroidal nucleus can lead to marked changes in their physiological activity [10]. Therefore, aiming to improve their pharmacological properties and/or develop compounds with different bioactivities, structural modifications of steroids have been an important focus of research over the last decades [11][12][13][14][15].
2. Synthetic Approaches to Prepare Arylidene Steroidal Derivatives
Arylidenesteroids are usually obtained through an aldol condensation between a steroid and an aldehyde. In an aldol condensation, an enol or enolate reacts with a carbonyl in the presence of an acid or base catalyst to form a β-hydroxyaldehyde or a β-hydroxyketone, followed by dehydration to afford a conjugated enone [16]. In general, the reactions to prepare arylidenesteroids occur at room temperature (RT), under basic catalysis, and the solvent is, in most cases, methanol (MeOH) or ethanol (EtOH) (Figure 1).
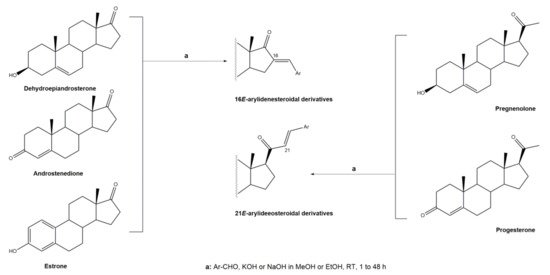
Figure 1. Claisen–Schmidt condensation to prepare 16E- and 21E-arylidenesteroids.
3. Bioactivity of 2- and 16E-arylideneandrostane Derivatives
The 16E-arylideneandrostane steroidal skeleton has emerged as a relevant template to develop potential anticancer agents. In addition to cytotoxic effects, several studies reported other biological activities, such as aromatase inhibition, anti-inflammatory, neuroprotective and skeletal muscle relaxing activities and tissue-selective androgen receptor modulator effects [17][18][19]. A selection of the most relevant 16E-arylideneandrostane derivatives is presented in Table 1.
Table 1. Examples of relevant bioactive 2- and 16E-arylideneandrostane derivatives. Positive controls, when shown, are identified by (+) symbol.
| Compound | Bioactivity Data | Ref. | |||||
|---|---|---|---|---|---|---|---|
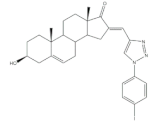 2 |
Antiproliferative activity (IC50 μM) | [11] | |||||
| Cell line | 2 | 5-FU (+) | |||||
| HepG2 | 9.10 | 10.59 | |||||
| MCF-7 | 9.18 | 28.11 | |||||
| Cell cycle arrest at G2 phase in HepG2 | |||||||
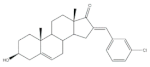 3 |
Antiproliferative activity (IC50 ± SEM μM) | [20] | |||||
| Cell line | 3 | Etoposide (+) | |||||
| KB | 0.6 ± 2.0 | 2.8 ± 16.8 | |||||
| T47-D | 1.7 ± 14.8 | 1.2 ± 8 | |||||
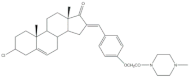 4 |
Antiproliferative activity (IC50 μM) | [21] | |||||
| Cell line | 4 | ||||||
| CCRF-CEM | 3.94 | ||||||
| K-562 | 2.61 | ||||||
| RPMI-8226 | 6.90 | ||||||
| SR | 1.79 | ||||||
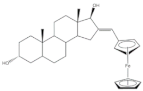 5 |
Antiproliferative activity (IC50 μM ± SD μM) | [22] | |||||
| Cell line | 5 | Cis (+) | |||||
| HT-29 | 1.2 ± 0.4 | 66 ± 2 | |||||
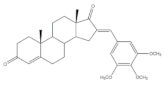 6 |
Cytotoxic activity–in vivo hollow fiber assay (score) | [18] | |||||
| 6 | Taxol (+) | ||||||
| I.P. | 2 | Data not shown | |||||
| S.C. | 8 | ||||||
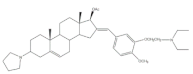 7 |
Cytotoxic activity–in vivo hollow fiber assay (score) | [23] | |||||
| 7 | Taxol (+) | ||||||
| I.P. | 12 | Data not shown | |||||
| S.C. | 8 | ||||||
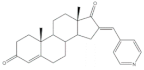 8 |
Aromatase inhibitory activity (IC50 μM) | [24] | |||||
| 8 | Aminoglutethimide (+) | ||||||
| 5.2 | 28.5 | ||||||
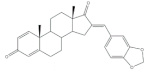 9 |
Aromatase inhibitory activity (IC50 μM) | [24] | |||||
| 9 | Aminoglutethimide (+) | ||||||
| 6.4 | 28.5 | ||||||
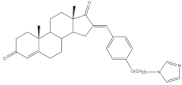 10 |
Aromatase inhibitory activity (IC50 μM) | [25] | |||||
| 10 | Aminoglutethimide (+) | ||||||
| 4.4 | 28.5 | ||||||
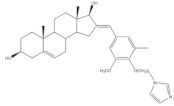 11 |
Aromatase inhibitory activity (IC50 μM) | [25] | |||||
| 11 | Aminoglutethimide (+) | ||||||
| 2.4 | 28.5 | ||||||
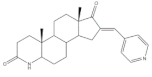 12 |
Anti-inflammatory activity TNF-α levels (pg.mg−1protein ± SD) |
[26] | |||||
| 12 | CEL (+) | DEX (+) | |||||
| 88.6 ± 1.8 | 68.2 ± 1.1 | 89.6 ± 2.0 | |||||
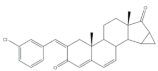 13 |
Anti-inflammatory activity (IC50 μM) (NO release of LPS-activated mouse microglial cell line BV2) |
[27] | |||||
| 13 | Minocycline (+) | ||||||
| 2.69 | 5.97 | ||||||
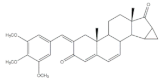 14 |
Anti-inflammatory activity (IC50 μM) (NO release of LPS-activated mouse microglial cell line BV2) |
[27] | |||||
| 14 | Minocycline (+) | ||||||
| 3.28 | 5.97 | ||||||
4. Bioactivity of 21E-arylidenepregnene Derivatives
Arylidenesteroidal derivatives of progesterone and pregnenolone and other similar pregnanes constitute a smaller group than arylideneandrostanes, and they have mainly been studied as potential antitumoral agents. Of these, the most potent arylidenepregnene derivatives reported until now are presented in Table 2.
Table 2. The most active 21E-arylidenepregnene derivatives. Positive controls, when shown, are identified by (+) symbol.
| Compound | Bioactivity Data | Ref. | ||
|---|---|---|---|---|
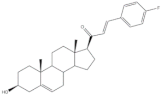 15 |
Antiproliferative activity (IC50 μM) | [28] | ||
| Cell line | 15 | |||
| HCT-15 | 0.81 | |||
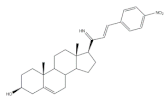 16 |
Antiproliferative activity (IC50 μM) | [28] | ||
| Cell line | 16 | |||
| MCF-7 | 0.60 | |||
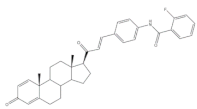 17 |
Antiproliferative activity Growth inhibition (%) |
[17] | ||
| Cell line | 17 | Cis (+) | ||
| HeLa | 58 | 99 | ||
| MCF-7 | 64 | 88 | ||
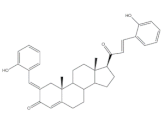 18 |
Antimicrobial activity Zone of inhibition (mm) |
[29] | ||
| Gram positive | 18 | AMP (+) | ||
| Streptococcus pneumoniae | 30 | 20 | ||
| Staphylococcus aureus | 24 | 22 | ||
5. Bioactivity of 16E-arylidenoestrone and 16E-arylidenoestradiol Derivatives
It is well known that estrogenic hormones have an important contribution to estrogen-dependent diseases, being breast cancers primarily initiated and stimulated by estrogens the majority of these conditions [30]. Consequently, the structural modification of estrone and estradiol in different positions to prepare bioactive compounds in this context has been the focus of intensive research.
Table 3. The most active 16E-arylidenoestrone and -estradiol derivatives. Positive controls, when shown, are identified by (+) symbol.
| Compound | Bioactivity Data | Ref. | |
|---|---|---|---|
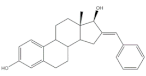 19 |
17β-HSD1 inhibitory activity (IC50 μM) | [31] | |
| 19 | Estradiol (+) | ||
| 3.4 | 7.3 | ||
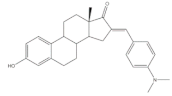 20 |
17β-HSD inhibitory activity (% at 10 μM) | [32] | |
| 20 | |||
| Type 1 | Type 2 | ||
| 72 | 13 | ||
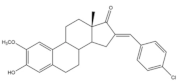 21 |
Tumor weight reduction (%) Human breast cancer (MCF-7) xenografts models |
[33] | |
| 21 (45 mg/kg/day) |
2ME (+) (45 mg/kg/day) | ||
| About 50% Significative reduction compared with negative control |
About 50% Significative reduction compared with negative control |
||
Of these prepared compounds, steroid 21 (Table 3) showed a potent antiangiogenic activity. Moreover, further studies suggested that this compound suppresses the tumor growth in about 50% in human breast cancer (MCF-7) xenograft models without relevant side effects. The action mechanism studies suggested that steroid 21 targeted the epithelial to mesenchymal transition process in MCF-7 cells and inhibited human umbilical vein endothelial cells (HUVEC) migration, contributing to angiogenesis interruption [33].
6. Importance of Steroidal Arylidene Derivatives as Synthetic Intermediates of Bioactive Molecules
In addition to their biological activity, steroidal arylidenes are also versatile synthetic intermediates in the preparation of other bioactive structures. In fact, these steroids have been used in the introduction of diverse chemical groups present in bioactive compounds, such as oximes, hydroxyl and hydrazones [28][34][35][36][37], and are particularly useful in several heterocyclization reactions. In this context, over the years, a large number of bioactive heterocyclic steroidal derivatives have been synthesized, and some of them are already being clinically used [38][39]. Interestingly, diverse heterocyclic compounds, including arylpyrazolines and pyrazoles, arylpyrimidines, oxindoles, pyridones and pyridines as well as spiro-pyrrolidines were prepared from arylidenesteroids [4][13][40][41][42][43][29][37][44][45][46][47][48][49][50][51][52][53][54]. The most promising steroidal derivatives prepared from arylidenesteroids that have been reported until the moment are presented in Table 4.
Table 4. The most active steroidal derivatives obtained from arylidenesteroids. Positive controls, when shown, are identified by (+) symbol.
| Compound | Bioactivity Data | Ref. | |||
|---|---|---|---|---|---|
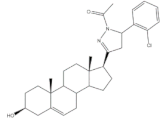 24 |
Antiproliferative activity (IC50 μM) | [4] | |||
| Cell line | 24 | ||||
| HT-29 | 0.24 | ||||
| HCT-15 | 0.25 | ||||
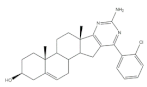 25 |
Antiproliferative activity (IC50 μM) | [41] | |||
| Cell line | 25 | 5-FU (+) | |||
| HepG-2 | 5.41 | >100 | |||
| Huh-7 | 5.65 | >95 | |||
| SGC-790 | 10.64 | >100 | |||
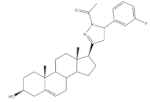 26 |
5AR-1 Inhibition (IC50 ± SEM μM) | [44] | |||
| 26 | Finasteride (+) | ||||
| 14.50 ± 0.48 | 21.6 ± 0.62 | ||||
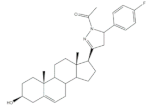 27 |
5AR-2 Inhibition (IC50 ± SEM μM) | [44] | |||
| 27 | Finasteride (+) | ||||
| 13.90 ± 0.75 | 15.4 ± 0.58 | ||||
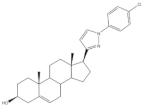 28 |
5AR-2 Inhibition (IC50 ± SEM μM) | [44] | |||
| 28 | Finasteride (+) | ||||
| 14.20 ± 0.75 | 15.4 ± 0.58 | ||||
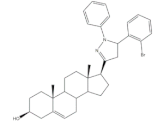 29 |
5AR-2 Inhibition (IC50 ± SEM nM) | [46] | |||
| 29 | Finasteride (+) | ||||
| 7.30 ± 0.62 | 2.4 ± 0.15 | ||||
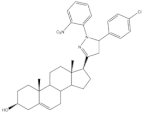 30 |
5AR-2 Inhibition (IC50 ± SEM nM) | [46] | |||
| 30 | Finasteride (+) | ||||
| 8.20 ± 0.55 | 2.4 ± 0.58 | ||||
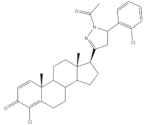 31 |
Antiproliferative activity (IC50 μg.mL−1) | [13] | |||
| Cell line | 31 | 5-FU (+) | Cis (+) | ||
| NCI-H460 | 10.30 | 2.48 | 0.699 | ||
| HeLa | 12.50 | 0.887 | 2.03 | ||
| Cell cycle arrest at S phase in HeLa cells | |||||
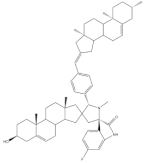 32 |
Antiproliferative activity (IC50 μM) | [43] | |||
| Cell line | 32 | 5-FU (+) | |||
| SMMC-7721 | 4.30 | 9.78 | |||
| MCF-7 | 2.06 | 7.54 | |||
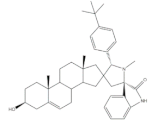 33 |
Antiproliferative activity (IC50 ± SEM μM) | [43] | |||
| Cell line | 33 | 5-FU (+) | |||
| SMMC-7721 | 6.05 ± 0.48 | 9.78 ± 0.99 | |||
| MGC-803 | 5.79 ± 0.76 | 6.92 ± 0.35 | |||
| Cell cycle arrest at G2/M phase in MGC cells | |||||
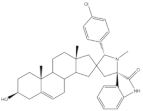 34 |
Antiproliferative activity (IC50 ± SEM μM) | [43] | |||
| Cell line | 34 | 5-FU (+) | |||
| SMMC-7721 | 0.71 ± 0.11 | 9.78 ± 0.99 | |||
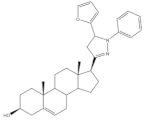 35 |
Antiproliferative activity (IC50 μM) | [37] | |||
| Cell line | 35 | DOX (+) | |||
| MDA-MB 231 | 0.91 | 1.23 | |||
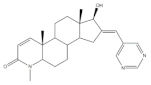 36 |
Osteoanabolic activity and tissue-selectivity (% of the effect of DHT) |
[55] | |||
| OVX | 36 | DHT | |||
| BRF | 120 | 100 | |||
| ORX | |||||
| VP | 3 | 100 | |||
| SV | 21 | 100 | |||
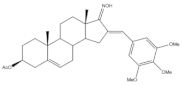 37 |
Cytotoxic activity-in vivo hollow fiber assay (score) | [18] | |||
| 37 | Taxol (+) | ||||
| I.P. | 4 | Data not shown | |||
| S.C. | 6 | ||||
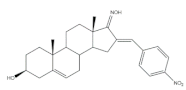 38 |
Antiproliferative activity Cell growth (%) |
[56] | |||
| Cell line | 38 | ||||
| NCI-H460 | −44 | ||||
| MFC-7 | −44 | ||||
| SF-268 | −79 | ||||
 39 |
Antiproliferative activity Cell growth (%) |
[36] | |||
| Cell line | 39 | ||||
| NCI-H460 | −11 | ||||
| MCF-7 | 5 | ||||
| SF-268 | −8 | ||||
7. Summary
Steroids constitute an important group of structurally related natural, semi-synthetic, and synthetic compounds with remarkable functions, including regulatory and signaling activities. In the last three decades, steroidal arylidene derivatives have been prepared and screened for a range of biological activities and used as synthetic intermediates, with special attention to bioactive heterocyclic steroids. In conclusion, due to the straightforward synthesis of arylidenesteroids and their bioactivity, as well as the inherent chemical reactivity of α,β-unsaturated ketones, useful in the preparation of other derivatives, this class of compounds has been of high interest in the last years.
This entry is adapted from the peer-reviewed paper 10.3390/molecules26072032
References
- Lednicer, D. Steroid Chemistry at a Glance; Wiley: Hoboken, NJ, USA, 2011; ISBN 9780470660850.
- Burger, A.; Abraham, D.J.; Rotella, D.P. Burger’s Medicinal Chemistry, Drug Discovery and Development; Wiley: Hoboken, NJ, USA, 2010; Volume 7, ISBN 9780470770085.
- Latham, K.A.; Zamora, A.; Drought, H.; Matejuk, A.; Offner, H.; Edward, F. Estradiol Treatment Redirects the Isotype of the Autoantibody Response and Prevents the Development of Autoimmune Arthritis. J. Immunol. 2003, 171, 5820–5827.
- Banday, A.H.; Mir, B.P.; Lone, I.H.; Suri, K.A.; Kumar, H.M.S. Studies on Novel D-Ring Substituted Steroidal Pyrazolines as Potential Anticancer Agents. Steroids 2010, 75, 805–809.
- Tantawy, M.A.; Nafie, M.S.; Elmegeed, G.A.; Ali, I.A.I. Auspicious Role of the Steroidal Heterocyclic Derivatives as a Platform for Anti-Cancer Drugs. Bioorg. Chem. 2017, 73, 128–146.
- Vil, V.A.; Terent, A.O.; Savidov, N.; Gloriozova, T.A. Hydroperoxy Steroids and Triterpenoids Derived from Plant and Fungi: Origin, Structures and Biological Activities. J. Steroid Biochem. Mol. Biol. 2019, 190, 76–87.
- Dembitsky, V.M. Progress in Lipid Research Antitumor and Hepatoprotective Activity of Natural and Synthetic Neo Steroids. Prog. Lipid Res. 2020, 79, 101048.
- Xiao, J.; Gao, M.; Fei, B.; Huang, G.; Diao, Q. Fitoterapia Nature-Derived Anticancer Steroids Outside Cardica Glycosides. Fitoterapia 2020, 147, 104757.
- Na, M.S.; Tantawy, M.A.; Elmgeed, G.A. Screening of Different Drug Design Tools to Predict the Mode of Action of Steroidal Derivatives as Anti-Cancer Agents. Steroids 2019, 152.
- Lemke, T.L.; Williams, D.A.; Roche, V.F.; Zito, S.W. Foye’s Principles of Medicinal Chemistry, 7th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012.
- Huang, X.; Shen, Q.; Zhang, H.; Li, J.; Tian, Y.; Quan, Z. Design and Synthesis of Novel Dehydroepiandrosterone Analogues as Potent Antiproliferative Agents. Molecules 2018, 23, 2243.
- Acharya, P.C.; Bansal, R.; Kharkar, P.S.; Res, D.; Bansal, R. Hybrids of Steroid and Nitrogen Mustard as Antiproliferative Agents: Synthesis, in Vitro Evaluation and in Silico Inverse Screening Authors. Drug Res. (Stuttg). 2017.
- Fan, N.; Tang, J.; Li, H.; Li, X.; Luo, B.; Gao, J. Synthesis and Cytotoxic Activity of Some Novel Steroidal C-17 Pyrazolinyl Derivatives. Eur. J. Med. Chem. 2013, 69, 182–190.
- Gogoi, J.; Bezbaruah, P.; Saikia, P.; Goswami, J.; Gogoi, P.; Boruah, R.C. Synthesis of a Novel Class of Steroidal Tetrazolo[1,5-a]Pyridines Via Intramolecular 1,3-Dipolar Cycloadditions. Tetrahedron Lett. 2012, 53, 1497–1500.
- Chowdhury, P.; Borah, J.M.; Goswami, P.; Das, A.M. A Convenient Synthesis of the Side Chain of Loteprednol Etabonate - an Ocular Soft Corticosteroid from 20-Oxopregnanes Using Metal-Mediated Halogenation as a Key Reaction. Steroids 2011, 76, 497–501.
- Furniss, B.S.; Hannaford, A.J.; Smith, P.W.G.; Tatchell, A.R. Vogel’s Textboom of Practical Organic Chemistry, 5th ed.; Pearson: Delhi, India, 1989.
- Fan, N.; Han, Y.; Li, Y.; Gao, J.; Tang, J. Synthesis of Novel 4′-Acylamino Modified 21E-Benzylidene Steroidal Derivatives and Their Cytotoxic Activities. Steroids 2017, 123, 20–26.
- Chattopadhaya, R.; Jindal, D.P.; Minu, M.; Gupta, R. Synthesis and Cytotoxic Studies of Hydroximino Derivatives of Some 16E-Arylidenosteroids. Arzneim. Forshung Drug Res. 2004, 556, 551–556.
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of Action and Clinical Strategies. Nat. Rev. Cancer 2003, 3, 330–338.
- Vosooghi, M.; Yahyavi, H.; Divsalar, K.; Shamsa, H.; Kheirollahi, A.; Safavi, M.; Ardestani, S.K.; Sadeghi-Neshat, S.; Mohammadhosseini, N.; Edraki, N.; et al. Synthesis and in Vitro Cytotoxic Activity Evaluation of (E)-16-(Substituted Benzylidene) Derivatives of Dehydroepiandrosterone. Daru 2013, 21, 34.
- Bansal, R.; Acharya, P.C. Synthesis and Antileukemic Activity of 16E-[4-(2-Carboxy)Ethoxybenzylidene]-Androstene Amides. Steroids 2012, 77, 552–557.
- Narváez-Pita, X.; Rheingold, A.L.; Meléndez, E. Ferrocene-Steroid Conjugates: Synthesis, Structure and Biological Activity. J. Organomet. Chem. 2017, 846, 113–120.
- Bansal, R.; Guleria, S. Synthesis of 16E-[3-Methoxy-4-(2-Aminoethoxy)Benzylidene]Androstene Derivatives as Potent Cytotoxic Agents. Steroids 2008, 73, 1391–1399.
- Bansal, R.; Thota, S.; Karkra, N.; Minu, M.; Zimmer, C.; Hartmann, R.W. Synthesis and Aromatase Inhibitory Activity of Some New 16E-Arylidenosteroids. Bioorg. Chem. 2012, 45, 36–40.
- Bansal, R.; Guleria, S.; Thota, S.; Hartmann, R.W.; Zimmer, C. Synthesis of Imidazole-Derived Steroidal Hybrids as Potent Aromatase Inhibitors. Med. Chem. Res. 2013, 22, 692–698.
- Singh, R.; Bansal, R. Investigations on 16-Arylideno Steroids as a New Class of Neuroprotective Agents for the Treatment of Alzheimer’s and Parkinson’s Diseases. ACS Chem. Neurosci. 2016.
- Zhu, L.; Yang, Y.; Gao, P.; An, X.; Sun, Y.; Sun, X.; Hou, Y.; Shan, L. Synthesis and Anti-Inflammatory Activity Evaluation of 2-Dehydroepiandrosterone Benzene Methyl Derivatives. Chinese J. Org. Chem. 2019, 39, 2625–2631.
- Banday, A.H.; Akram, S.M.M.; Shameem, S.A. Benzylidine Pregnenolones and Their Oximes as Potential Anticancer Agents: Synthesis and Biological Evaluation. Steroids 2014, 84, 64–69.
- Abood, N.K.; Ibraheem, H.H. Synthesis, Characterize and Antimicrobial Study of New Chalcones and Pyrazole Derivatives from Progesterone. Int. J. Sci. Res. 2016, 5, 2319–7064.
- Bernstein, L.; Ross, R.K. Endogenous Hormones and Breast Cancer Risk. Epidemiol. Rev. 1993, 15, 48–65.
- Poirier, D.; Chang, H.; Azzi, A.; Boivin, R.P.; Lin, S. Estrone and Estradiol C-16 Derivatives as Inhibitors of Type 1 17b-Hydroxysteroid Dehydrogenase. Mol. Cell. Endocrinol. 2006, 248, 236–238.
- Allan, G.M.; Lawrence, H.R.; Cornet, J.; Bubert, C.; Fischer, D.S.; Vicker, N.; Smith, A.; Tutill, H.J.; Purohit, A.; Day, J.M.; et al. Modification of Estrone at the 6, 16, and 17 Positions: Novel Potent Inhibitors of 17 -Hydroxysteroid Dehydrogenase Type 1. J. Med. Chem. 2006, 49, 1325–1345.
- Wang, C.; Li, L.; Fu, D.; Qin, T.; Ran, Y.; Xu, F.; Du, X. Discovery of Chalcone-Modified Estradiol Analogs as Antitumour Agents That Inhibit Tumour Angiogenesis and Epithelial to Mesenchymal Transition. Eur. J. Med. Chem. 2019, 176, 135–148.
- Kolo, A.M.; İpek, E.; Çapan, İ.; Servi, S. Synthesis of Heterocyclic-Substituted Novel Hydroxysteroids with Regioselective and Stereoselective Reactions. J. Heterocycl. Chem. 2018, 55, 492–497.
- Thamotharan, S.; Parthasarathi, V.; Gupta, R. Two Androst-5-Ene Derivatives: 16-[4-(3-Chloropropoxy)-3-Methoxybenzylidene]-17-Oxoandrost-5-En-3β-Ol and 16-[3-Methoxy-4-(2-Pyrrolidin-1-Ylethoxy)Benzylidene]-3β-Pyrrolidinoandrost-5-En-17β-Ol Monohydrate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2004, 60, 75–78.
- Dubey, S.; Piplani, P.; Jindal, D.P. Synthesis and Evaluation of Some 16-Benzylidene Substituted 3,17-Dioximino Androstene Derivatives as Anticancer Agents. Lett. Drug Des. Discov. 2005, 2, 537–545.
- Choudhary, M.I.; Alam, M.S.; Yousuf, S.; Wu, Y.; Lin, A.; Shaheen, F. Pregnenolone Derivatives as Potential Anticancer Agents. Steroids 2011, 76, 1554–1559.
- Bryce, A.; Ryan, C.J. Development and Clinical Utility of Abiraterone Acetate as an Androgen Synthesis Inhibitor. Clin. Pharmacol. Ther. 2009, 91, 101–108.
- Bastos, D.A.; Antonarakis, E.S. Galeterone for the Treatment of Advanced Prostate Cancer: The Evidence to Date. Drug Des. Devel. Ther. 2016, 10, 2289–2297.
- Huang, L.-H.; Zheng, Y.-F.; Lu, Y.-Z.; Song, C.-J.; Wang, Y.-G.; Yu, B.; Liu, H.-M. Synthesis and Biological Evaluation of Novel Steroidal[17,16-d][1,2,4]Triazolo[1,5-a]Pyrimidines. Steroids 2012, 77, 710–715.
- Ke, S.; Shi, L.; Zhang, Z.; Yang, Z. Pyrimidines Derived from Dehydroepiandrosterone: A Convenient Synthesis, Antiproliferation Activity, Structure-Activity Relationships, and Role of Heterocyclic Moiety. Nat. Publ. Gr. 2017, 7, 1–7.
- Mótyána, G.; Molnár, B.; Wölfling, J.; Frank, É. Microwave-Assisted Stereoselective Heterocyclization to Novel Ring D-Fused Arylpyrazolines in the Estrone Series. Molecules 2019, 24, 1–15.
- Yu, B.; Shi, X.; Qi, P.; Yu, D.; Liu, H. Design, Synthesis and Biological Evaluation of Novel Steroidal Spiro-Oxindoles as Potent Antiproliferative Agents. J. Steroid Biochem. Mol. Biol. 2014, 141, 121–134.
- Banday, A.H.; Shameem, S.A.; Jeelani, S. Steroidal Pyrazolines and Pyrazoles as Potential 5α-Reductase Inhibitors: Synthesis and Biological Evaluation. Steroids 2014, 92, 13–19.
- Singh, R.; Thota, S.; Bansal, R. Studies on 16,17-Pyrazoline Substituted Heterosteroids as Anti-Alzheimer and Anti-Parkinsonian Agents Using LPS Induced Neuroinflammation Models of Mice and Rats. ACS Chem. Neurosci. 2017.
- Banday, A.H.; Shameem, S.A.; Banday, J.A.; Ganaie, B.A. Synthesis, 17α-Hydroxylase-C 17,20-Lyase Inhibitory and 5AR Reductase Activity of Novel Pregnenolone Derivatives. Anticancer. Agents Med. Chem. 2018, 18, 1919–1926.
- El-Naggar, M.; Amr, A.E.E.; Fayed, A.A.; Elsayed, E.A. Potent Anti-Ovarian Cancer with Inhibitor Activities on Both Topoisomerase II and V600E BRAF of Synthesized Substituted Estrone Candidates. Molecules 2019, 24, 2054.
- Amr, A.E.E.; Abdel-latif, N.A.; Abdalla, M.M. Synthesis and Antiandrogenic Activity of Some New 3-Substituted Androstano [17,16-c]-5′-Aryl-Pyrazoline and Their Derivatives. Bioorg. Med. Chem. 2006, 14, 373–384.
- Shi, Y.; Wang, B.; Shi, X.; Zhao, Y. Synthesis and Biological Evaluation of New Steroidal Pyridines as Potential Anti-Prostate Cancer Agents. Eur. J. Med. Chem. 2018, 145, 11–22.
- Amr, A.E.E.; Elsayed, E.A.; Al-omar, M.A.; Eldin, H.O.B.; Nossier, E.S.; Abdallah, M.M. Design, Synthesis, Anticancer Evaluation and Molecular Modeling of Novel Estrogen Derivatives. Molecules 2019, 24, 416.
- Babu, A.R.S.; Raghunathan, R. An Easy Access to Novel Steroidal Dispiropyrrolidines through 1, 3-Dipolar Cycloaddition of Azomethine Ylides. Tetrahedron Lett. 2008, 49, 4618–4620.
- Gavaskar, D.; Babu, A.R.S.; Raghunathan, R.; Dharani, M.; Balasubramanian, S. An Expedient Sequential One-Pot Four Component Synthesis of Novel Steroidal Spiro-Pyrrolidine Heterocycles in Ionic Liquid. Steroids 2016, 109, 1–6.
- Mótyána, G.; Gopisetty, M.K.; Kiss-Faludy, R.E.; Kulmány, Á.; Zupkó, I.; Frank, É.; Kiricsi, M. Anti-Cancer Activity of Novel Dihydrotestosterone-Derived Ring A-Condensed Pyrazoles on Androgen Non-Responsive Prostate Cancer Cell Lines. Int. J. Mol. Sci. 2019, 20, 2170.
- Iványi, Z.; Szabó, N.; Wölfling, J.; Szécsi, M.; Julesz, J.; Schneider, G. Novel Series of 17β-Pyrazolylandrosta-5,16-Diene Derivatives and Their Inhibitory Effect on 17α-Hydroxylase/C17,20-Lyase. Steroids 2012, 77, 1152–1159.
- Mitchell, H.J.; Dankulich, W.P.; Hartman, G.D.; Prueksaritanont, T.; Schmidt, A.; Vogel, R.L.; Bai, C.; Mcelwee-witmer, S.; Zhang, H.Z.; Chen, F.; et al. Design, Synthesis, and Biological Evaluation of 16-Substituted 4-Azasteroids as Tissue-Selective Androgen Receptor Modulators (SARMs). J. Med. Chem. 2009, 52, 4578–4581.
- Dubey, S.; Jindal, D.P.; Piplani, P. Synthesis and Antineoplastic Activity of Some 16-Benzylidene Substituted Steroidal Oximes. Indian J. Chem. 2005, 44, 2126–2137.
