The global energy crisis and environmental problems are becoming increasingly serious. It is now urgent to vigorously develop an efficient energy storage system. Lithium-sulfur batteries (LSBs) are considered to be one of the most promising candidates for next-generation energy storage systems due to their high energy density. Sulfur is abundant on Earth, low-cost, and environmentally friendly, which is consistent with the characteristics of new clean energy. Although LSBs possess numerous advantages, they still suffer from numerous problems such as the dissolution and diffusion of sulfur intermediate products during the discharge process, the expansion of the electrode volume, and so on, which severely limit their further development. Graphene is a two-dimensional crystal material with a single atomic layer thickness and honeycomb bonding structure formed by sp2 hybridization of carbon atoms. Since its discovery in 2004, graphene has attracted worldwide attention due to its excellent physical and chemical properties.
- graphene
- lithium-sulfur battery
- cathode
- polysulfide
- composites
1. Introduction
Facing the depletion of fossil fuels and gradual serious environmental pollution problems, people have slowly realized the necessity of clean energy development. Sustainable energy such as solar and wind energy has been extensively developed [1]. However, this decentralized energy supply is not a long-term solution for social energy storage. Therefore, it is urgent to develop a stable high-capacity clean energy storage system to handle the social energy demand problem. Among the many new energy battery systems, lithium-ion batteries (LIBs) have attracted much attention due to their high discharge specific capacity, high safety, long service life, and environmental friendliness advantages [2][3].
Unfortunately, LIB cathode materials (layered metal oxides and spinel structures) are expensive and their performance has approached the theoretical limit [4][5], making it difficult to meet the long-term battery life requirements represented by electric vehicles. Hence, researching new low-cost cathode materials is an effective strategy to deal with market demand. As one of the basic elements on Earth, sulfur is abundant in nature and environmentally friendly. In particular, it has an ultra-high theoretical capacity of 1675 mAh·g−1 and a theoretical energy density of 2600 Wh·kg−1 [6][7][8][9][10]. These advantages have promoted the development of sulfur cathode materials. After decades of research, LSBs have made great progress [11][12][13]. However, there are still many problems to be solved in sulfur cathodes:
-
The insulation of sulfur reduces the electron transfer rate (conductivity: 5 × 10−30 S·cm−1 at 25 °C) [14].
-
The volume expansion of the sulfur cathode material after multiple electrode reactions destroys the electrode structure.
-
The dissolution of soluble lithium polysulfide triggers a “shuttle effect”, which causes energy loss and low battery life.
In order to solve the above problems and achieve high electrochemical activity, a feasible and effective method is to establish abundant electron and ion transport channels inside the cathode material and provide a compatible surface for insoluble Li2S and S. Researchers have designed sulfur cathodes into zero-dimensional [15], one-dimensional [16], two-dimensional [17], and three-dimensional materials [18][19], rationally designing sulfur host materials from multiple dimensions to explore higher performance LSBs. Until 2009, Nazar et al. prepared a cathode composed of mesoporous carbon (CMK-3) and sulfur for the first time, obtaining an initial discharge capacity of 1000 mAh·g−1 at 0.1 C [20]. Their study proved the feasibility of utilizing mesoporous carbon to adsorb polysulfides. This research achievement has attracted the interest of more and more researchers and promoted the development of various forms of carbon-based materials in the field of sulfur cathodes.
Among the many carbon materials, graphene has attracted attention for its high conductivity, high specific surface area, and excellent mechanical properties. It has an electron mobility of 15,000 cm2·V−1·S−1 at room temperature and a theoretical surface area of 2630 m2·g−1 [21][22][23][24]. Graphene is a two-dimensional material composed of a layer of sp2-bonded carbon atoms [21][25]. It has excellent flexibility, which is benefit to deal with the volume change of sulfur cathode in the redox reaction and also provides a material basis for the preparation of flexible devices. Second, graphene as a matrix material can provide good conductive network support for the sulfur cathode and further improve the sulfur utilization rate. Furthermore, the programmable assembly characteristics of graphene can be flexibly changed. It not only has a large specific surface area, but also facilitates the construction of interconnected and layered macroporous networks, which is an effective method to inhibit the diffusion of polysulfides. However, non-polar graphene has a weak adsorption capacity for polar polysulfides. Graphene doping by heteroatoms can produce polar electroactive sites, which effectively overcome this shortcoming. In addition to the high conductivity and good mechanical properties of graphene, a large number of functional groups on the surface of graphene can achieve interaction with lithium polysulfides (LiPSs).
With the deep understanding of the interaction mechanism between graphene and LSBs, a variety of effective methods have been explored to prevent the shuttle effect. Correspondingly, the electrochemical performance of LSBs has also been greatly improved. In recent years, the overall structure of LSB has been summarized including cathodes, anodes, electrolytes, and separators. For instance, Pang et al. summarized the new electrolyte and intelligent cathode system in LSBs that control the dissolution of polysulfides [26]. Yang et al. and Li et al. focused on summarizing the stable performance of LSB cathode materials [27][28]. There are also examples of scientific researchers summarizing the battery from the perspective of materials. Shao et al. discussed the challenges and prospects of LSBs from the two-dimensional material level [29]. Dai et al. mainly summarized the application of graphene in the field of flexible batteries including various metal-ion batteries, metal-air batteries, and LSBs [30]. Fang et al. classified LIBs and LSBs from two materials: carbon nanotubes and graphene [31]. Wu et al. respectively summarized the development of core-shell structured S electrodes, freestanding and flexible sulfur cathodes, and functionalization of graphene-based carbon in LSBs [32]. The most recent review describing the progress of graphene in LSBs is the application of porous graphene in sulfur cathodes and lithium anodes introduced by Sun et al. [33], who looked at aspects of sulfur utilization, cathode volume change, and the reduction of lithium loss.
2. Graphene as the Positive Electrode Skeleton
Since graphene was mechanically exfoliated by Geim et al. in 2004 [34], the preparation methods, characterization methods, and physical and chemical properties of graphene have been extensively developed. These important studies laid the foundation for the application of graphene in electrode materials. Since then, the superior conductivity and higher chemical stability of graphene have been widely recognized by scientific researchers. Graphene-based materials as the positive electrode framework of LSBs have made rapid progress in recent years. Its relationship with sulfur and its advantages and disadvantages as a pure cathode framework are introduced and summarized in this section.
2.1. The Interactions between Sulfur and Graphene
There are both physical and chemical interactions between sulfur molecules and graphene and these two interactions complement each other. In terms of physical interactions, first, graphene’s various geometric features act as a substrate material for coating sulfur particles on a macroscopic scale (Figure 1a) [35]. Besides, its flexibility is also suitable for electrode applications. The stretching ability of the sulfur cathode framework is essential for the improvement of electrochemical performance. In fact, graphene boosts the charge transfer between sulfur particles and electrolytes due to its unique structure. The physical coating of graphene can prevent the spreading of dissolved polysulfides. Porous graphene can accommodate the volume expansion and improve the utilization of sulfur during the charge–discharge process. Moreover, graphene is a hexagonal honeycomb lattice structure composed of carbon atoms with sp² hybrid orbitals. The S8 molecule is a zigzag ring with eight sulfur atoms, which is called a double-layer octagonal structure. The crystal structures of graphene and element S8 molecules are both highly symmetrical and both possess non-polar properties, as shown in Figure 1b. Therefore, van der Waals forces are very strong. The interaction between the two is reflected in the lone pairs of the S 3pz2 electrons and the antibonding conjugated π* states of the graphene plane [36][37]. Intriguingly, during the discharge process, the larger the electron density of the polysulfide, the stronger the above interaction will be. Therefore, a graphene-based material can not only immobilize the element S8 molecule, but also fix LiPSs, which is a good choice for the sulfur cathode framework.
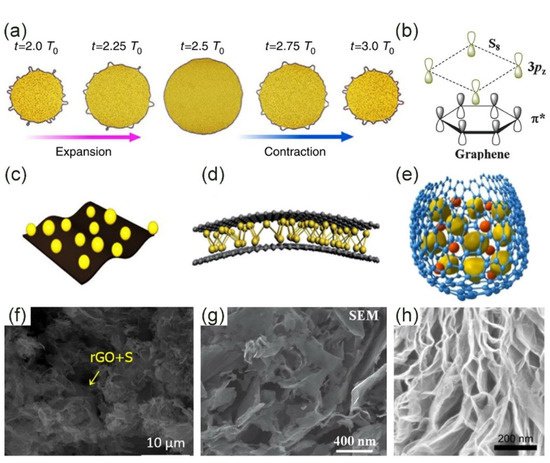
Figure 1. Interaction between graphene and sulfur and its configuration. (a) Macroscopic volume change of graphene-coated sulfur particles [35]. Copyright 2014, Springer Nature. (b) Microscopic symmetry and non-polarity of graphene and sulfur [36]. Copyright 2013, Royal Society of Chemistry. (c) The in-plane structure has the advantage of flexible electrodes [38]. Copyright 2016, Elsevier. (d) Sandwich structure: sulfur is confined between two or more layers of graphene sheets [36]. Copyright 2013, Royal Society of Chemistry. (e) Core-shell structure: sulfur and LiPSs are coated by graphene to prevent leakage [39]. Copyright 2013, Elsevier. (f) Scanning Electron Microscope (SEM) image of complex of sulfur and graphene oxide (S/GO) [38]. Copyright 2016, Elsevier. (g) The sandwich structure of sulfur is evenly distributed on the graphene sheet [36]. Copyright 2013, Royal Society of Chemistry. (h) SEM image of core-shell structure [39]. Copyright 2013, Elsevier.
2.2. Configurations of Pure Graphene and Sulfur
In recent years, a variety of graphene/sulfur configuration materials have been developed as sulfur cathodes. In order to maximize the utilization of active materials, researchers have studied the graphene/sulfur configuration from a variety of perspectives. The basic configuration is divided into the following types: in-plane, three-dimensional sandwich, three-dimensional core-shell, and so on. This section summarizes the various configurations of sulfur cathodes based on unmodified graphene.
There are several forms for in-plane: graphene sheets, graphene paper, graphene nanoribbons, etc. [40][41][42][43][44]. Their names are different according to different preparation methods. Among them, graphene nanoribbons are the most special. Graphene nanoribbons are intermediary products of graphene and carbon nanotubes. The graphene nanotubes can be cut and expanded longitudinally to obtain graphene nanoribbons. Compared with other paper-like structures, graphene nanoribbons are composed of a large number of quasi-one-dimensional graphene nanoribbons that are closely connected to each other, which is beneficial to improve the stability of the electrode. The self-assembly process induced by water evaporation was developed by Liu et al. [40]. The obtained graphene nanoribbons made the internal structure network tightly connected due to the benefits of evaporation, which can not only promote charge transfer but also physically limit LiPSs. In-plane means that the redox reaction takes place on the surface of the graphene paper (Figure 1c). Therefore, with the progress of the reaction, the inner part of the graphene paper surface is etched, and the intermediate pore and fold structure are gradually formed, thus increasing the specific surface area of the material (Figure 1f) [42][43][44]. The advantage of the in-plane structure is that its flexible electrode can also show good electrochemical performance in the bent state. Moreover, the utilization rate of the graphene/sulfur active material with a paper-like structure is very high. However, it is a flat structure after all and cannot achieve the effect of macro-physical packaging.
The sandwich structure is a simple physical packaging scheme, and the sulfur is confined between two or more layers of graphene nanosheets (Figure 1d,g) [45][46][47][48]. Recently, Li et al. wrapped ultrafine nano-sulfur particles between two layers of graphene to form a sandwich structure [46]. Ultra-fine sulfur particles have a larger electronic contact area than bulk materials, avoiding the “dead sulfur” problem. The electrode material’s capacity of 1208 mAh·g−1 at 0.1 C was also due to the effective physical limitation of the sandwich structure. Fang et al. prepared a full graphene sandwich structure composed of high-porosity graphene (HPG), highly conductive graphene (HCG), and partial graphene oxide (POG) (Figure 2a) [48]. Sulfur was mainly in HPG. HCG was used as a current collector and POG was used as an adsorption layer for polysulfides. Three kinds of graphene played their respective roles, making the initial discharge capacity as high as 1500 mAh·g−1 at 0.34 A·g−1. Surprisingly, the electrode still had an area capacity of 4.2 mAh·cm−2 after 400 cycles (Figure 2d). There was also a very special sandwich structure in which sulfur and LiPSs were confined between the graphene and the separator. Graphene was coated on the sulfur and separator, respectively (Figure 2b). This structure adapts to the volume expansion in the lithiation process to a greater extent. Graphene on the surface of the separator also effectively reduces the shuttle effect [45]. Obviously, the sandwich structure has excellent electron transfer capabilities and can also alleviate volume shrinkage/expansion to a certain extent. However, for a long-term cycle, the dissolved polysulfides will inevitably leak from the edge of the graphene, which is very detrimental to the battery life.
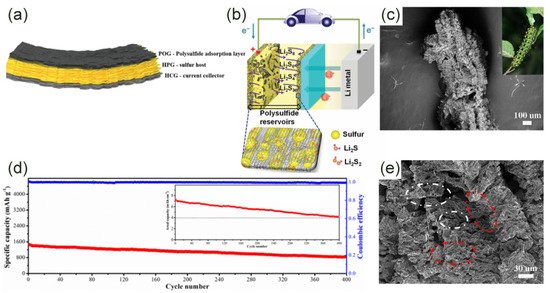
Figure 2. (a) Schematic diagram of all-graphene sandwich structure [48]. Copyright 2016, American Chemical Society. (b) Electrode configuration diagram: LiPSs are confined between graphene and diaphragm [45]. Copyright 2014, Springer Nature. (c) SEM image of caterpillar-like graphene [49]. Copyright 2017, Elsevier. (d) The long cycle test and the corresponding area capacity of the sulfur cathode at 0.34 A·g−1 [48]. Copyright 2016, American Chemical Society. (e) Layered (red circles) porous (white circles) structure shown by SEM image [49]. Copyright 2017, Elsevier.
The core-shell structure has been proven to be able to solve the above-mentioned problems of LiPS leakage and poor volume buffer capacity. Graphene is used as an outer packaging to firmly bind the sulfur particles in the shell (Figure 1e,h). The LiPS intermediate product of the redox reaction cannot break the boundaries of graphene packaging. In addition, the core-shell structure is often very flexible, and has a good ability to deal with the volume shrinkage/expansion caused by the electrode reaction [39][50]. Although the core-shell structure can solve the problem of volume change, the large accumulation of sulfur particles in the center of the shell inevitably reduces the electron transfer rate between lithium and sulfur, and the synthesis process of the core-shell structure is complicated and cumbersome.
In order to obtain better electrochemical performance, researchers are more willing to effectively combine three-dimensional, in-plane, sandwich, core-shell, and other structures, even though the synthesis process is complex and cumbersome. For instance, Yoo et al. transformed graphene nanosheets with an in-plane structure into a columnar structure of graphene nanorolls through a freeze-casting process [38]. This structure combines the characteristics of in-plane and sandwich. Sulfur is wrapped inside the nano-volume to act as a physical barrier to the diffusion of LiPSs. A similar structure is caterpillar-like graphene (Figure 2c), which is different from graphene nano volumes in that it has a layered, dense porous, and wrinkled structure inside (Figure 2e) [49]. In particular, the dense porous structure enables the track-like graphene to contain more sulfur particles. Similar to the core-shell structure is a microsphere-type graphene structure, where the combination of sandwich structure and core-shell structure is characteristic. The rod-shaped nano-sulfur is uniformly deposited in the reduced graphene oxide structure by spray freezing and the combination of spray-freezing components and ozonation is used to control the size and pore structure of the microspheres [51]. Therefore, this structure achieves a higher sulfur utilization rate. Graphene aerogels also have good electrochemical benefits as an electrode material. A reduced graphene oxide aerogel as a stable interconnected porous conductive scaffold can promote the reaction of Li+ with polysulfides. The oxygen-containing groups and a large amount of space in the rGO aerogel inhibit the migration of LiPSs from the cathode [52][53]. The graphene framework of these multiple configurations is stronger than the graphene framework of a single configuration in terms of rate capability and cycle capability. However, its complicated preparation procedure also inevitably increases the cost.
3. Graphene-Based Composite Materials
Nowadays, more and more researchers tend to choose graphene composite materials as S electrodes. For example, the combination of metal compounds and graphene alleviates the shuttle effect from both physical and chemical aspects. The combination of other carbon materials and graphene allows them to perform their own duties, not only to obtain a composite network conductive structure support, but also to expand the pore area to improve the sulfur loading and sulfur utilization. The improvement of electrochemical performance is even more multifaceted.
3.1. Metal Compound/Graphene Composite
Inspired by the immobilization of LiPSs by single-atom chemical bonds, numerous studies have focused on the effect of polar metal compounds on the performance of the graphene electrodes. Metal particles such as cobalt, vanadium, and nickel are generally used as single-atom catalysts, and the high surface free energy of the metal center is used to catalyze polysulfides. However, due to the disadvantage that a single atom is easy to expose catalytic sites, it has to be introduced into materials with high specific surface area. The non-polar properties of graphene are a good choice for materials with large specific surface areas. The composite material of metal compounds and graphene is of great significance for suppressing the shuttle effect. Recently, Cui reported that bivalent metal oxides (MO) and tetravalent transition metal sulfides with M:O = 1:1 can maintain a high reversible capacity to promote battery electrochemical performance [54]. This research provides important insights into the design principles of transition metal compounds and graphene composite cathodes. The catalysis of polysulfide conversion is still in the early stages of research. This part mainly introduces the development of composite materials formed by different metal oxides, sulfides, and graphene.
3.1.1. Metal Oxide
Oxygen vacancy (OV) defects are widely used in the field of catalysis because OVs can not only capture the adsorbent, but also inhibit the recombination of carriers. The O2− state of the oxygen has a strong interaction with LiPSs, which reduces the amount of the migrated LiPSs [55][56][57][58][59]. Pt and Ni have been shown to have the ability to adsorb soluble polysulfides and can also accelerate the conversion of insoluble LiPSs to long-chain LiPSs and sulfur [60][61]. Consequently, adding metal oxides into graphene electrodes for catalysis is a feasible strategy.
Tang used the advantages of metal oxides in the electrode preparation process [62]. Porous CaO is used as a CVD growth template to prepare graphene materials. CaO has two roles in this process: on one hand, CaO stimulates the rapid growth of graphene. On the other hand, it provides the electrode frame with medium- and large-pore sizes. This structure shortens the diffusion path of ions and also reduces interfacial resistance. In the electrochemical performance test, when the current rate was 0.5 C, the initial discharge capacity was 434 mAh·g−1, and the capacity decay rate for 150 cycles was 0.11% (coulomb efficiency was 90%). Zheng et al. prepared a three-dimensional Fe2O3–graphene hybrid (Fe–PGM) by a one-pot method [63]. It was found that α–Fe2O3 greatly enhances the interaction between the sulfur host and LiPSs by comparing the adsorption energy of S-containing clusters on graphene and α–Fe2O3. At the same time, it also promotes the conversion of soluble LiPSs to insoluble LiPSs during the charge and discharge process, which in turn improves the utilization rate of sulfur (Figure 3a). The maximum discharge capacity of the cathode compounded with sulfur (Fe–PGM–S) at 0.3 C is 1571 mAh·g−1 and the decay rate corresponding to 1000 cycles at a high current rate of 5 C is 0.049%. This also reflects the contribution of metal oxides in improving cycle stability.
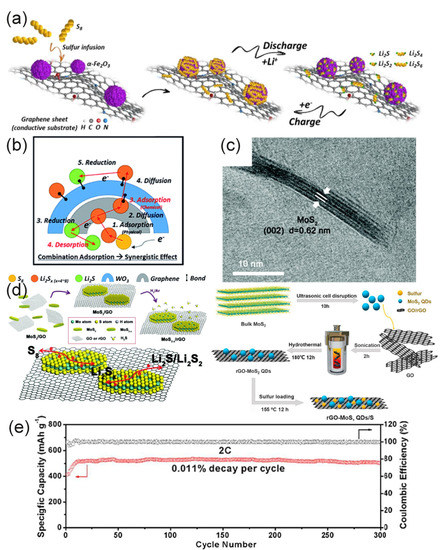
Figure 3. Metal compound/graphene composite sulfur cathode. (a) The Fe2O3 nanoparticles on the graphene sheet fix LiPSs by thermodynamic adsorption and promote the conversion of soluble LiPSs into insoluble LiPSs [63]. Copyright 2011, American Chemical Society. (b) WO3 in S@G@WO3 composites forms chemical bonds with LiPSs to inhibit the dissolution of LiPSs [64]. Copyright 2019, Royal Society of Chemistry. (c) HRTEM image of MoS2 nanosheets. (d) Schematic of the synthesis of the MoS2–x/rGO and rGO–MoS2 QDs/S. (e) Electrochemical performance of rGO–MoS2 QDs/S electrode [65][66]. Copyright 2019, Elsevier. Copyright 2017, Royal Society of Chemistry.
Transition metal oxide films have received more attention in the field of sulfur cathodes due to their unique properties [67][68][69][70]. In consideration of the unique perovskite structure of tungsten trioxide (WO3) and excellent electrochromic, photochromic, and gasochromic characteristics, Choi combined WO3 film with graphene/sulfur nanomaterials (S@G@WO3) and used it in LSBs [64]. They found that WO3 could adsorb LiPSs with different chain lengths and concentrations during the redox reaction (Figure 3b). The use of WO3 film solves the problem of weak interaction between graphene and polysulfide, which makes the dissolution of polysulfide more difficult, thereby reducing the shuttle effect. Therefore, the cycle capacity is obviously increased (the capacity retention rate was 95% after 500 cycles).
Song et al. prepared vanadium dioxide/graphene sulfur cathode material (VO2/G/S), which possesses both trapping and catalytic effects [71]. VO2 has the advantages of high abundance, low cost, and rapid ion diffusion rate. In the VO2/G/S cathode, it showed strong anchoring of LiPSs, and the sulfur redox reaction was faster. It showed excellent electrochemical performance in the Li–S full battery. The initial discharge capacity at 0.2 C was 1405 mAh·g−1. Their research results provide a new way to prepare low-cost and environmentally-friendly Li–S cathodes. The proper polarity and high chemical stability of MoO2 are also make it a good choice of metal oxide as sulfur host materials. Feng et al. prepared one-dimensional hollow reduced graphene oxide-coated MoO2 nanotubes (H-S@MoO2/rGO) and achieved 84% high sulfur loading [72]. The special structure of nanotubes shortens the electron transmission path. The non-polar Mo–Mo metal bond in the MoO2 lattice exhibits metallic characteristics, and the combination with polarity increases the adsorption and charge transfer functions during the discharge process.
4.1.2. Metal Sulfide
Metal sulfides have obvious advantages than metal oxides in LSBs. First, metal sulfides can reduce the lithiation voltage to avoid overlap with the operating voltage window; second, its conductivity is higher than that of metal oxides, which can significantly improve the utilization rate of the material. The third point is the redox reaction during the process, there is a strong interaction between metal sulfides and Li2Sx, this can lower the barrier and catalyze the reaction process. The polar sites provided by metal sulfides inhibit the migration of polysulfides, at the same time, it can also be used as an active material for storing lithium to provide additional capacity. The addition of metal sulfides maximizes the catalysis and capture effects [27][28][33][73][74][75][76].
Since the introduction of the catalytic redox reaction strategy, researchers have tried to use various electrocatalysts to improve the electrochemical performance of materials. Yuan et al. mixed cobalt disulfide and graphene and used it in LiSBs [77]. As a conductive sulfiphilic host, CoS2 plays an indispensable role in the catalysis of redox reactions. Highly shrunken graphene was used as a substrate, and CoS2 clusters with a diameter of 1 μm were mechanically ground and mixed into it to obtain a CoS2/graphene composite (CoS2 + G). In the experiment of simulating a highly polar polysulfide as a statically adsorbed adsorbate, they found that the existence of aa Co–S bond greatly increased the affinity of Li2S4 molecules for heteropolar CoS2. When the CoS2 content was increased up to 30%, the adsorption effect of LiPSs in the electrolyte was not significantly enhanced. Therefore, CoS2 does not simply prevent polysulfides from penetrating into the electrolyte through chemical adsorption. Although this structure effectively improves the electron path, the small size of the CoS2 particles leads to insufficient distribution on the graphene framework. Thus, how to make up for the shortage of metal sulfides to further improve the performance of electrode materials should become one of the research hotspots in the future.
MoS2 is also a low-cost catalyst. Its high surface area and abundant active sites have attracted special attention from researchers. It has shown highly efficient catalytic ability in industrial reactions such as hydrogen evolution reaction (HER), oxygen reduction reaction (ORR), and oxygen evolution reaction (OER) [78][79][80][81]. Lin et al. decorated MoS2 nanoflakes on rGO [66]. As shown in Figure 3c, MoS2 nanoflakes have a structure of 6–8 layers, each layer is about 3–5 nm. MoS2 and RGO, as two-dimensional materials, have good contact compatibility, which not only improves the quality of the contact interface, but also successfully exposes the sulfur defects on the catalyst surface. This sulfur defect is an important factor in the catalytic conversion of polysulfides. When the sulfur cathode contained 4% MoS2-x/rGO, the capacity at an 8 C rate was 826.5 mAh·g−1. Moreover, 600 cycles could be carried out at 0.5 C rate and the capacity decay rate of each cycle was 0.083%. Both high rate performance and excellent cycle stability were obtained. The rGO-MoS2QDs (quantum dot)/S electrode prepared by Wei et al. through a hydrothermal reaction reached an ultra-low capacity decay rate of 0.011% per cycle in 300 cycles at the 2 C rate, and the coulombic efficiency was close to 100% (Figure 8e) [65]. Their preparation methods are similar, as shown in Figure 3d. Two kinds of MoS2 disulfide were obtained under the assistance of ultrasound and GO was prepared by the modified Hummer method [82], and finally, the sulfur cathode was prepared by the melt diffusion method. The difference between them was that when Lin synthesized MoS2-x/rGO, the amount of sulfur deficiency was changed by changing the heat treatment time and temperature in hydrogen. However, Wei et al. directly made the mixture solution undergo a hydrothermal reaction and then freeze-drying, which did not change the situation of sulfur deficiency [65]. These studies confirm the effectiveness of MoS2 as a polysulfide conversion catalyst.
In recent years, nickel sulfide (Ni3S2) has also received attention in supercapacitors. Ni3S2 is not only low-cost but is also highly conductive. Guo et al. synthesized a Ni3S2/(N, S)-rGO hybrid material [83]. The excellent effect brought by the N/S co-doping modification above-mentioned was still effective. The co-doping of Ni3S2 and N/S synergistically improved the material’s conductivity and polysulfide adsorption capacity. Both chemical bonding and catalytic effects were reflected. The recombination with a 3D-rGO structure suppressed the shuttle effect from both physical and chemical aspects. The composite with 28.2 wt% Ni3S2 content showed good performance in the charge–discharge test. A total of 1000 cycles were reached at a current density of 3 C, and the capacity decay rate per cycle was 0.023%. When the sulfur loading density per unit area was as high as 5.8 mg·cm−2, the capacity remained above 72.5% after 200 cycles at 1 C.
3.2. Other Carbon Materials/Graphene Composite Materials
The graphene-based material as the sulfur host does provide a large specific surface area for the electrode and sufficient space for sulfur loading. However, the long-term electrostatic attraction inside the graphene sheets makes the graphene sheets continuously gather and accumulate, which destroys the large specific surface area of sulfur loading to a certain extent. The specific capacity and rate capability also gradually decrease [84][85]. Along with the self-supporting carbide-derived carbon/CNT/S composite cathode first proposed by the Kaskel group, the advantages of the conductivity and cycle stability of this dual carbon material mixture have gradually been recognized by researchers [86]. As such, researchers’ attention to the dual carbon material mixture has risen to a new level.
Carbon nanotubes (CNTs) are multi-purpose matrix materials with high conductivity and large specific surface area. However, CNTs have limited performance as a conductive matrix. Both CNT and graphene are representative carbon allotropes, and their basic structural units are hexagonal honeycomb lattices of carbon. CNT is a one-dimensional nanotube structure. It is not limited by the area of the active material and CNTs with a large aspect ratio can promote the rapid transmission of lithium ions and electrons. Graphene is a two-dimensional nanosheet. Its planar structure will prevent the transmission of lithium ions, which is particularly obvious at high current densities [31][87][88]. From the analysis of material surface properties, graphene had a rich pore structure and more surface functional groups and defects. The theoretical specific surface area was 2630 m2·g−1. The various atomic doping behaviors described in the previous sections are important factors that limit the diffusion of polysulfides. The rich pore structure also helps electrolyte penetration. CNTs are usually obtained by CVD, and the lack of surface functional groups can be attributed to the high purity [89]. Of course, a large number of functional group modifications will inevitably lead to a decrease in the conductivity of graphene. Thus, the advantages of CNTs will emerge at this time. The above analysis indicated that graphene and CNTs have complementary characteristics in many aspects. Rather than treating them as competitors, it is better to combine them cleverly to exert beneficial synergies. Breaking their limited characteristics by constructing a CNT/graphene hybrid structure is an effective method.
Graphene/single-walled CNT (SWCNT) hybrid materials are considered to have superior performance in constructing conductive networks than other sp2 nano carbons [39][90]. Such G/SWCNT preparation strategies usually choose layered double hydroxides (LDHs) [91] or layered double oxides (LDO) [92] as the 2D lamellar substrates for graphene CVD growth to achieve the inherent position of SWCNT and graphene. This method usually chooses methane as the growth carbon source and removes the calcined LDH or LDO after the high-temperature CVD process to obtain a G/SWCNT hybrid. The hybrid prepared by this method anchors SWCNTs on the surface of the graphene layer, thereby inhibiting the aggregation of SWCNTs. The G/SWCNT–S cathode prepared by Zhao had a capacity of 650 mAh·g−1 after 100 cycles at a current rate of 5 C, which proves the feasibility of this scheme [91].
In the initial studies, researchers started to combine graphene and CNTs from the basic shape. For example, the combination of ultra-long CNT and graphene spheres (GS) (Figure 4b) [93], graphene and CNT co-growth seamlessly symbiosis model (Figure 4a) [94], and the combination of the three-dimensional sponge-like morphology of ordinary CNT and graphene [95]. Ultra-long CNTs have excellent flexibility and can also provide a highly conductive network, providing an effective way for flexible electronic devices with high energy density. The internal size of hollow graphene nanospheres is about 15–30 nm, which has dual functions: on one hand, it provides a tight load space for active sulfur; on the other hand, it maintains volume fluctuations during the cycle to prevent the dissolution of LiPSs (Figure 4e) [93]. A three-dimensional CNTs/graphene-sulfur (3DCGS) sponge structure prepared by He et al. enabled the sulfur loading to reach 80.1% [95]. CNTs were used to enhance conductivity and adjust the mesoporous structure. Compared with the three-dimensional graphene-sulfur (3DGS) sponge without carbon nanotubes, the conductivity was increased by 324.7%. Furthermore, of prominence is that the pore size in 3DG was only 3.5 nm, and 3DCG formed a new mesopore with a size of 32.1 nm, so a large amount of active sulfur was incorporated into it. The electrolyte permeability also improved greatly (Figure 4c,f). The capacity of the entire electrode at a high rate of 4 C was 653.4 mA·hg−1.
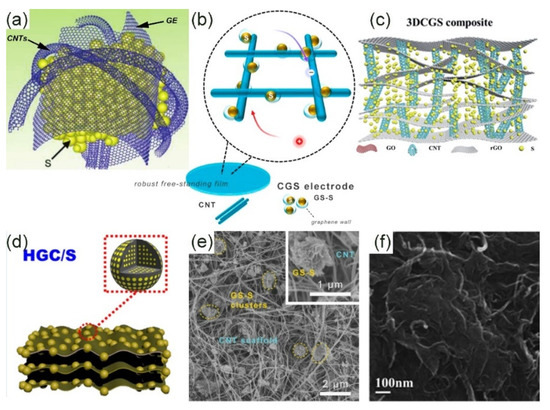
Figure 4. Various carbon/G composite cathode configurations. (a) Schematic diagram of G/CNT hybrid [94]. Copyright 2017, John Wiley and Sons. Schematic diagram of ultra-long CNT/GS-S structure (b) and its SEM image (e) [93]. Copyright 2015, Elsevier. 3DCGS composite cathode structure (c) and its SEM image (f) [95]. Copyright 2015, Royal Society of Chemistry. (d) Graphene and carbon nanospheres structure diagram [96]. Copyright 2019, Elsevier.
The amount and form of loading sulfur are also some of the evaluation criteria for electrode performance. For GO/CNT/S prepared by the freeze-drying method, Hwa first added sulfur and its sulfides during the electrode preparation process and finally removed the impurities [19], while Gómez-Urbano first completed the preparation of GO-CNT and finally added S through the melt diffusion method [97]. In the former, due to the advantages of the preparation process and the clever embedding of CNTs, there was no obvious S agglomeration when the S content was as high as 87%. The latter electrode with a sulfur loading exceeding 4.0 mg·cm−2 had a better specific capacity when doped with 2 wt% CNT (the specific capacity value was 500 mAh·g−1 after 100 charge/discharge cycles at 0.1 C).
The abundant functional groups on graphene are also one of the breakthrough points of G/CNT composites. Combined with the heteroatom-modified graphene structure mentioned in previous sections, single-atom doping or diatomic co-doping are both effective ways to improve the adsorption performance of G/CNT. For example, Su et al. converted Prussian blue (dehydrated sodium ferrocyanide) into a N-doped graphene-carbon nanotube hybrid material through one-step pyrolysis [94]. The active sites generated by nitrogen doping effectively trap polysulfides, greatly improving the cycle performance. Wen et al. prepared N-GCNT composites using a one-step ultrasonic spraying deposition method and successfully penetrated N atoms into the carbon lattice [98]. The interaction between N and S atoms limited the dissolution of LiPSs. Interestingly, the dopant atoms here are effective for the modification of carbon materials. Wu et al. used egg white as a precursor for the inherent N/P co-doping [99]. This preparation method was economical and environmentally friendly. N/P elements were uniformly doped on the carbon framework and the chemical adsorption capacity of the composite material was improved. The N-doped carbon/graphene sheet was designed by Xu et al. and used as cathode material for fixing sulfur [100]. According to reports, the shuttle of polysulfides was still greatly inhibited in the cathode without LiNO3 in the electrolyte. Lee et al. prepared a graphene-loaded N-doped carbon framework (NCF-G) [101]. The initial capacity of the NCF-G electrode compounded with sulfur at 0.1 C was 1359.7 mAh·g−1. Sun et al. decorated graphene with N and S co-doped carbon nanowalls (NSCNWs) to effectively trap polysulfides [102]. The NSCNW-G/S cathode exhibited an ultra-low capacity decay rate (the capacity decay rate for 100 cycles at 0.2 C was 0.078%). The above studies of heteroatom-modified dual carbon materials have shown their practicality for battery performance.
In addition to the CNT structure, electrodes with spherical structures have also been studied accordingly. The nanosphere structure can hold a large amount of sulfur and increase the contact area with the electrolyte to promote the transport of Li+. The most important thing is that it can fix polysulfides well. Zhou et al. designed N-doped double-shelled hollow carbon spheres (G–NDHCS–S) coated with graphene to capture sulfur [84]. Due to the highly electronic conductive network provided by the graphene package, the material did not need to add additional conductive additives and binders. The design of the porous double-shelled hollow structure significantly improves the electrochemical performance of lithium-sulfur batteries. The dual-confined flexible cathode configuration wrapped with carbon spheres and graphene enables the initial discharge capacity to reach 1360 mAh·g−1 at a current rate of 0.2 C. The graphene/carbon nanosphere composite material synthesized by Jia et al. possessed a specific surface area of 3200 m2·g−1 [96]. Its structure is shown in Figure 4d. This structure made the sulfur content 74.5 wt%. The layered porous structure and macropore volume could still effectively fix sulfur. The electrode still had a capacity of 916 mAh·g−1 after 100 cycles. Therefore, the potential performance of the spherical structure electrode in the LSBs has a very broad development prospect.
Additionally, current collectors based on other carbon materials and graphene have shown multiple advantages in LSBs. First, they show excellent flexibility. This is particularly prominent in CNT/graphene electrodes and provides an important foundation for the research of flexible batteries. Second, they mostly act as porous containers to hold polysulfides during the oxidation–reduction reaction process and improve the cycle stability of the battery.
This entry is adapted from the peer-reviewed paper 10.3390/molecules26092507
References
- Chu, S.; Cui, Y.; Liu, N. The Path Towards Sustainable Energy. Nat. Mater. 2016, 16, 16–22.
- Goodenough, J.B.; Park, K.S. The Li-ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176.
- Kim, T.; Song, W.; Son, D.Y.; Ono, L.K.; Qi, Y. Lithium-ion Batteries: Outlook on Present, Future, and Hybridized Technologies. J. Mater. Chem. A 2019, 7, 2942–2964.
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion Battery Materials: Present and Future. Mater. Today 2015, 18, 252–264.
- Whittingham, M.S. Lithium Batteries and Cathode Materials. Chem. Rev. 2004, 104, 4271–4302.
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.M. Erratum: Li–O2 and Li–S Batteries with High Energy Storage. Nat. Mater. 2011, 11, 172.
- Song, M.K.; Cairns, E.J.; Zhang, Y. Lithium/Sulfur Batteries with High Specific Energy: Old Challenges and New Opportunities. Nanoscale 2013, 5, 2186–2204.
- Wang, D.W.; Zeng, Q.; Zhou, G.; Yin, L.; Li, F.; Cheng, H.M.; Gentle, I.R.; Lu, G.Q.M. Carbon–Sulfur Composites for Li–S Batteries: Status and Prospects. J. Mater. Chem. A 2013, 1.
- Zhang, S.S. Liquid Electrolyte Lithium/Sulfur Battery: Fundamental Chemistry, Problems, and Solutions. J. Power Sour. 2013, 231, 153–162.
- Xu, J.; Zhou, K.; Chen, F.; Chen, W.; Wei, X.; Liu, X.W.; Liu, J. Natural Integrated Carbon Architecture for Rechargeable Lithium–Sulfur Batteries. ACS Sustain. Chem. Eng. 2016, 4, 666–670.
- Huang, J.Q.; Zhuang, T.Z.; Zhang, Q.; Peng, H.J.; Chen, C.M.; Wei, F. Permselective Graphene Oxide Membrane for Highly Stable and Anti-Self-Discharge Lithium–Sulfur Batteries. ACS Nano 2015, 9, 3002–3011.
- Jin, F.; Xiao, S.; Lu, L.; Wang, Y. Efficient Activation of High-Loading Sulfur by Small CNTs Confined Inside a Large CNT for High-Capacity and High-Rate Lithium-Sulfur Batteries. Nano Lett. 2016, 16, 440–447.
- Li, B.; Li, S.; Xu, J.; Yang, S. A New Configured Lithiated Silicon–Sulfur Battery Built on 3D Graphene with Superior Electrochemical Performances. Energy Environ. Sci. 2016, 9, 2025–2030.
- Liang, C.; Dudney, N.J.; Howe, J.Y. Hierarchically Structured Sulfur/Carbon Nanocomposite Material for High-Energy Lithium Battery. Chem. Mater. 2009, 21, 4724–4730.
- Jayaprakash, N.; Shen, J.; Moganty, S.S.; Corona, A.; Archer, L.A. Porous Hollow Composites for High-Power Lithium-Sulfur Batteries. Angew. Chem. Int. Ed. Engl. 2011, 50, 5904–5908.
- Fang, R.; Li, G.; Zhao, S.; Yin, L.; Du, K.; Hou, P.; Wang, S.; Cheng, H.M.; Liu, C.; Li, F. Single-Wall Carbon Nanotube Network Enabled Ultrahigh Sulfur-Content Electrodes for High-Performance Lithium-Sulfur Batteries. Nano Energy 2017, 42, 205–214.
- Xu, J.; Shui, J.; Wang, J.; Wang, M.; Liu, H.K.; Dou, S.X.; Jeon, I.Y.; Seo, J.M.; Baek, J.B.; Dai, L. Sulfur-Graphene Nanostructured Cathodes via Ball-Milling for High-Performance Lithium-Sulfur Batteries. ACS Nano 2014, 8, 10920–10930.
- Chong, W.G.; Huang, J.Q.; Xu, Z.L.; Qin, X.; Wang, X.; Kim, J.K. Lithium-Sulfur Battery Cable Made from Ultralight, Flexible Graphene/Carbon Nanotube/Sulfur Composite Fibers. Adv. Funct. Mater. 2017, 27.
- Hwa, Y.; Seo, H.K.; Yuk, J.M.; Cairns, E.J. Freeze-Dried Sulfur-Graphene Oxide-Carbon Nanotube Nanocomposite for High Sulfur-Loading Lithium/Sulfur Cells. Nano Lett. 2017, 17, 7086–7094.
- Ji, X.; Lee, K.T.; Nazar, L.F. A Highly Ordered Nanostructured Carbon-Sulphur Cathode for Lithium-Sulphur Batteries. Nat. Mater. 2009, 8, 500–506.
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. In Nanoscience and Technology; Macmillan Publishers Ltd.: Basingstoke, UK, 2009; pp. 11–19.
- Chen, F.; Tao, N.J. Electron Transport in Single Molecules: From Benzene to Graphene. Acc. Chem. Res. 2009, 42, 429–438.
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H.L. Ultrahigh Electron Mobility in Suspended Graphene. Solid State Commun. 2008, 146, 351–355.
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502.
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-Dimensional Atomic Crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451.
- Pang, Q.; Liang, X.; Kwok, C.Y.; Nazar, L.F. Advances in Lithium–Sulfur Batteries Based on Multifunctional Cathodes and Electrolytes. Nat. Energy 2016, 1.
- Yang, L.; Li, Q.; Wang, Y.; Chen, Y.; Guo, X.; Wu, Z.; Chen, G.; Zhong, B.; Xiang, W.; Zhong, Y. A Review of Cathode Materials in Lithium-Sulfur Batteries. Ionics 2020, 26, 5299–5318.
- Li, F.; Liu, Q.; Hu, J.; Feng, Y.; He, P.; Ma, J. Recent Advances in Cathode Materials for Rechargeable Lithium-Sulfur Batteries. Nanoscale 2019, 11, 15418–15439.
- Shao, Q.; Wu, Z.S.; Chen, J. Two-Dimensional Materials for Advanced Li-S Batteries. Energy Storage Mater. 2019, 22, 284–310.
- Dai, C.; Sun, G.; Hu, L.; Xiao, Y.; Zhang, Z.; Qu, L. Recent Progress in Graphene-Based Electrodes for Flexible Batteries. InfoMat 2019, 2, 509–526.
- Fang, R.; Chen, K.; Yin, L.; Sun, Z.; Li, F.; Cheng, H.M. The Regulating Role of Carbon Nanotubes and Graphene in Lithium-Ion and Lithium-Sulfur Batteries. Adv. Mater. 2019, 31, e1800863.
- Wu, S.; Ge, R.; Lu, M.; Xu, R.; Zhang, Z. Graphene-Based Nano-Materials for Lithium–Sulfur Battery and Sodium-ion Battery. Nano Energy 2015, 15, 379–405.
- Sun, C.; Liu, Y.; Sheng, J.; Huang, Q.; Lv, W.; Zhou, G.; Cheng, H.M. Status and Prospects of Porous Graphene Networks for Lithium–Sulfur Batteries. Mater. Horiz. 2020, 7, 2487–2518.
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666.
- Zhao, Y.; Feng, J.; Liu, X.; Wang, F.; Wang, L.; Shi, C.; Huang, L.; Feng, X.; Chen, X.; Xu, L.; et al. Self-Adaptive Strain-Relaxation Optimization for High-Energy Lithium Storage Material through Crumpling of Graphene. Nat. Commun. 2014, 5, 4565.
- Lin, T.; Tang, Y.; Wang, Y.; Bi, H.; Liu, Z.; Huang, F.; Xie, X.; Jiang, M. Scotch-Tape-Like Exfoliation of Graphite Assisted with Elemental Sulfur and Graphene–Sulfur Composites for High-Performance Lithium-Sulfur Batteries. Energy Environ. Sci. 2013, 6.
- Zhang, Y.; Gao, Z.; Song, N.; He, J.; Li, X. Graphene and Its Derivatives in Lithium–Sulfur Batteries. Mater. Today Energy 2018, 9, 319–335.
- Yoo, S.; Lee, J.; Kim, J.M.; Seong, C.Y.; Seong, K.D.; Piao, Y. Well-Dispersed Sulfur Wrapped in Reduced Graphene Oxide Nanoscroll as Cathode Material for Lithium–Sulfur Battery. J. Electroanal. Chem. 2016, 780, 19–25.
- Huang, J.Q.; Liu, X.F.; Zhang, Q.; Chen, C.M.; Zhao, M.Q.; Zhang, S.M.; Zhu, W.; Qian, W.Z.; Wei, F. Entrapment of Sulfur in Hierarchical Porous Graphene for Lithium–Sulfur Batteries with High Rate Performance from −40 to 60°C. Nano Energy 2013, 2, 314–321.
- Liu, Y.; Wang, X.; Dong, Y.; Tang, Y.; Wang, L.; Jia, D.; Zhao, Z.; Qiu, J. Self-Assembled Sulfur/Reduced Graphene Oxide Nanoribbon Paper as a Free-Standing Electrode for High Performance Lithium-Sulfur Batteries. Chem. Commun. 2016, 52, 12825–12828.
- Chen, H.; Wang, C.; Dai, Y.; Qiu, S.; Yang, J.; Lu, W.; Chen, L. Rational Design of Cathode Structure for High Rate Performance Lithium-Sulfur Batteries. Nano Lett. 2015, 15, 5443–5448.
- Yang, J.; Shan, X.; Guo, Z.; Duan, L.; Zhang, X.; Lü, W. A Facile Synthetic Strategy of Free-Standing Holey Graphene Paper as Sulfur Host for High-Performance Flexible Lithium Sulfur Batteries. J. Electroanal. Chem. 2020, 876.
- Wang, C.; Wang, X.; Wang, Y.; Chen, J.; Zhou, H.; Huang, Y. Macroporous Free-Standing Nano-Sulfur/Reduced Graphene Oxide Paper as Stable Cathode for Lithium-Sulfur Battery. Nano Energy 2015, 11, 678–686.
- Cao, J.; Chen, C.; Zhao, Q.; Zhang, N.; Lu, Q.; Wang, X.; Niu, Z.; Chen, J. A Flexible Nanostructured Paper of a Reduced Graphene Oxide-Sulfur Composite for High-Performance Lithium-Sulfur Batteries with Unconventional Configurations. Adv. Mater. 2016, 28, 9629–9636.
- Zhou, G.; Pei, S.; Li, L.; Wang, D.W.; Wang, S.; Huang, K.; Yin, L.C.; Li, F.; Cheng, H.M. A Graphene-Pure-Sulfur Sandwich Structure for Ultrafast, Long-Life Lithium-Sulfur Batteries. Adv. Mater. 2014, 26, 625–631, 664.
- Li, Y.; Guan, Q.; Cheng, J.; Wang, B. Ultrafine Nanosulfur Particles Sandwiched in Little Oxygen-Functionalized Graphene Layers as Cathodes for High Rate and Long-Life Lithium-Sulfur Batteries. Nanotechnology 2020, 31, 245404.
- Papandrea, B.; Xu, X.; Xu, Y.; Chen, C.Y.; Lin, Z.; Wang, G.; Luo, Y.; Liu, M.; Huang, Y.; Mai, L.; et al. Three-Dimensional Graphene Framework with Ultra-High Sulfur Content for a Robust Lithium–Sulfur Battery. Nano Res. 2016, 9, 240–248.
- Fang, R.; Zhao, S.; Pei, S.; Qian, X.; Hou, P.X.; Cheng, H.M.; Liu, C.; Li, F. Toward More Reliable Lithium-Sulfur Batteries: An All-Graphene Cathode Structure. ACS Nano 2016, 10, 8676–8682.
- Xu, G.; Yuan, J.; Geng, X.; Dou, H.; Chen, L.; Yan, X.; Zhu, H. Caterpillar-Like Graphene Confining Sulfur by Restacking Effect for High Performance Lithium Sulfur Batteries. Chem. Eng. J. 2017, 322, 454–462.
- Xu, H.; Deng, Y.; Shi, Z.; Qian, Y.; Meng, Y.; Chen, G. Graphene-Encapsulated Sulfur (GES) Composites with a Core–Shell Structure as Superior Cathode Materials for Lithium-Sulfur Batteries. J. Mater. Chem. A 2013, 1.
- Yeon, J.S.; Yun, S.; Park, J.M.; Park, H.S. Surface-Modified Sulfur Nanorods Immobilized on Radially Assembled Open-Porous Graphene Microspheres for Lithium-Sulfur Batteries. ACS Nano 2019, 13, 5163–5171.
- He, Y.; Bai, S.; Chang, Z.; Li, Q.; Qiao, Y.; Zhou, H. Porous Hybrid Aerogels with Ultrahigh Sulfur Loading for Lithium–Sulfur Batteries. J. Mater. Chem. A 2018, 6, 9032–9040.
- Cavallo, C.; Agostini, M.; Genders, J.P.; Abdelhamid, M.E.; Matic, A. A Free-Standing Reduced Graphene Oxide Aerogel as Supporting Electrode in a Fluorine-Free Li2S8 Catholyte Li-S Battery. J. Power Sour. 2019, 416, 111–117.
- Cui, M.; Zheng, Z.; Wang, J.; Wang, Y.; Zhao, X.; Ma, R.; Liu, J. Rational Design of Lithium-Sulfur Battery Cathodes Based on Differential Atom Electronegativity. Energy Storage Mater. 2021, 35, 577–585.
- Rehman, S.; Guo, S.; Hou, Y. Rational Design of Si/SiO2 @Hierarchical Porous Carbon Spheres as Efficient Polysulfide Reservoirs for High-Performance Li-S Battery. Adv. Mater. 2016, 28, 3167–3172.
- Fan, F.Y.; Chiang, Y.M. Electrodeposition Kinetics in Li-S Batteries: Effects of Low Electrolyte/Sulfur Ratios and Deposition Surface Composition. J. Electrochem. Soc. 2017, 164, A917–A922.
- Chen, X.; Yuan, L.; Hao, Z.; Liu, X.; Xiang, J.; Zhang, Z.; Huang, Y.; Xie, J. Free-Standing Mn3O4@CNF/S Paper Cathodes with High Sulfur Loading for Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 13406–13412.
- Ni, L.; Wu, Z.; Zhao, G.; Sun, C.; Zhou, C.; Gong, X.; Diao, G. Core-Shell Structure and Interaction Mechanism of Gamma-MnO2 Coated Sulfur for Improved Lithium-Sulfur Batteries. Small 2017, 13.
- Hu, B.; Mai, L.; Chen, W.; Yang, F. From MoO3 Nanobelts to MoO2 Nanorods: Structure Transformation and Electrical Transport. ACS Nano 2009, 3, 478–482.
- Al Salem, H.; Babu, G.; Rao, C.V.; Arava, L.M. Electrocatalytic Polysulfide Traps for Controlling Redox Shuttle Process of Li-S Batteries. J. Am. Chem. Soc. 2015, 137, 11542–11545.
- He, Y.B.; Liu, M.; Xu, Z.L.; Zhang, B.; Li, B.; Kang, F.; Kim, J.K. Li-ion Reaction to Improve the Rate Performance of Nanoporous Anatase TiO2 Anodes. Energy Technol. 2013, 1, 668–674.
- Tang, C.; Li, B.Q.; Zhang, Q.; Zhu, L.; Wang, H.F.; Shi, J.L.; Wei, F. CaO-Templated Growth of Hierarchical Porous Graphene for High-Power Lithium-Sulfur Battery Applications. Adv. Funct. Mater. 2016, 26, 577–585.
- Zheng, C.; Niu, S.; Lv, W.; Zhou, G.; Li, J.; Fan, S.; Deng, Y.; Pan, Z.; Li, B.; Kang, F.; et al. Propelling Polysulfides Transformation for High-Rate and Long-Life Lithium–Sulfur Batteries. Nano Energy 2017, 33, 306–312.
- Choi, S.; Seo, D.H.; Kaiser, M.R.; Zhang, C.; van der laan, T.; Han, Z.J.; Bendavid, A.; Guo, X.; Yick, S.; Murdock, A.T.; et al. WO3 Nanolayer Coated 3D-Graphene/Sulfur Composites for High Performance Lithium/Sulfur Batteries. J. Mater. Chem. A 2019, 7, 4596–4603.
- Wei, H.; Ding, Y.; Li, H.; Zhang, Q.; Hu, N.; Wei, L.; Yang, Z. MoS2 Quantum Dots Decorated Reduced Graphene Oxide as a Sulfur Host for Advanced Lithium-Sulfur Batteries. Electrochim. Acta 2019, 327.
- Lin, H.; Yang, L.; Jiang, X.; Li, G.; Zhang, T.; Yao, Q.; Zheng, G.W.; Lee, J.Y. Electrocatalysis of Polysulfide Conversion by Sulfur-Deficient MoS2 Nanoflakes for Lithium–Sulfur Batteries. Energy Environ. Sci. 2017, 10, 1476–1486.
- Park, S.K.; Lee, H.J.; Lee, M.H.; Park, H.S. Hierarchically Structured Reduced Graphene Oxide/WO3 Frameworks for an Application into Lithium ion Battery Anodes. Chem. Eng. J. 2015, 281, 724–729.
- Guan, X.H.; Zhang, Z.W.; Yang, L.; Wang, G.S. One-Pot Hydrothermal Synthesis of Hexagonal WO3 Nanorods/Graphene Composites as High-Performance Electrodes for Supercapacitors. Chempluschem 2017, 82, 1174–1181.
- Wu, X.; Yao, S. Flexible Electrode Materials Based on WO3 Nanotube Bundles for High Performance Energy Storage Devices. Nano Energy 2017, 42, 143–150.
- Liu, X.; Huang, J.Q.; Zhang, Q.; Mai, L. Nanostructured Metal Oxides and Sulfides for Lithium-Sulfur Batteries. Adv. Mater. 2017, 29.
- Song, Y.; Zhao, W.; Zhu, X.; Zhang, L.; Li, Q.; Ding, F.; Liu, Z.; Sun, J. Vanadium Dioxide-Graphene Composite with Ultrafast Anchoring Behavior of Polysulfides for Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 15733–15741.
- Feng, Y.; Liu, H.; Zhao, F.; Liu, Y.; Li, J.; Liu, X. Simultaneous Defect-Engineered and Thiol Modified of MoO2 for Improved Catalytic Activity in Lithium-Sulfur Batteries: A Study of Synergistic Polysulfide Adsorption-Conversion Function. Chem. Eng. J. 2021, 409.
- Deng, C.; Wang, Z.; Wang, S.; Yu, J. Inhibition of Polysulfide Diffusion in Lithium–Sulfur Batteries: Mechanism and Improvement Strategies. J. Mater. Chem. A 2019, 7, 12381–12413.
- Liu, D.; Zhang, C.; Zhou, G.; Lv, W.; Ling, G.; Zhi, L.; Yang, Q.H. Catalytic Effects in Lithium-Sulfur Batteries: Promoted Sulfur Transformation and Reduced Shuttle Effect. Adv. Sci. 2018, 5, 1700270.
- Rout, C.S.; Kim, B.H.; Xu, X.; Yang, J.; Jeong, H.Y.; Odkhuu, D.; Park, N.; Cho, J.; Shin, H.S. Synthesis and Characterization of Patronite Form of Vanadium Sulfide on Graphitic Layer. J. Am. Chem. Soc. 2013, 135, 8720–8725.
- Huo, H.; Zhao, Y.; Xu, C. 3D Ni3S2 Nanosheet Arrays Supported on Ni Foam for High-Performance Supercapacitor and Non-Enzymatic Glucose Detection. J. Mater. Chem. A 2014, 2.
- Yuan, Z.; Peng, H.J.; Hou, T.Z.; Huang, J.Q.; Chen, C.M.; Wang, D.W.; Cheng, X.B.; Wei, F.; Zhang, Q. Powering Lithium-Sulfur Battery Performance by Propelling Polysulfide Redox at Sulfiphilic Hosts. Nano Lett. 2016, 16, 519–527.
- Chang, K.; Mei, Z.; Wang, T.; Kang, Q.; Ouyang, S.; Ye, J. MoS2/Graphene Cocatalyst for Efficient Photocatalytic H2 Evolution under Visible Light Irradiation. ACS Nano 2014, 8, 7078–7087.
- Kibsgaard, J.; Chen, Z.; Reinecke, B.N.; Jaramillo, T.F. Engineering the Surface Structure of MoS2 to Preferentially Expose Active Edge Sites for Electrocatalysis. Nat. Mater. 2012, 11, 963–969.
- Kiriya, D.; Lobaccaro, P.; Nyein, H.Y.; Taheri, P.; Hettick, M.; Shiraki, H.; Sutter-Fella, C.M.; Zhao, P.; Gao, W.; Maboudian, R.; et al. General Thermal Texturization Process of MoS2 for Efficient Electrocatalytic Hydrogen Evolution Reaction. Nano Lett. 2016, 16, 4047–4053.
- Asadi, M.; Kumar, B.; Liu, C.; Phillips, P.; Yasaei, P.; Behranginia, A.; Zapol, P.; Klie, R.F.; Curtiss, L.A.; Salehi-Khojin, A. Cathode Based on Molybdenum Disulfide Nanoflakes for Lithium-Oxygen Batteries. ACS Nano 2016, 10, 2167–2175.
- Tang, W.; Goh, B.M.; Hu, M.Y.; Wan, C.; Tian, B.; Deng, X.; Peng, C.; Lin, M.; Hu, J.Z.; Loh, K.P. In Situ Raman and Nuclear Magnetic Resonance Study of Trapped Lithium in the Solid Electrolyte Interface of Reduced Graphene Oxide. J. Phys. Chem. C 2016, 120, 2600–2608.
- Guo, D.; Zhang, Z.; Xi, B.; Yu, Z.; Zhou, Z.; Chen, X.a. Ni3S2 Anchored to N/S Co-Doped Reduced Graphene Oxide with Highly Pleated Structure as a Sulfur Host for Lithium–Sulfur Batteries. J. Mater. Chem. A 2020, 8, 3834–3844.
- Zhou, G.; Zhao, Y.; Manthiram, A. Dual-Confined Flexible Sulfur Cathodes Encapsulated in Nitrogen-Doped Double-Shelled Hollow Carbon Spheres and Wrapped with Graphene for Li-S Batteries. Adv. Energy Mater. 2015, 5.
- Park, S.K.; Lee, J.; Hwang, T.; Piao, Y. Sulfur-Loaded Monodisperse Carbon Nanocapsules Anchored on Graphene Nanosheets as Cathodes for High Performance Lithium–Sulfur Batteries. J. Mater. Chem. A 2017, 5, 975–981.
- Thieme, S.; Brückner, J.; Bauer, I.; Oschatz, M.; Borchardt, L.; Althues, H.; Kaskel, S. High Capacity Micro-Mesoporous Carbon–Sulfur Nanocomposite Cathodes with Enhanced Cycling Stability Prepared by a Solvent-Free Procedure. J. Mater. Chem. A 2013, 1.
- Su, F.Y.; He, Y.B.; Li, B.; Chen, X.C.; You, C.H.; Wei, W.; Lv, W.; Yang, Q.H.; Kang, F. Could Graphene Construct an Effective Conducting Network in a High-Power Lithium ion Battery? Nano Energy 2012, 1, 429–439.
- Wei, W.; Lv, W.; Wu, M.B.; Su, F.Y.; He, Y.B.; Li, B.; Kang, F.; Yang, Q.H. The Effect of Graphene Wrapping on the Performance of LiFePO4 for a Lithium ion Battery. Carbon 2013, 57, 530–533.
- Zhang, Q.; Huang, J.Q.; Qian, W.Z.; Zhang, Y.Y.; Wei, F. The Road for Nanomaterials Industry: A Review of Carbon Nanotube Production, Post-Treatment, and Bulk Applications for Composites and Energy Storage. Small 2013, 9, 1237–1265.
- Zhang, S.M.; Zhang, Q.; Huang, J.Q.; Liu, X.F.; Zhu, W.; Zhao, M.Q.; Qian, W.Z.; Wei, F. Composite Cathodes Containing Coaxial Nanocables: Facile Synthesis, Surface Modification, and Enhanced Performance for Li-Ion Storage. Part. Part. Syst. Charact. 2013, 30, 158–165.
- Zhao, M.Q.; Liu, X.F.; Zhang, Q.; Tian, G.L.; Huang, J.Q.; Zhu, W.; Wei, F. Graphene/Single-Walled Carbon Nanotube Hybrids: One-Step Catalytic Growth and Applications for High-Rate Li–S Batteries. ACS Nano 2012, 6, 10759–10769.
- Peng, H.J.; Huang, J.Q.; Zhao, M.Q.; Zhang, Q.; Cheng, X.B.; Liu, X.Y.; Qian, W.Z.; Wei, F. Nanoarchitectured Graphene/ Carbon with Extraordinary Electrical Conductivity and Interconnected Micro/Mesopores for Lithium-Sulfur Batteries. Adv. Funct. Mater. 2014, 24, 2772–2781.
- Zhu, L.; Peng, H.J.; Liang, J.; Huang, J.Q.; Chen, C.M.; Guo, X.; Zhu, W.; Li, P.; Zhang, Q. Interconnected Carbon Nanotube/Graphene Nanosphere Scaffolds as Free-Standing Paper Electrode for High-Rate and Ultra-Stable Lithium–Sulfur Batteries. Nano Energy 2015, 11, 746–755.
- Su, D.; Cortie, M.; Wang, G. Fabrication of N-Doped Graphene-Carbon Nanotube Hybrids from Prussian Blue for Lithium-Sulfur Batteries. Adv. Energy Mater. 2017, 7.
- He, J.; Chen, Y.; Li, P.; Fu, F.; Wang, Z.; Zhang, W. Three-Dimensional CNT/Graphene–Sulfur Hybrid Sponges with High Sulfur Loading as Superior-Capacity Cathodes for Lithium–Sulfur Batteries. J. Mater. Chem. A 2015, 3, 18605–18610.
- Jia, J.; Wang, K.; Zhang, X.; Sun, X.; Zhao, H.; Ma, Y. Graphene-Based Hierarchically Micro/Mesoporous Nanocomposites as Sulfur Immobilizers for High-Performance Lithium–Sulfur Batteries. Chem. Mater. 2016, 28, 7864–7871.
- Gómez-Urbano, J.L.; Gómez-Cámer, J.L.; Botas, C.; Rojo, T.; Carriazo, D. Graphene Oxide-Carbon Nanotubes Aerogels with High Sulfur Loadings Suitable as Binder-Free Cathodes for High Performance Lithium Sulfur Batteries. J. Power Sour. 2019, 412, 408–415.
- Wen, X.; Xiang, K.; Zhu, Y.; Xiao, L.; Liao, H.; Chen, W.; Chen, X.; Chen, H. 3D Hierarchical Nitrogen-Doped Graphene/CNTs Microspheres as a Sulfur Host for High-Performance Lithium-Sulfur Batteries. J. Alloys Compd. 2020, 815.
- Wu, H.; Xia, L.; Ren, J.; Zheng, Q.; Xu, C.; Lin, D. A High-Efficiency N/P Co-Doped Graphene/ Carbon Hybrid Matrix as a Cathode Host for High Performance Lithium–Sulfur Batteries. J. Mater. Chem. A 2017, 5, 20458–20472.
- Xu, H.; Jiang, Q.; Zhang, B.; Chen, C.; Lin, Z. Integrating Conductivity, Immobility, and Catalytic Ability into High-N Carbon/Graphene Sheets as an Effective Sulfur Host. Adv. Mater. 2020, 32, e1906357.
- Lee, J.; Park, S.K.; Piao, Y. N-doped Carbon Framework/Reduced Graphene Oxide Nanocomposite as a Sulfur Reservoir for Lithium-Sulfur Batteries. Electrochim. Acta 2016, 222, 1345–1353.
- Sun, J.; Liu, Y.; Du, H.; He, S.; Liu, L.; Fu, Z.; Xie, L.; Ai, W.; Huang, W. Molecularly Designed N, S Co-Doped Carbon Nanowalls Decorated on Graphene as a Highly Efficient Sulfur Reservoir for Li–S Batteries: A Supramolecular Strategy. J. Mater. Chem. A 2020, 8, 5449–5457.
