We utilized Rapha Myr®, a novel blend of broccoli seed extract (Brassica Oleracea s.e., Sulforaphane glucosinolate titer 11%) plus active myrosinase, to treat the human astrocytoma cell line (1321N1). Rapha Myr® exhibited low antioxidant capability and exerted antiproliferative and genotoxic effects on 1321N1 cells by blocking the cell cycle, disarranging cytoskeleton structure and focal adhesions, decreasing the integrin α5 expression, renewing anoikis and modulating some important epigenetic pathways independently of the cellular p53 status. In addition, Rapha Myr® suppresses the expression of the oncogenic p53 mutant protein. These findings promote Rapha Myr® as a promising chemotherapeutic agent for integrated cancer therapy of human astrocytoma.
- brain cancer
- astrocytoma 1321N1 cells
- sulforaphane
- myrosinase
- cytoskeleton morphology
- cell migration
- anoikis
- apoptosis
- oxidative stress
- global DNA methylation
- sirtuins
- mutated p53 R213Q
1. Introduction
Currently, the strategies involved in the prevention, progression, remission and relapse of cancer represent the key priority aims in public health worldwide [1][2].
Anaplastic astrocytomas and glioblastomas (GBM) represent the most frequent and aggressive primary malignancies of the central nervous system. So far, they are incurable diseases due mostly to their highly invasive phenotype, and the poor efficiency of the available therapies further reduced by the blood brain barrier that limits the delivery of drugs into the brain. Moreover, the current chemotherapy may lead to drug resistance in GBM treatment. Therefore, the treatment of these tumors constitutes a fundamental challenge for neuro-oncology and there is an urgent need to fully understand the molecular background of these cancers and to develop new promising therapeutic strategies [3].
The hallmarks of tumorigenesis and cancer progression are genetic instability, deregulation of epigenetic mechanisms, oxidative homeostasis, cell cycle progression, tumor microenvironment, and cell death program activation (e.g., the apoptotic cascade) [4]. Anoikis, a greek word meaning loss of “home” or “homelessness”, is a specific form of caspase-mediated programmed cell death that occurs when the cell cycle is arrested due to the loss of the normal cell–matrix interactions. Anoikis represents an essential defense for the organism by inhibiting detached cells’ re-adhesion to new matrices in inappropriate environments and their dysplastic growth. As a result, the resistance to anoikis program execution, an emerging hallmark of cancer cells, is a crucial step in the acquisition of malignancy as cancer cells can migrate throughout the body, spread into the surrounding tissues and distant organs, and grow metastatically [5]. A main role in anoikis is played by Integrins, surface receptors composed of two non-covalently associated α and β subunits that sense the extracellular matrix (ECM) and connect it to the cytoskeleton, therefore regulating many important cell processes. In adherent cells, in fact, cell-specific activation of Integrins and their downstream signaling mediators are responsible for anoikis protection by transducing signals, which are necessary for cell proliferation, survival and migration, from the extracellular matrix (ECM) to the cell [5][6]. Moreover, unligated Integrins can also act as cell-death promoters, through a process named ‘integrin-mediated death’ (IMD). In neuroblastoma cells, anoikis obtained by unligated integrin-induced caspase 8 activation prevents metastasis and the caspase-8 suppression is associated with increased invasive capacity in vivo [7].
In addition, a body of literature data highlights that epigenetic aberrations such as alterations in chromatin state, DNA methylation, histone modifications and the posttranscriptional regulation of gene expression by miRNAs play a crucial role in the tumor suppressor silencing, oncogene activation, or in the cell fate transitions, thus giving rise to all of the classic hallmarks of cancer [8][9][10].
All these aspects of cancer biology are widely regulated by environmental and lifestyle factors including physical activity, nutrition, dietary supplements (herbs, nutraceuticals and phytochemicals) and synthetic agents. Many natural bioactive compounds have demonstrated their capability in counteracting tumorigenesis as well as tumor progression in various cancer types, through their impact on genomic instability, tumor-promoting inflammation, dysregulated metabolism, immune system evasion and epigenetics [4][11][12]. These findings have encouraged the research and the use of diet and/or nutraceutical supplementation as new integrated therapies in primary cancer prevention, in improving the efficiency of chemotherapy and in survivors’ management [13].
Cruciferous vegetables (broccoli, broccoli sprouts, cabbage or kale), a rich source of glucosinolates, which are metabolized to isothiocyanate compounds (ITCs), exert many anticancer effects both in vitro and in vivo models [14]. Sulforaphane (1-siothiocyanato-4-methylsulfinylbutane, SFN), obtained from myrosinase-catalyzed hydrolysis of glucoraphanin (GR), is one of the major ITCs that regulates carcinogenesis through the targeting of multiple mechanisms within the cell [15].
Notably, SFN exerts its effects through the Nrf2-mediated induction of phase II detoxification enzymes, with the suppression of oxidative stress-induced DNA damage, inhibition of proliferation, histones modulation, anti-inflammatory and pro-apoptotic activity [15][16][17][18][19]. Moreover, SFN is able to enhance the anticancer activity of chemotherapeutic agents [20][21] and to renew anoikis resistance in many common cancers, e.g., prostate, lung and colorectal cancers [22][23][24].
Although SFN is water-soluble and well absorbed after oral administration both in humans and animals [25][26], the SFN bioavailability from cruciferous vegetables varies widely based upon many factors, mainly stemming from the conversion rate of GR to SNF by the enzyme myrosinase. This plant enzyme is commonly segregated from the glucosinolates until the cells are disrupted and is also produced by gut microbiome [27]. The inactivation of myrosinase, as it may be in extract supplementation, significantly reduces SFN absorption [28].
Sita et al. (2018) reviewed a number of studies reporting the use of adjunctive SFN therapy in GBM for better targeting resistance and synergistically complementing and improving standard treatments as it induces apoptosis, and inhibits both growth and invasion of GBM cells [3].
Moreover, we chose the 1321N1 cell line with mutated cellular tumor antigen p53 (mut p53 R213Q) as a model, because the p53 gene is mutated in approximately 40% of astrocytic tumours and dissection of the molecular pathogenesis of astrocytic tumors has identified defects in the p53 pathway as one of the most common molecular alterations observed in human astrocytoma involved in both the early transforming events and progression from low-grade to high-grade neoplasms. The functional elimination of these critical cell cycle regulatory and apoptosis-inducing factors is believed to contribute to the aggressive and invasive nature of these tumors [29].
For the first time, we utilized Rapha Myr®, a novel blend of broccoli seed extract (Brassica Oleracea s.e., Sulforaphane glucosinolate titer 11%) plus active myrosinase, to treat the human astrocytoma cell line (1321N1). In the present study, we investigated the anticancer activity of Rapha Myr®, demonstrating that Rapha Myr® elicited antiproliferative effects by inducing cell cycle arrest, oxidative stress and genotoxicity accompanied by global DNA hypermethylation and increased levels of DNA methyltransferase 1 (DNMT1), and changes in sirtuins’ expression and activity. Moreover, after Rapha Myr® treatment, the cells lose migratory and proliferative properties as proved by cell migration inhibition, cytoskeleton network destructuration, and the blocking of integrin α5 translocation and expression. As result, the cell cycle is arrested and an anoikis-like death is induced via p53-independent mechanisms and under the epigenetic control of gene expression.
2. Antioxidant Capability of Rapha Myr®
The results of antioxidant capability refer to different concentrations of Rapha Myr® extract measured by DPPH assay and are reported in Figure 1. The data shows that an important antioxidant activity is exhibited only at a concentration of Rapha Myr® higher than 2.5% v/v.
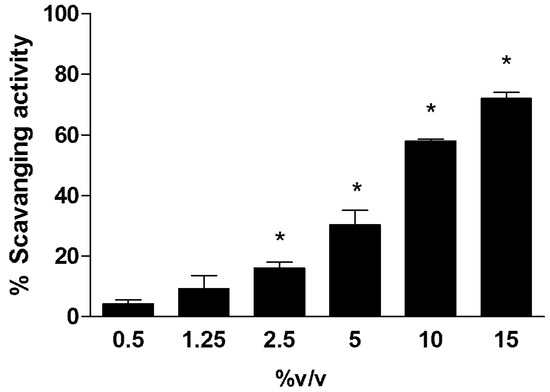
Figure 1. Antioxidant capability of Rapha Myr® extract (0.5–1.25–2.5–5–10–15% v/v) evaluated by DPPH assay. The scavenging activity was expressed as percentage decrease in absorbance with respect to control. Values are mean ± SD of three experiments in triplicate. * p < 0.05 vs. control.
3. MTT Assay, Cell Morphological Analysis, DNA Integrity and Redox Status
We compared the cytotoxicity of Rapha Myr® extract in tumour and non-tumour cells by evaluating the IC50 values and cell morphology in 1321N1 (human astrocytoma cell line), U87 (human glioblastoma cell line), SHSY5Y (human neuroblastoma cell line) and HFF1 (Human Foreskin Fibroblast cell line).
MTT assay was performed on all cell lines treated with Rapha Myr® extract (0.5–10% v/v) for 24 h. The treatment of both glioma cells (1321N1 and U87) and neuroblastoma cells (SHSY5Y) with Rapha Myr® extract results in various degrees of inhibition of cell viability based on the extract concentration. The results highlight that Rapha Myr® extract mainly reduces, in a dose-dependent manner, the viability of both 1321N1 astrocytoma cells and SHSY5Y neuroblastoma cells compared with U87 glioblastoma cells (Figure 2A). The IC50 values of Rapha Myr® extract are 2.95%, 2.72% and 4.66% in these tumour cell lines after 24 h, respectively. The IC50 value of Rapha Myr® extract is 9.93% in the HFF1 non-tumour cell line, suggesting that Rapha Myr® is cytotoxic towards cancer cells.
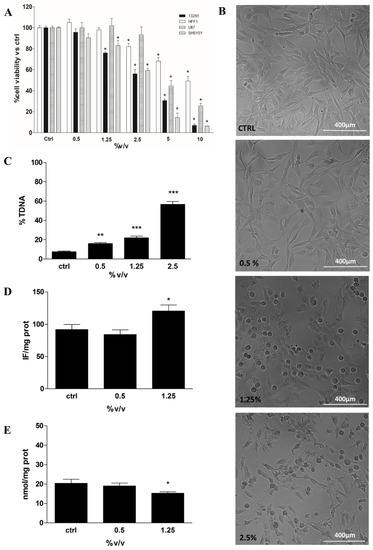
Figure 2. Cell viability, cell morphological analysis, DNA integrity and Redox Status in 1321N1 cells untreated and treated for 24 h with different concentrations of Rapha Myr® extract depending on each cell-based assay protocol. (A) Cell viability in HFF1,1321N1, SHSY5Y and U87 cells evaluated by MTT assay. The results were expressed as a percentage of cell viability compared to untreated control viable cells, which had a value equal to 100%. (B) Representative images of 1321N1 cell morphology acquired by optical inverted light microscopy. Original magnification 10×, scale bar 400 µm. (C) DNA damage was evaluated by alkaline comet assay. The results were expressed as the percentage of DNA present in the comet tail (%TDNA). (D) Intracellular reactive oxygen species (ROS) levels evaluated by using 2′,7′-dichlorofluorescein diacetate (DCFH-DA); the results were expressed as intensity of fluorescence (IF) per mg protein. (E) Non-protein thiol levels in 1321N1 cells; the results were expressed as nmol GSH/mg proteins. All values are mean ± SD of three experiments in triplicate. * p < 0.05 vs. control; ** p < 0.01 vs. control; *** p < 0.001 vs. control.
Moreover, a morphological change in 1321N1 was induced by exposure to Rapha Myr® extract (0.5–1.25–2.5% v/v) for 24 h (Figure 2B). Mainly at 1.25 and 2.5% v/v, the cells did not grow on the plate surface uniformly but were dispersed, showing an altered shape and a reduced proliferation compared with the control cells. Rapha Myr® 2.5% induced the death of cancer cells, with many cells floating in the medium. Besides, the inverted light microscope images of SHSY5Y and U87 tumour cells show dose-dependent morphological modifications (rounding, shrinkage and reduced adhesion) upon 24 h exposure to Rapha Myr® (0.5–1.25–2.5% v/v), which are more evident in SHY5Y (Figures S1 and S2). Conversely, the viability, cell morphology and adhesion of HFF1 normal cells were not affected by the Rapha Myr® concentrations that were effective on cancer cells (1321N1 ≥ SHSY5Y >> U87 > HFF1) (Figure S3).
Taking in to account these preliminary results and considering drug resistance of brain tumours harbouring mutated p53 [29], we selected the human astrocytoma 1321N1 cell line to test the biological effects of Rapha Myr®.
The genotoxicity of Rapha Myr® extract (0.5–1.25–2.5% v/v) was evaluated on 1321N1 by alkaline comet assay in order to assess whether our extract could cause DNA damage responsible for its anti-tumour effects. After 24 h of treatment, a dose-dependent increase in DNA damage was observed, being 16.05%, 22.15% and 56.66%, respectively (Figure 2C).
The involvement of redox status has been investigated by the determination of both ROS (DCFDA assay) and non-protein thiols levels (DTNB method). The data indicates that Rapha Myr® extract increases ROS production and concurrently decreases GSH levels in human astrocytoma 1321N1 cells (Figure 2D,E).
4. Cell Migration Inhibition and Cytoskeleton Structure Alteration
Figure 3A shows the results of cell migration analysis by wound healing assay on 1321N1 untreated and treated with different concentrations of Rapha Myr® extract for 72 h. The width and the closure capacity of the wound, relative to initial width, was measured at different time points (0, 24, 48 and 72 h). The results point out that wound healing capacity was severely reduced in Rapha Myr®-treated cells compared with untreated control cells. Particularly, Rapha Myr® extract 1.25% v/v totally inhibited the wound closure. (Figure 3B).
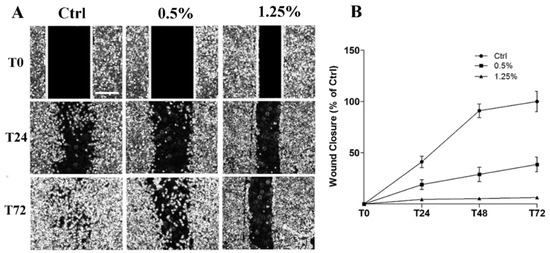
Figure 3. Cell migration evaluated by Wound Healing assay in 1321N1 cells untreated and treated with different concentrations of Rapha Myr® extract (0.5 and 1.25% v/v). (A) Phase contrast microscope images at 0, 24 and 72 h post-scratch. Original magnification 4×, scale bar 1000 µm. (B) The migratory capacity of 1321N1 cells was measured at 24, 48 and 72 h as the percentage of wound closure compared to time 0 h when the scratch was made.
Cell migration is a complex multi-step process, also driven by cytoskeleton proteins, utilized by cancer cells to invade and metastasize other tissues. The structural analysis of the cytoskeleton by immunofluorescence after 24 h and 72 h exposure to Rapha Myr® (0.5% and 1.25%) is reported in Figure 4 and Figure 5. The cytoskeleton of untreated astrocytoma cells shows microfilaments organized in stress fibers, filipodia and lamellipodia, and in the cortex (Figure 4A and Figure 5A). Microtubules are assembled into a fine fibrillary network, and some normal mitotic spindles are also visible (Figure 4D). At 72 h, the cell monolayer still shows an apparently continuous microtubular network that is an evident sign of cell–cell adhesion (Figure 5D).
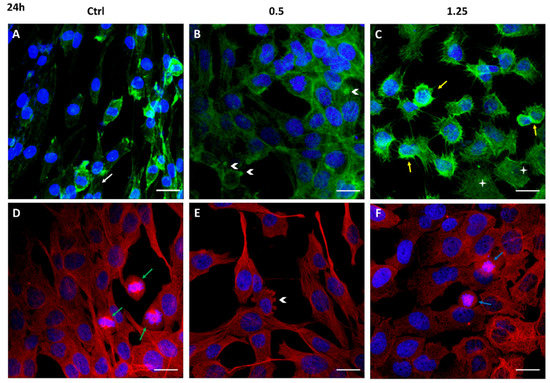
Figure 4. Cytoskeleton structure analysis of 1321N1 cells untreated (A,D) and treated with 0.5% (B,F) and 1.25% v/v (C,D) Rapha Myr® extract for 24 h. Representative IF images for actin stained with FITC-Phalloidin (green; A,B,C), microtubules stained with anti–α-tubulin antibody (red; D,E,F) and nuclei stained with DAPI (blue), a merge was made. White arrows: stress fibers; yellow arrows: cellular cortex; arrowheads: blebs; green arrows: mitosis; blue arrows: abnormal mitosis. Scale Bar: 20 μm.
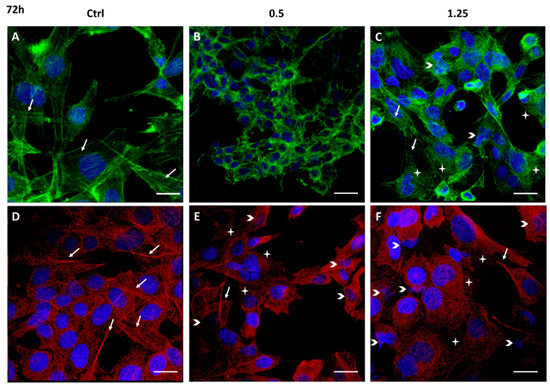
Figure 5. Cytoskeleton structure analysis of 1321N1 cells untreated (A,D) and treated with 0.5% (B,E) and 1.25% v/v (C,F) Rapha Myr® extract for 72 h. Representative IF images for actin stained with FITC-Phalloidin (green; A,B,C), microtubules stained with anti–α-tubulin antibody (red; D,E,F) and nuclei with DAPI (blue); a merge was made. White arrows: stress fibers in green fluorescent images and bundles of microtubules in red fluorescent images; arrowheads: small or misshapen nuclei; stars: disorganized actin or microtubule network. Scale Bar: 20 μm.
After 24 h of treatment with Rapha Myr® 0.5% v/v and 1.25% v/v, the cell architecture is strongly altered. In particular, at 0.5% v/v the cell cortex appears more evident around the small nucleus while microfilaments are depolymerized in the cytoplasm of cells flattened onto substratum (Figure 4B). The microtubular network is compact, forming thick bundles above all in the cytoplasmatic protrusion (Figure 4E). The plasma membrane shows some blebs fluorescent in green (FITC-Phalloidin) or red (Alexa Fluor 594).
After treatment with Rapha Myr® 1.25% v/v, the microfilaments thicken in the cell cortex and in the numerous thin filopodia protruding radially from the cell body, indicating that the cell migratory capacity and the adhesion onto the substrate are impaired. Spread cells show a pale-green cytoplasmic fluorescence, an evident sign of F-actin depolymerization (Figure 4C). Conversely, microtubules are irregularly localized in the cytoplasm and around the nucleus (Figure 4F). The inhibition of microtubule function results in abnormal mitotic spindles (Figure S4), multinucleated cells and decondensed nuclei (Figure 4F).
All concentrations of Rapha Myr® extract cause strong changes in the cytoskeleton at 72 h (Figure 5B,C,E,F). At 0.5%, microfilaments and microtubules appear strictly condensed around nuclei that are smaller than control cells (Figure 5B,E). After Rapha Myr® 1.25%, the actin cortex is well-organized only in rounded cells while the green fluorescence is weak and widespread in the cytoplasm of spreading cells (Figure 5C). The microtubule network is totally disarranged and tubulin appears like bright fluorescent spots instead of microtubules (Figure 5F). Moreover, nuclei of different shapes and sizes were observed and nuclear chromatin fragmentation was detected, a clear feature of apoptosis (Figure S5).
5. Discussion
Cancer treatment is still a major challenge; in fact, although chemotherapy/radiotherapy are the major therapeutic approaches, their efficiency is limited by toxicity and poor utilization of personalized oncology therapies [30][31]. Moreover, the “resistance to anoikis”, a peculiar kind of apoptosis characterized by anchorage-independent growth of cancer cells, is now considered a hallmark of cancer cells [32]. Surprisingly, literature data suggests that both the introduction of appropriate nutrients, both by diet or as supplements, and improving lifestyle could prevent 30–40% of cancers, improve responses to conventional therapies and reduce their adverse effects [33]. Among the different valuable nutrients, sulforaphane (SFN) has largely demonstrated its beneficial potentiality in prevention and in multistage cancer development, by affecting important cellular mechanisms including the epigenetic pathway modulation, the sensitivity restoration to anoikis in some cancer cell types and animal models, the alteration in cell cycle control with p21 up-regulation and a block in the PI3K/Akt signaling pathway [12][15][21][22][34]. Several studies also highlight the potent activity of SFN versus the most malignant brain tumor, glioblastoma, targeting apoptosis and cell survival pathways [3].
At the beginning, we carried out the evaluation of viability and cell morphology on three brain tumor cell lines (1321N1, U87 and SHSY5Y) and one normal cell line (HFF1), demonstrating that Rapha Myr® is safer for normal cells and more active against tumor cells, mainly to both astrocytoma (1321N1) and neuroblastoma (SHSY5Y) cells. Although a lot of studies report the use of adjunctive SFN therapy for targeting glioblastoma, no evidence supports its use in astrocytoma treatment [3]. Moreover, we decided to choose the 1321N1 cell line as a model with mut p53 R213Q since about 40% of astrocytic tumours express mutant forms of p53 but its role in the molecular pathogenesis of these tumours deserves further study [29].
To the best of our knowledge, this is the first study that investigates 1321N1 human astrocytoma cell lines harbouring mut p53, the effect of Rapha Myr®, a novel blend of sulforaphane and hydrolytic enzymes myrosinase capable of improving isothiocyanates’ bioavailability [15]. Our experimental setup tackled some aspects implicated in the potential chemotherapeutic activity of Rapha Myr®, focusing on some pathways involved in cytotoxicity and DNA damage, as well as cell cycle arrest, disarrangement of cytoskeleton and epigenetic modulation.
In this study, we found that Rapha Myr® may be a promising anticancer compound for astrocytoma mostly due to anoikis induction. Rapha Myr® exerted it effects in a dose-dependent manner, particularly, after 2.5% treatment most cells changed their morphology, floated in the medium and then underwent apoptosis. Rapha Myr®-induced anoikis may be associated with cytoarchitecture alteration at 1.25%, as shown by immunofluorescence analysis. In astrocytoma cells, we demonstrated that Rapha Myr® provoked protein–cytoskeletal reorganization with growth arrest, shape modifications and inhibition of migration capability, probably resulting from its effects on the microtubule polymerization by interaction with cysteine residues of tubulin [35] and on the Rho pathway alteration leading to the disarrangement of the actin-cytoskeleton [36]. Our results agree with previous data reporting that sulforaphane causes changes in the shape and migration processes of cells, altering the enormous dynamism of actin, and influences microtubule polymerization and depolymerization, regulating cell polarity, mitosis and cell motility. In addition, sulforaphane acts as an inhibitor of microtubule polymerization in rapidly growing colon cancer cells with a block of proliferation and cell cycle arrest [37].
It is well known that tumour cells escape anoikis through diverse mechanisms such as changes in the integrins’ repertoire, the activation of oncogenes and pro-survival signal pathways, or upregulation of growth factor receptor and their transduction pathways. Notably, integrins play a role in cell growth, differentiation, and death by regulating the interaction between cell and ECM [5][6]. In GBM α6β4, α5β1, αvβ6, and αvβ3 are upregulated and correlated with poor patient survival [38]. Besides, α5β1 integrin reduces GBM cell proliferation when inhibited [39] and was shown to mediate EMT in GBM cells [40] which is one of the strategies adopted by cancer cells to avoid anoikis.
In the extracellular matrix, the molecules of fibronectin assemble into fibrils only on the cell surface in a process guided by additional proteins, especially Integrin α5β1, which mediates the interactions between the extracellular fibronectin fibrils and intracellular actin filaments and promotes fibronectin fibril polymerization and matrix assembly [41].
In our study, the distension of untreated 1321N1 cells on ECM-coated surface was clearly mediated by cytoskeletal components, mainly microfilaments. At the same time, integrin α5 allowed cells to anchor onto ECM by arranging correctly on the entire cell surface. Unlike the untreated cells, the exposure to Rapha Myr® 2.5% for 72 h inhibited integrin α5 translocation from the cell center towards the cell surface, and reduced its expression in a dose-dependent manner. We can speculate that the destructive effect of Rapha Myr® on actin polymerization may be responsible for the alteration in adhesion molecules’ pattern, which is associated with the reduced adhesion to ECM due to the absence of microfilaments organized in stress fibers, as demonstrated by immunofluorescence. Consequently, the loss of adhesion may lead to cell cycle arrest and anoikis of 1321N1, as observed by us.
We also found a reduction in proliferation and cell cycle block as a consequence of genomic DNA damage. Consistent with this, we observed a high DNA fragmentation associated with a moderate cleavage of PARP-1, a hallmark of apoptosis, and the increase in γH2AX level indicating that Rapha Myr® increased double strand break damage. Many anticancer therapies (i.e., radiation or drugs) induce DNA damage directly or interfere with DNA metabolism achieving DNA double-strand breaks (DSBs) responsible for apoptotic cell death. γH2AX protein is one of the main actors in DNA repair and damage response (DDR) pathways forming a focus at the DSB site. PARPs are also activated by DNA damage including DNA single- and double-strand breaks [42].
One of the mechanisms associated with SFN cytotoxic activity in HeLa, HCT116 and HT29 cell lines is its genotoxicity [43][44]. For instance, DNA damage, ATM and Chk2 kinase activation, γH2AX nuclear foci formation and subsequent G2/M cell cycle arrest have been observed in SFN-treated prostate cancer cells [45][46]. Similarly to these previous studies, here we also documented a cell cycle arrest at the G2/M phase following treatment with 2.5% Rapha Myr®. All these alterations triggered proapoptotic signals in a concentration-dependent way, which results in apoptotic cell death, as indicated by the increase in percentage of apoptotic 1321N1 cells exposed to 2.5% of Rapha Myr®. Furthermore, the investigation of the molecular mechanism involved in 1321N1 cell cycle and cell death by studying expression levels of p53, the “guardian of genome”, and its downstream effector p21, the cyclin-dependent kinase inhibitor, allows us to demonstrate that Rapha Myr® would seem to regulate both these cellular processes in a p53-independent manner. P53 gene is frequently mutated in human cancer and the mutant p53 proteins not only lose their tumor-suppressor function, but may also gain new oncogenic functions and promote tumorigenesis [47]. This potential mechanism activated by Rapha Myr® can be related to the mutation state of p53, in fact, it is reported that the mutation c.638G > A (p.R213Q) is present in 1321N1 cells, severely compromising the capability to induce p53-regulated genes; mainly harbouring amino acid changes in its DNA-binding domain, it does not transactivate p21 [48][49]. In our model, the oncogenic function of mutant p53-R213Q may promote both cell survival and anoikis resistance of 1321N1 cancer cells, also being less efficient in binding the consensus sequence in the p21 gene-regulatory region [49]. This supports our result showing a reduction in p21 at 1.25% concentration of Rapha Myr ®, despite the presence of p53 (Figure 8H). Rapha Myr®.2.5% also decreases the concentration of the oncogenic p53 mutant protein and several papers report that SFN-mediated apoptosis was independent of a mutated p53 status [50]. At the same time, Rapha Myr®.2.5% increases the p21 levels with respect to 1.25%, thus inhibiting survival by induction of cell cycle arrest, apoptosis and anoikis. Our data partially agrees with previous studies demonstrating that p53 and p21 are involved in cell detachment and anoikis resistance, as well as that the anoikis escaping from cancer cells is favored either by suppression of p53 activation or p53 mutations [49][50][51]. In addition, Tsai and colleagues showed that A549 cells with wild-type p53 are more sensitive to SFN-induced anoikis than p53-mutant CL1–5 cells and also demonstrate that p53 and its downstream effector p21 are negative regulators of anchorage-independent growth [22]. However, p21 can be transactivated by other transcription factors than p53. In our model, it seems likely that other factors might contribute to p21 regulation via different mechanisms, independent of p53, to convey different oncosuppressive signals able to hinder aggressiveness in human cancers [52]. We think that the effects observed may not only be linked to a reduction in p53 mutant form or dephosphorylation of p53 influencing other p53 targets, but also to direct and/or indirect action of Rapha Myr®; for instance, its impact on cellular oxidative balance with the increase in ROS production and the reduction in free thiols groups, as observed by us. Many authors showed that p53 mutant isoforms maintain high ROS levels in cancer cells through a coordinated control of various redox-related enzymes and signaling pathways, thus favoring cancer cell proliferation [53][54]. We can speculate that 131N1 cancer cells expressing mutant p53 proteins can be significantly more sensitive to a pro-oxidant environment, thus Rapha Myr® induced an anoikis-like death by provoking redox unbalance following modulation of both mutant p53 isoform and p21 expression. Intriguingly, this cellular context driven by inhibition of mutant p53 might be responsible for the cytoskeleton disarrangement and inhibition of cell migration contributing to the promotion of anoikis-like apoptosis and reducing metastatic spread.
A significant amount of evidence indicates that SFN also promotes secondary chemoprevention by targeting the transcription of apoptotic and cell cycle arrest genes through regulating activities or expression of DNMTs and HDACs that modify cancer’s epigenetic signature [11][34]. However, there appears to be a dearth of studies investigating the role of sulforaphane as an epigenetic modulator in brain cancer.
Rapha Myr® induces the upregulation of DNA methyltransferase1 (DNMT1) mRNA level and global DNA hypermethylation, unlike the results from other authors reporting that SFN induces DNA hypomethylation and decreased levels of DNMT1 in several types of cancer cells (breast, prostate, colon and liver) [55]. Among epigenetic markers, the sirtuins, a family of NAD+ −dependent histone deacetylases comprising seven proteins from Sirt1 to Sirt7, are involved in many crucial cellular processes [56][57]. Their role in cancer is dual depending on the type of tumor, stage and microenvironment. Notably, sirtuins promote migration, growth, epithelial–mesenchymal transition (EMT) and metastasis [57][58]. We demonstrated that Rapha Myr® influences most of the sirtuins except the mitochondrial SIRT3, considered an EMT-repressor and tumor-suppressor protein principally regulating oxidative response, energetic balance, and cellular metabolism, thus promoting genomic and mitochondrial DNA instability. Although the involvement of Sirt 5 in cancer has not yet been investigated, we found out Rapha Myr® modulates its expression, reducing it at its highest concentration [59].
As regards nuclear sirtuins, both Sirt6 and Sirt 7 are considered well-established tumor suppressors and when they are upregulated, they induce apoptosis and cell cycle arrest through HIF1/2 modulation [58][60]. Moreover, Sirt7 is an EMT-promoter. Unlike the literature data, our extract reduces both Sirt6 and Sirt7 expression in astrocytoma cells. Sirt1 instead plays a dual role in tumorigenesis and EMT. Numerous studies report Sirt1 as tumor promoter in several malignant cancers such as breast, colon and prostate cancer. Moreover, SIRT1 also acts as a tumor suppressor directly or by repressing other oncogenes (e.g., Myc). Consistent with this, some tumors including glioma, bladder and ovarian cancer show a low level of SIRT1 [59]. In the context of EMT, SIRT1 has an ambivalent role both as EMT-promoter and EMT-suppressor. Sirt1 increases cellular resistance to apoptosis, senescence, and anoikis and promotes migration and invasion, for instance in both gastric and gastroesophageal junction cancer. On the other hand, Sirt1 suppresses migration and invasion in oral squamous cell carcinoma, lung and ovarian cancer [58].
As regards the nuclear Sirt1, we only observed an increase in the phosphorylated form expression and in the ratio between pSirt1/Sirt1 as well (Figure 9G,H); this data is supported by a moderately significant increase in nuclear activity (Figure 9I). Studies point out that SIRT1 phosphorylation played an important role in regulating the activity of SIRT1 deacetylation, which is responsible for most nuclear deacetylation activity inside the cell [59][61].
All together, the results on epigenoma, stimulating future research direction, suggest Rapha Myr® extract as an epi-drug that could be utilized in human astrocytoma as an integrated therapeutic compound able to modulate cancer development through epigenetic regulation mainly mediated by sirtuins.
This entry is adapted from the peer-reviewed paper 10.3390/ijms21155328
References
- Loomans-Kropp, H.A.; Umar, A. Cancer prevention and screening: The next step in the era of precision medicine. npj Precis. Oncol. 2019, 3, 1–8.
- Palumbo, M.O.; Kavan, P.; Miller, W.H., Jr.; Panasci, L.; Assouline, S.; Johnson, N.; Cohen, V.; Patenaude, F.; Pollak, M.; Jagoe, R.T.; et al. Systemic cancer therapy: Achievements and challenges that lie ahead. Front. Pharmacol. 2013, 4, 57.
- Sita, G.; Hrelia, P.; Graziosi, A.; Morroni, F. Sulforaphane from Cruciferous Vegetables: Recent Advances to Improve Glioblastoma Treatment. Nutrients 2018, 10, 1755.
- Block, K.I.; Gyllenhaal, C.; Lowe, L.; Amedei, A.; Amin, A.R.M.R.; Amin, A.; Aquilano, K.; Arbiser, J.; Arreola, A.; Arzumanyan, A. Designing a broad-spectrum integrated approach for cancer prevention and treatment. Semin. Cancer Biol. 2015, 35, S276–S304.
- Guadamillas, M.C.; Cerezo, A.; del Pozo, M.A. Overcoming anoikis-pathways to anchorage-independent growth in cancer. J. Cell Sci. 2011, 124, 3189–3197.
- Paoli, P.; Giannoni, E.; Chiarugi, P. Anoikis molecular pathways and its role in cancer progression. Biochim. Biophys. Acta 2013, 1833, 3481–3498.
- Stupack, D.G.; Teitz, T.; Potter, M.D.; Mikolon, D.; Houghton, P.J.; Kidd, V.J.; Lahti, J.M.; Cheresh, D.A. Potentiation of neuroblastoma metastasis by loss of caspase-8. Nature 2006, 439, 95–99.
- Flavahan, W.A.; Gaskell, E.; Bernstein, B.E. Epigenetic plasticity and the hallmarks of cancer. Science 2017, 357, eaal2380.
- Kanwala, R.; Gupta, S. Epigenetic modifications in cancer. Clin. Genet. 2012, 81, 303–311.
- Falzone, L.; Romano, G.L.; Salemi, R.; Bucolo, C.; Tomasello, B.; Lupo, G.; Anfuso, C.A.; Spandidos, D.A.; Libra, M.; Candido, S. Prognostic significance of deregulated microRNAs in uveal melanomas. Mol. Med. Rep. 2019, 19, 2599–2610.
- Carlos-Reyes, Á.; López-González, J.S.; Meneses-Flores, M.; Gallardo-Rincón, D.; Ruíz-García, E.; Marchat, L.A.; Astudillo-de la Vega, H.; Hernández de la Cruz, O.N.; López-Camarillo, C. Dietary Compounds as Epigenetic Modulating Agents in Cancer. Front. Genet. 2019, 10, 79.
- Shukla, S.; Penta, D.; Mondal, P.; Meeran, S.M. Epigenetics of Breast Cancer: Clinical Status of Epi-drugs and Phytochemicals. Adv. Exp. Med. Biol. 2019, 1152, 293–310.
- Ranjan, A.; Ramachandran, S.; Gupta, N.; Kaushik, I.; Wright, S.; Srivastava, S.; Das, H.; Srivastava, S.; Prasad, S.; Srivastava, S.K. Role of Phytochemicals in Cancer Prevention. Int. J. Mol. Sci. 2019, 20, 4981.
- Wu, X.; Zhou, Q.H.; Xu, K. Are isothiocyanates potential anti-cancer drugs? Acta Pharmacol. Sin. 2009, 30, 501–512.
- Bayat Mokhtari, R.; Baluch, N.; Homayouni, T.S.; Morgatskaya, E.; Kumar, S.; Kazemi, P.; Yeger, H. The role of Sulforaphane in cancer chemoprevention and health benefits: A mini-review. J. Cell Commun. Signal. 2018, 12, 91–101.
- Kaufman-Szymczyk, A.; Majewski, G.; Lubecka-Pietruszewska, K.; Fabianowska-Majewska, K. The Role of Sulforaphane in Epigenetic Mechanisms, Including Interdependence between Histone Modification and DNA Methylation. Int. J. Mol. Sci. 2015, 16, 29732–29743.
- Liu, P.; Atkinson, S.J.; Akbareian, S.E.; Zhou, Z.; Munsterberg, A.; Robinson, S.D.; Bao, Y. Sulforaphane exerts anti-angiogenesis effects against hepatocellular carcinoma through inhibition of STAT3/HIF-1α/VEGF signalling. Sci. Rep. 2017, 7, 12651.
- Kensler, T.W.; Egner, P.A.; Agyeman, A.S.; Visvanathan, K.; Groopman, J.D.; Chen, J.G.; Chen, T.Y.; Fahey, J.W.; Talalay, P. Keap1-nrf2 signaling: A target for cancer prevention by sulforaphane. Top. Curr. Chem. 2013, 329, 163–177.
- Lewinska, A.; Adamczyk-Grochala, J.; Deregowska, A.; Wnuk, M. Sulforaphane-Induced Cell Cycle Arrest and Senescence are accompanied by DNA Hypomethylation and Changes in microRNA Profile in Breast Cancer Cells. Theranostics 2017, 7, 3461–3477.
- Burnett, J.P.; Lim, G.; Li, Y.; Shah, R.B.; Lim, R.; Paholak, H.J.; McDermott, S.P.; Sun, L.; Tsume, Y.; Bai, S.; et al. Sulforaphane enhances the anticancer activity of taxanes against triple negative breast cancer by killing cancer stem cells. Cancer Lett. 2017, 394, 52–64.
- Lubecka-Pietruszewska, K.; Kaufman-Szymczyk, A.; Stefanska, B.; Cebula-Obrzut, B.; Smolewski, P.; Fabianowska-Majewska, K. Sulforaphane Alone and in Combination with Clofarabine Epigenetically Regulates the Expression of DNA Methylation-Silenced Tumour Suppressor Genes in Human Breast Cancer Cells. J. Nutr. Nutr. 2015, 8, 91–101.
- Tsai, J.Y.; Tsai, S.H.; Wu, C.C. The chemopreventive isothiocyanate sulforaphane reduces anoikis resistance and anchorage-independent growth in non-small cell human lung cancer cells. Toxicol. Appl. Pharmacol. 2019, 362, 116–124.
- Pereira, L.P.; Silva, P.; Duarte, M.; Rodrigues, L.; Duarte, C.M.; Albuquerque, C.; Serra, A.T. Targeting Colorectal Cancer Proliferation, Stemness and Metastatic Potential Using Brassicaceae Extracts Enriched in Isothiocyanates: A 3D Cell Model-Based Study. Nutrients 2017, 9, 368.
- Ming, Y.; Meng, R.; Yue, Q.; Wendi, T.; Zhongpeng, W.; Hao, L.; Qipeng, Y. Sulforaphene inhibits hepatocellular carcinoma through repressing keratin 8 and activating anoikis. RSC Adv. 2016, 6, 70326–70334.
- Fahey, J.W.; Wade, K.L.; Stephenson, K.K.; Panjwani, A.A.; Liu, H.; Cornblatt, G.; Cornblatt, B.S.; Ownby, S.L.; Fuchs, E.; Holtzclaw, W.D.; et al. Bioavailability of Sulforaphane Following Ingestion of Glucoraphanin-Rich Broccoli Sprout and Seed Extracts with Active Myrosinase: A Pilot Study of the Effects of Proton Pump Inhibitor Administration. Nutrients 2019, 11, 1489.
- Curran, K.M.; Bracha, S.; Wong, C.P.; Beaver, L.M.; Stevens, G.F.; Ho, E. Sulforaphane absorption and histone deacetylase activity following single dosing of broccoli sprout supplement in normal dogs. Vet. Med. Sci. 2018, 4, 357–363.
- Fahey, J.W.; Wehage, S.L.; Holtzclaw, W.D.; Kensler, T.W.; Egner, P.A.; Shapiro, T.A.; Talalay, P. Protection of humans by plant glucosinolates: Efficiency of conversion of glucosinolates to isothiocyanates by the gastrointestinal microflora. Cancer Prev. Res. 2012, 5, 603–611.
- Clarke, J.D.; Hsu, A.; Riedl, K.; Bella, D.; Schwartz, S.J.; Stevens, J.F.; Ho, E. Bioavailability and inter-conversion of sulforaphane and erucin in human subjects consuming broccoli sprouts or broccoli supplement in a cross-over study design. Pharmacol. Res. 2011, 64, 456–463.
- Rutka, J.T.; Akiyama, Y.; Lee, S.P.; Ivanchuk, S.; Tsugu, A.; Hamel, P.A. Alterations of the p53 and pRB Pathways in Human Astrocytoma. Rev. Brain Tumor Pathol. 2000, 17, 65–70.
- Kalia, M. Biomarkers for personalized oncology: Recent advances and future challenges. Metabolism 2015, 64, S16–S21.
- Cirrone, G.A.P.; Margarone, D.; Maggiore, M.; Anzalone, A.; Borghesi, M.; Jia, S.B.; Bulanov, S.S.; Bulanov, S.; Carpinelli, M.; Cavallaro, S.; et al. ELIMED: A New Hadron Therapy Concept Based on Laser Driven Ion Beams. In Proceedings of the SPIE Optics + Optoelectronics, Prague, Czech Republic, 18–21 April 2011; The International Society for Optical Engineering: Bellingham, DC, USA, 2013; Volume 8779, p. 87791I.
- Zhong, X.; Rescorla, F.J. Cell surface adhesion molecules and adhesion-initiated signaling: Understanding of anoikis resistance mechanisms and therapeutic opportunities. Cell Signal. 2012, 24, 393–401.
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and Mediterranean Diet: A Review. Nutrients 2019, 11, 2059.
- Paul, B.; Li, Y.; Tollefsbol, T.O. The Effects of Combinatorial Genistein and Sulforaphane in Breast Tumor Inhibition: Role in Epigenetic Regulation. Int. J. Mol. Sci. 2018, 19, 1754.
- Azarenko, O.; Okouneva, T.; Singletary, K.W.; Jordan, M.A.; Wilson, L. Suppression of microtubule dynamic instability and turnover in MCF7 breast cancer cells by sulforaphane. Carcinogenesis 2008, 29, 2360–2368.
- Lockett, S.; Verma, C.; Brafman, A.; Gudla, P.; Nandy, K.; Mimaki, Y.; Fuchs, P.L.; Jaja, J.; Reilly, K.M.; Beutler, J.; et al. Quantitative Analysis of F-Actin Redistribution in Astrocytoma Cells Treated with Candidate Pharmaceuticals. Cytom. Part A 2014, 85, 512–521.
- Byun, S.; Shin, S.H.; Park, J.; Lim, S.; Lee, E.; Lee, C.; Sung, D.; Farrand, L.; Lee, S.R.; Kim, K.H.; et al. Sulforaphene suppresses growth of colon cancer-derived tumors via induction of glutathione depletion and microtubule depolymerization. Mol. Nutr. Food Res. 2016, 60, 1068–1078.
- Malric, L.; Monferran, S.; Gilhodes, J.; Boyrie, S.; Dahan, P.; Skuli, N.; Sesen, J.; Filleron, T.; Kowalski-Chauvel, A.; Cohen-Jonathan Moyal, E.; et al. Interest of integrins targeting in glioblastoma according to tumor heterogeneity and cancer stem cell paradigm: An update. Oncotarget 2017, 8, 86947–86968.
- Renner, G.; Noulet, F.; Mercier, M.C.; Choulier, L.; Etienne- Selloum, N.; Gies, J.P.; Lehmann, M.; Lelong-Rebel, I.; Martin, S.; Dontenwill, M. Expression/activation of alpha5beta1 integrin is linked to the beta-catenin signaling pathway to drive migration in glioma cells. Oncotarget 2016, 7, 62194–62207.
- Maglott, A.; Bartik, P.; Cosgun, S.; Klotz, P.; Ronde, P.; Fuhrmann, G.; Takeda, K.; Martin, S.; Dontenwill, M. The small alpha5beta1 integrin antagonist, SJ749, reduces proliferation and clonogenicity of human astrocytoma cells. Cancer Res. 2006, 66, 6002–6007.
- Pankov, R.; Cukierman, E.; Katz, B.Z.; Matsumoto, K.; Lin, D.C.; Lin, S.; Hahn, C.; Yamada, K.M. Integrin Dynamics and Matrix Assembly: Tensin-dependent Translocation of α5β1 Integrins Promotes Early Fibronectin Fibrillogenesis. J. Cell Biol. 2000, 148, 1075–1090.
- Redon, C.E.; Nakamura, A.J.; Zhang, Y.W.; Ji, J.J.; Bonner, W.M.; Kinders, R.J.; Parchment, R.E.; Doroshow, J.H.; Pommier, Y. Histone gammaH2AX and poly(ADP-ribose) as clinical pharmacodynamic biomarkers. Clin. Cancer Res. 2010, 16, 4532–4542.
- Sekine-Suzuki, E.; Yu, D.; Kubota, N.; Okayasu, R.; Anzai, K. Sulforaphane induces DNA double strand breaks predominantly repaired by homologous recombination pathway in human cancer cells. Biochem. Biophys. Res. Commun. 2008, 377, 341–345.
- Hoffman, J.D.; Ward, W.M.; Loo, G. Effect of antioxidants on the genotoxicity of phenethyl isothiocyanate. Mutagenesis 2015, 30, 421–430.
- Żuryń, A.; Litwiniec, A.; Safiejko-Mroczka, B.; Klimaszewska-Wiśniewska, A.; Gagat, M.; Krajewski, A.; Gackowska, L.; Grzanka, D. The effect of sulforaphane on the cell cycle, apoptosis and expression of cyclin D1 and p21 in the A549 non-small cell lung cancer cell line. Int. J. Oncol. 2016, 48, 2521–2533.
- Clarke, J.D.; Hsu, A.; Yu, Z.; Dashwood, R.H.; Ho, E. Differential effects of sulforaphane on histone deacetylases, cell cycle arrest and apoptosis in normal prostate cells versus hyperplastic and cancerous prostate cells. Mol. Nutr. Food Res. 2011, 55, 999–1009.
- Olivier, M.; Hollstein, M.; Hainaut, P. TP53 mutations in human cancers: Origins, consequences, and clinical use. Cold Spring Harb. Perspect. Biol. 2010, 2, a001008.
- Pan, Y.; Haines, D.S. Identification of a tumor-derived p53 mutant with novel transactivating selectivity. Oncogene 2000, 19, 3095–3100.
- Zhang, Y.; Zhang, Y.J.; Zhao, H.Y.; Zhai, Q.L.; Zhang, Y.; Shen, Y.F. The impact of R213 mutation on p53-mediated p21 activity. Biochimie 2014, 99, 215–218.
- Lenzi, M.; Fimognari, C.; Hrelia, P. Sulforaphane as a Promising Molecule for Fighting Cancer. Cancer Treat. Res. 2014, 159, 207–223.
- Powell, E.; Piwnica-Worms, D.; Piwnica-Worms, H. Contribution of p53 to metastasis. Cancer Discov. 2014, 4, 405–414.
- El-Deiry, W.S. P21 (WAF1) mediates cell-cycle inhibition, relevant to cancer suppression and therapy. Cancer Res. 2016, 76, 5189–5191.
- Cordani, M.; Butera, G.; Pacchiana, R.; Masetto, F.; Mullappilly, N.; Riganti, C.; Donadelli, M. Mutant p53-Associated Molecular Mechanisms of ROS Regulation in Cancer Cells. Biomolecules 2020, 10, 361.
- Naletova, I.; Satriano, C.; Curci, A.; Margiotta, N.; Natile, G.; Arena, G.; La Mendola, D.; Nicoletti, V.; Rizzarelli, E. Cytotoxic phenanthroline derivatives alter metallostasis and redox homeostasis in neuroblastoma cells. Oncotarget 2018, 9, 36289–36316.
- Pop, S.; Enciu, A.M.; Tarcomnicu, I.; Gille, E.; Tanase, C. Phytochemicals in cancer prevention: Modulating epigenetic alterations of DNA methylation. Phytochem. Rev. 2019, 18, 1005–1024.
- Tomasello, B.; Malaguarnera, M.; Renis, M.; Di Giacomo, C. Physical Exercise and oxidative stress biomarkers in the elderly. Biochim. Clin. 2020, 44, 36–44.
- Carafa, V.; Rotili, D.; Forgione, M.; Cuomo, F.; Serretiello, E.; Hailu, G.S.; Jarho, E.; Lahtela-Kakkonen, M.; Mai, A.; Altucci, L. Sirtuin functions and modulation: From chemistry to the clinic. Clin. Epigenet. 2016, 8, 61.
- Palmirotta, R.; Cives, M.; Della-Morte, D.; Capuani, B.; Lauro, D.; Guadagni, F.; Silvestris, F. Sirtuins and Cancer: Role in the Epithelial-Mesenchymal Transition. Oxid. Med. Cell. Longev. 2016, 3031459.
- Carafa, V.; Altucci, L.; Nebbioso, A. Dual Tumor Suppressor and Tumor Promoter Action of Sirtuins in Determining Malignant Phenotype. Front. Pharmacol. 2019, 10, 38.
- Gilkes, D.M.; Xiang, L.; Lee, S.J.; Chaturvedi, P.; Hubbi, M.E.; Wirtz, D.; Semenza, G.L. Hypoxia-inducible factors mediate coordinated RhoA-ROCK1 expression and signaling in breast cancer cells. Proc. Natl. Acad. Sci. USA 2014, 111, E384–E393.
- Sasaki, T.; Maier, B.; Koclega, K.D.; Chruszcz, M.; Gluba, W.; Stukenberg, P.T.; Minor, W.; Scrable, H. Phosphorylation regulates SIRT1 function. PLoS ONE 2008, 3, e4020.
